
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 19 April 2024
Sec. Pediatric Infectious Diseases
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1355502
Introduction: Despite recommendations for COVID-19 vaccination in pregnant people, the effect of vaccination on neonatal outcomes remains unknown. We sought to determine the association between COVID-19 vaccination status in pregnancy and presence of neonatally diagnosed congenital anomalies.
Methods: A comprehensive vaccine registry was combined with a delivery database to create a cohort including all patients aged 16–55 years with a delivery event between December 10, 2020 and December 31, 2021 at a hospital within the Mayo Clinic Health System. Pregnancy and neonatal outcomes were analyzed in relation to vaccination status and timing, including a composite measure of congenital anomalies diagnosed in neonatal life. Comparisons between cohorts were conducted using chi-square test for categorical and Kruskal–Wallis test for continuous variables. A multivariable logistic regression was modeled to assess the association with congenital anomalies.
Results: 5,096 mother-infant pairs were analyzed. A total of 1,158 were vaccinated, with 314 vaccinated in the first trimester. COVID-19 vaccination status, including vaccination during the first trimester of pregnancy, was not associated with an increased risk of composite congenital anomalies. When further examining congenital anomalies by organ system, we did demonstrate a significant difference in eye, ear, face, neck anomalies between vaccinated and not vaccinated groups (Table 3, Not vaccinated = 2.3%, Vaccinated = 3.3%, p-value 0.04) however we did not demonstrate this difference between the 1st trimester and not vaccinated groups (Not vaccinated = 2.3%, 1st Trimester = 2.5%, p-value 0.77). No differences were found between not vaccinated, vaccinated, or 1st trimester vaccinated groups for any other organ systems. There were no differences in birthweight by gestational age, APGAR scores, incidence of NICU admission, or living status of the neonate by vaccination status.
Conclusion: We add additional information regarding the safety of COVID-19 vaccination status and timing as it pertains to neonatal composite congenital anomalies, with no association demonstrated. Our findings agree with prior literature that COVID-19 vaccination is not associated with adverse pregnancy outcomes or small for gestational age neonates. Further research is needed to elucidate the association between COVID-19 vaccination and eye, ear, face, neck, anomalies.
SARS-COV-2 infection has been associated with increased risks in the pregnant compared to non-pregnant populations. Need for ICU admission, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) have been shown to be significantly higher among pregnant people with COVID-19 disease (1, 2). Pregnant people with SARS-COV-2 infection also have increased mortality compared to uninfected pregnant people (2, 3). In addition, SARS-COV-2 infection in pregnancy has been associated with adverse pregnancy outcomes including preterm delivery, stillbirth, and increased risk of maternal mortality and morbidity from obstetric complications such as hypertensive disorders of pregnancy and secondary infections (3–7).
In April 2021, the Centers for Disease Control and Prevention (CDC) announced that pregnant people who were eligible for the COVID-19 vaccine should receive it after data from 90,000 participants in vaccine safety registries did not identify any safety concerns in pregnant participants or their offspring (8, 9). Despite reassuring evidence and recommendations for vaccination, two global meta-analyses demonstrated only 49%–54% of pregnant women would be accepting of vaccination (10, 11). There is currently no guidance for vaccine administration at any particular gestational age, and many pregnant people choose to vaccinate in the second or third trimesters to avoid theoretical concerns surrounding the possible effects of vaccination on organogenesis (12). We report on the association between COVID-19 vaccination timing in pregnancy and congenital anomalies as diagnosed in neonatal life.
Patient information was collected using a comprehensive vaccine registry that was linked to Mayo Clinic as well as the Mayo Clinic Health System delivery registry. The Mayo Clinic Health System is as system of community-based medical facilities owned by Mayo Clinic. The vaccine registry captured COVID-19 vaccine administrations, manufacturers, and patients, as well as identifying information from Mayo Clinic vaccination sites and other sites across the states of Minnesota and Wisconsin. Our delivery registry data are directly derived from elements in the electronic medical record and were used in a previous study (13). All fields were validated manually during development. The creation of the registries and subsequent analysis were performed under approval by the Mayo Clinic Institutional Review Board.
This was a retrospective cohort study including all patients aged 16–55 years with a delivery event between December 10, 2020 and December 31, 2021 at hospitals within an integrated healthcare system. To be included, pregnancy must have achieved at least 20 weeks gestation at time of delivery. Gestational age was established using American College of Obstetrics and Gynecology (ACOG) criteria (14). In accordance with Minnesota law, patients who opted out of using their medical records for research were excluded from the study. Corresponding infants were included if the infant had research authorization as well.
To assess vaccination status and pregnancy outcomes, vaccinated individuals were defined as those receiving any dosage or formulation of the COVID-19 vaccine from 30 days prior to pregnancy onset, defined as 30 days prior to day 1 of pregnancy by ACOG dating, until delivery. Dates of each dose were captured as well as the timing in relation to the pregnancy (pre-pregnancy, 1st, 2nd, 3rd trimester). Vaccination status was categorized as none, at least one dose during the first trimester (1st Tri) and all other vaccinations (Other), which may have occurred pre-pregnancy or during pregnancy as long as no doses occurred during the first trimester (Supplementary Appendix A). Timing of vaccination was determined via electronic health record documentation. Most vaccinations were mRNA vaccines manufactured by either Pfizer or Moderna (Supplementary Appendix B). COVID-19 status indicates the presence of infection during the pregnancy, regardless of temporal relation to the vaccine, defined as a positive SARS-COV-2 result via reverse-transcription-polymerase chain reaction test documented in the medical record between day 1 of pregnancy and delivery.
The primary outcome of congenital anomalies is a composite outcome calculated as the summation of infants with one or more congenital anomaly diagnosis codes (Supplementary Table S1). Our congenital anomaly list most closely mirrors that used by the Metropolitan Atlanta Congenital Defects Program (MACDP), the reference used by the CDC (15). All anomalies were diagnosed in neonatal life, as opposed to prenatal ultrasound. We used ICD-10 diagnostic codes to identify anomalies which were then verified by manual medical record review of neonatal inpatient and outpatient records. All infants (living, stillbirth, neonatal demise, and therapeutic abortion) were examined for congenital anomalies. If infants had multiple anomalies, they were only counted once in the congenital anomaly composite. For each anomaly type (chromosomal, musculoskeletal, urinary, genital, digestive, respiratory, circulatory, nervous, the combined eye, ear, face and neck, and other) we assessed this as present vs. absent, where each system has only value per infant. Our end date for infant follow up was January 31, 2022, with mean follow up time of 242.3 days and standard deviation of 104.9 days.
The secondary outcome of birthweight by gestational age was calculated using a published United States birth weight reference (16). Large for gestational age (LGA) was defined as greater than the 90th percentile and small for gestational age (SGA) was defined as less than the 10th percentile of neonatal birth weight. Infants born at less than 24 weeks gestation were placed in their own category as there are no established birth weight curves below this gestational age. Other neonatal outcomes examined include NICU admission and living status.
Based on estimates from the CDC in conjunction with the MACDP, major structural or genetic birth defects affect approximately 3% of United States births (17). A power calculation was performed after data collection. This study has 80% power using a two-sided chi-square test with a type 1 error of 0.05, to detect a difference in the congenital anomaly rate of 3% (3% vs. 6%) between infants who were and were not exposed to COVID-19 vaccination during the first trimester (at least one dose during first trimester) compared to unvaccinated cohorts.
Comparisons between groups were evaluated using the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. A multivariable logistic regression model to assess the association between the outcome of congenital anomalies and vaccination status was adjusted by maternal age at delivery, smoking status, illicit drug use, gravidity, COVID-19 during pregnancy, pre-gestational diabetes, and chronic hypertension. Pre-gestational diabetes was added to the regression model despite being non-significant in the univariate analysis due to the frequency of the condition and its known association with various congenital anomalies (18). Diagnostic codes used for maternal conditions are outlined in Supplementary Table S2.
For neonatal weight at delivery, a nominal regression model was conducted with appropriate for gestational age (AGA) as the reference group and adjusting for the same factors as referenced above. As only 23 infants from 20 patients were less than 24 weeks gestation, this group was removed for modeling purposes. Interactions between COVID-19 infection during pregnancy and COVID-19 vaccination (none, 1st Tri, other) were tested using the likelihood ratio test for both the primary and secondary outcome. All model assumptions were validated (19). Analysis was performed using SAS (version 9.4; SAS Institute Inc., Cary, NC). All calculated p-values were 2-sided and any values <0.05 were considered statistically significant.
A total of 5,096 patient-infant pairs delivering between December 10, 2020 and December 31, 2021 were included in our analysis. Of these, a total of 3,938 patients were not vaccinated and 1,158 were vaccinated (Figure 1). Of those vaccinated, 314 were vaccinated in the first trimester, with 57 out of those 314 having had at least one dose of the vaccine pre-pregnancy. Of the 844 patients in the other vaccinated group, 3 had doses only prior to pregnancy, 5 had doses prior to and during pregnancy (2nd and 3rd trimesters), and the remaining 836 had doses only in the second and/or third trimesters.
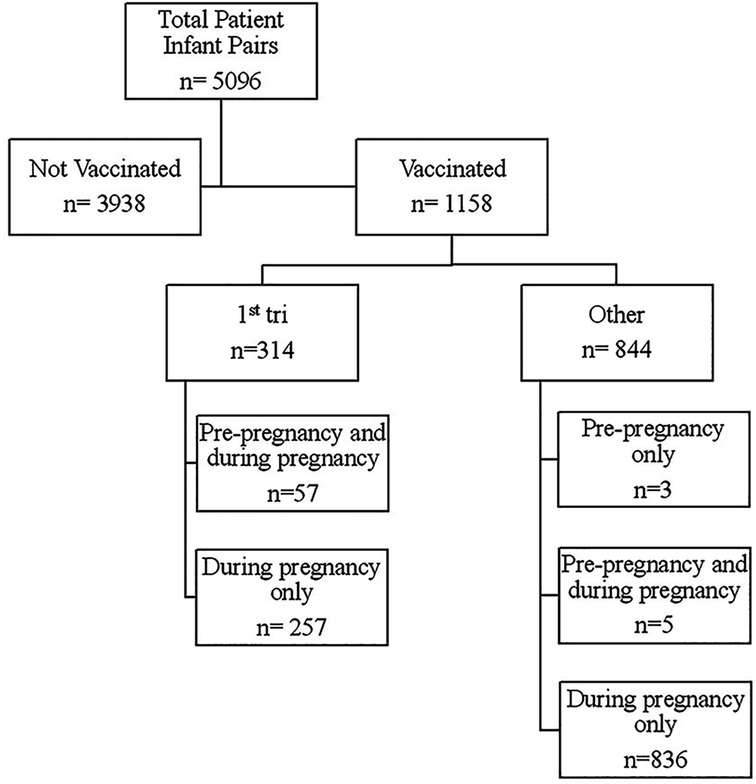
Figure 1. Flow diagram of vaccination timing in relation to pregnancy start. 1st tri = All patients receiving at least one dose of SARS-CoV-2 vaccine in the first trimester. Other = All patients receiving at least one dose of SARS-CoV-2 vaccine as long as no doses were received in the first trimester.
Several patient characteristics differed between vaccinated and unvaccinated groups. Vaccinated pregnant people were significantly older, more likely to be White non-Hispanic, were of lower gravidity and parity, and were more likely to have chronic hypertension. Vaccinated pregnant people were significantly less likely to be obese or have substance use disorder. We found no difference between vaccinated and unvaccinated groups regarding presence of pre-gestational diabetes or multiple pregnancy (Table 1). The rates of gestational diabetes, gestational hypertension, and preeclampsia did not differ significantly between vaccinated and not vaccinated groups. Gestational age at delivery did not differ amongst groups. However, it should be regarded that 6 infants in the unvaccinated group were missing gestational age information. Unvaccinated pregnant people were significantly more likely to have COVID-19 during pregnancy however, the rate of monoclonal antibody (MAB) treatment, the treatment recommended for outpatient COVID-19 illness during the study period, did not differ significantly (Table 1). Among those who were vaccinated and acquired COVID-19 during the pregnancy, most were diagnosed with COVID-19 prior to their first vaccination (Table 2).
No significant difference in the composite congenital anomalies outcome was observed when examining infant characteristics by not vaccinated, vaccinated, or 1st trimester vaccination groups (Table 3, 20.0% vs. 19.1% vs. 19.1%, 1st Trimester p-value 0.72, Vaccinated p-value 0.53). When further examining congenital anomalies by organ system, we did demonstrate a significant difference in eye, ear, face, neck anomalies between vaccinated and not vaccinated groups (Table 3, Not vaccinated = 2.3%, Vaccinated = 3.3%, p-value 0.04), but we did not demonstrate this difference between the 1st trimester and not vaccinated groups (Table 3, Not vaccinated = 2.3%, 1st Trimester = 2.5%, p-value 0.77). The relative risk for an ENT anomaly among vaccinated infants was 1.47 times more likely than unvaccinated infants, with the 95% confidence interval ranging from 1.02 to 2.13 times. No differences were found between not vaccinated, vaccinated, or 1st trimester vaccinated groups for any other organ systems. We manually reviewed the specific eye, ear, face, neck anomalies diagnosed in not vaccinated vs. vaccinated groups (Table 4). None of these anomalies would be considered major congenital anomalies, with all requiring either no intervention or minor surgical procedures (15). We found no difference for infant sex, NICU admission, and living status across all groups. When adjusting for covariates listed in the statistical section, no significant difference was found by vaccination status (Table 5, 1st Trimester Vaccine OR = 0.92 95% CI 0.69–1.24, Other Vaccine OR = 0.93 95% CI 0.77–1.13).
Lastly, we examined the association of birthweight with vaccination status. No difference in birthweight was observed when analyzed by vaccination status in any trimester or vaccination in first trimester (Table 3). Of note, there were 7 missing gestational ages in the unvaccinated group and so these could not be analyzed. No association was found when adjusting by covariates within a nominal multivariable model (Table 5). Interaction between birthweight by gestational age, vaccine status, and COVID-19 infection was found not to be statistically significant (Figure 2).
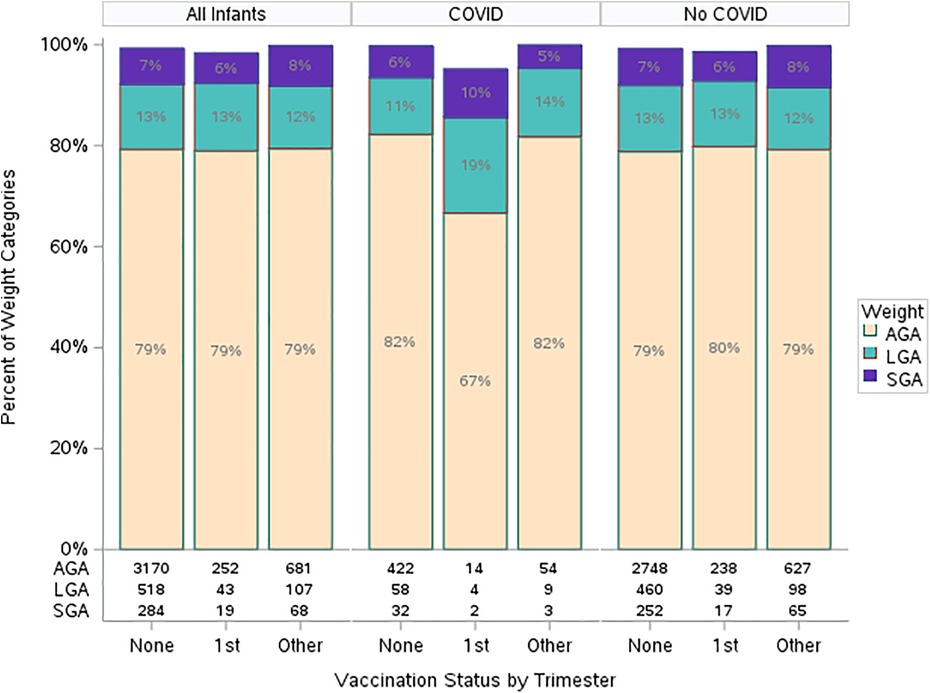
Figure 2. Gestational weight by vaccination and COVID-19 infection status. 1st trimester vaccine = All patients receiving at least one dose of SARS-CoV-2 vaccine in the first trimester. Other vaccine = All patients receiving at least one dose of SARS-CoV-2 vaccine as long as no doses were received in the first trimester. SGA, small for gestational age; LGA, large for gestational age; AGA, appropriate for gestational age; GA, gestational age.
In this prospective cohort study, COVID-19 vaccination status including vaccination during the first trimester was not associated with an increased risk of composite congenital anomalies. We did notice a significant difference in the presence of minor eye, ear, face, neck anomalies among vaccinated vs. not vaccinated groups (Table 3, Not vaccinated = 2.3%, Vaccinated = 3.3%, p-value 0.04). However, we did not demonstrate this difference between 1st trimester vaccination and not vaccinated groups (Table 3, Not vaccinated = 2.3%, 1st Trimester = 2.5%, p-value 0.77). No other associations were found between not vaccinated, vaccinated, and first trimester vaccination groups for anomalies by any other organ systems. We did not see differences in birthweight by gestational age, APGAR scores, incidence of NICU admission, or living status of the neonate. COVID-19 vaccination status in pregnancy was not associated with increased rates of pregnancy complications including gestational diabetes, gestational hypertension, and preeclampsia.
COVID-19 vaccination in pregnancy has been shown to be similarly effective as vaccination in the non-pregnant population, with one study demonstrating 89% (43%–100%) prevention of COVID-19-related hospitalizations in pregnant populations (20) and another demonstrating risk of progression to severe disease was reduced by 74% after the primary series and 91% after the booster dose (21). Given the known increased risk of mortality and severe COVID-19 disease in pregnant people, there is a clear patient benefit for vaccination (1, 2, 13). Additionally, data has shown vaccination is associated with improved pregnancy outcomes and may be beneficial to offspring. Several studies demonstrate associated reduced risk of stillbirth, neonatal death, and premature delivery with vaccination administration during pregnancy (22–24). One meta-analysis demonstrated significantly lower risk of stillbirth by 15% in vaccinated cohorts (25). Evidence has also accrued regarding vaccine efficacy in infants after vaccination administration during pregnancy. The presence of functional anti-spike IgG antibodies in umbilical cord blood has been shown (26, 27), with one study demonstrating 61% (95% CI = 31%–78%) effectiveness of COVID-19 vaccination during pregnancy against critical illness and hospitalization from COVID-19 infection among infants aged <6 months (28, 29). Infant COVID-19 infection and hospitalization prevention associated with maternal vaccination during pregnancy has now been corroborated in Canadian, Israeli, and Norwegian population studies (30–32).
Many trials have now examined neonatal outcomes as related to COVID-19 vaccination in pregnancy (22, 33–39). One large retrospective cohort study following 46,079 pregnant people demonstrated no increased risk of small for gestational age infants; however, only 1.7% of the study population received the vaccine in the first trimester (37). A large Swedish cohort of 94,303 neonates exposed to the vaccine during pregnancy exhibited no increased risk of adverse neonatal outcomes including complications such as disorders of the nervous, circulatory, respiratory and gastrointestinal systems as well as hematologic and infectious complications (40). Several studies have also emerged surrounding the safety of vaccination as it pertains to congenital anomalies. Another study examining the presence of congenital anomalies at time of anatomy ultrasound did not find a difference associated with vaccination. Remarkably, 1,149 (43.8%) of those vaccinated did receive the vaccine during the teratogenic window, however, this study remains limited in that it did not examine congenital anomalies as diagnosed in neonatal life (38). A population registry of all singleton livebirths in Israel demonstrated no association between neonatal anomalies and vaccine uptake during pregnancy, but by design excluded fetuses who miscarried or were terminated (36). Perhaps the most robust study to date on the subject was conducted by Calvert et al. This was a matched cohort population study in Scotland finding no association between vaccination 6 weeks pre-conception to 19 weeks gestational age and major congenital anomalies diagnosed in neonatal life (35). This study included all clinically recognized pregnancies ending in any outcome.
Evidence is rapidly accruing regarding the safety of COVID-19 vaccination for pregnant people and their offspring. Our study adds to this data and examines the association of vaccination in pregnancy and congenital anomalies diagnosed in neonatal life, with no increased risk of composite congenital anomalies. We did demonstrate a significant difference in eye, ear, face, neck anomalies between vaccinated and not vaccinated groups but did not demonstrate this difference between the first trimester and not vaccinated groups. It seems unlikely that vaccination itself would be the cause of the increase in ENT anomalies seen as this increased risk was not observed after vaccination in the first trimester when the fetus would be at highest teratogenic risk. It may be possible that there are other differences between populations vaccinated in first vs. later trimesters that could account for this difference. This finding may also be a type 1 error in our study. Calvert et al. does examine the presence of eye, ear, face, neck anomalies, however, their analysis is limited to major anomalies while our analysis includes all anomalies. Table 4 outlines the eye, ear, face, neck anomalies found in our study, with most being considered minor and/or cosmetic in nature, requiring no or minimal interventions (35). Based off the amounting data from other studies, we would encourage patients to pursue vaccination in all trimesters of pregnancy. In addition, our study agrees with previous studies on the lack of association between COVID-19 vaccination in pregnancy and small for gestational age neonates (22, 23, 34, 37). These results can be used to counsel pregnant patients making decisions regarding vaccination.
While our results are reassuring, a minority (n = 314, 27.1%) of our vaccinated cohort was vaccinated in the first trimester and additional studies will be needed to examine differences in rare adverse birth outcomes following early pregnancy vaccination. Further research needs to be conducted specifically examining the association of neonatal outcomes with first trimester vaccination—the time of organogenesis—to better define risks of COVID vaccination. In addition, future studies should include a diverse population at the multicenter level as our study was limited demographically. Lastly, our study was conducted prior to the introduction of the bivalent COVID-19 vaccine. Further studies are needed examining this formulation as this is the predominant COVID-19 vaccine given today.
The strengths of this study include the use of a comprehensive population level vaccine registry data in combination with a validated, all-inclusive delivery database including births at multiple community and teaching hospitals across two states. Data were extracted from the primary medical record, and all identified congenital anomalies were verified by medical record review. Limitations of this analysis include the small percentage of non-White subjects in taken from a small geographic region which may not be representative of a more diverse patient population. Additionally, a minority (n = 320, 20.1%) of our vaccinated cohort was vaccinated in the first trimester. We had 80% power to detect a difference between unvaccinated and first trimester vaccine cohorts at a 3% difference (3% vs. 6%). It is possible that a smaller difference exists that was unable to be detected. Although the incidence of chromosomal anomalies and monochorionic-diamniotic gestations appear to be similar in both groups, we did not control for this in our analysis. We did examine teratogenicity as a cause of congenital anomalies (ICD-10 code Q86.xx), however, this was also not controlled for in our analysis. Substance use was most common in the unvaccinated group, but we cannot report on the specific use of teratogenic substance or medications beyond this. Finally, this study was conducted prior to the advent of the bivalent version of the SARS-CoV-2 vaccine, protecting against the Omicron BA.4/BA.5 variant, the principal vaccine given today.
Our findings add to the existing research regarding the safety of COVID-19 vaccination as it pertains to pregnancy and neonatal outcomes. We corroborate prior evidence that COVID-19 vaccination is not associated with adverse pregnancy outcomes or increased incidence of small for gestational age neonates. With this study, we add new information regarding the safety of COVID-19 vaccination as it pertains to composite neonatal congenital anomalies, with no association demonstrated. This data should encourage patients and providers to pursue vaccination in all trimesters of pregnancy.
These findings were presented at the SMFM 43rd Annual Pregnancy Meeting in San Francisco, CA. February 9, 2023.
The data analyzed in this study is subject to the following licenses/restrictions: protected electronic health record information. Requests to access these datasets should be directed todGhlaWxlci5yZWdhbkBtYXlvLmVkdQ==.
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JS: Visualization, Writing – original draft, Writing – review & editing. MM: Writing – review & editing. MB: Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. RM: Data curation, Formal Analysis, Visualization, Writing – review & editing. RT: Conceptualization, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
RT reports having a know-how license and research funding from HeraMED and is on the medical advisory board for Delfina.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1355502/full#supplementary-material
1. Khan DSA, Pirzada AN, Ali A, Salam RA, Das JK, Lassi ZS. The differences in clinical presentation, management, and prognosis of laboratory-confirmed COVID-19 between pregnant and non-pregnant women: a systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18(11):5613. doi: 10.3390/ijerph18115613
2. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Br Med J. (2020) 370:m3320. doi: 10.1136/bmj.m3320
3. Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. (2022) 327(8):748–59. doi: 10.1001/jama.2022.1190
4. Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. (2021) 225(5):522.e1–e11. doi: 10.1016/j.ajog.2021.05.016
5. DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, Ellington SR. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1640–5. doi: 10.15585/mmwr.mm7047e1external
6. Ferrara A, Hedderson MM, Zhu Y, Avalos LA, Kuzniewicz MW, Myers LC, et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. (2022) 182(5):503–12. doi: 10.1001/jamainternmed.2022.0330
7. Schwartz DA, Mulkey SB, Roberts DJ. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: clinical-pathologic correlations. Am J Obstet Gynecol. (2023) 228(3):261–9. doi: 10.1016/j.ajog.2022.10.001
8. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. (2021) 384(24):2273–82. doi: 10.1056/NEJMoa2104983
9. Centers for Disease Control and Prevention. V-safe COVID-19 Vaccine Pregnancy Registry. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html (accessed May 16, 2022)
10. Carbone L, Di Girolamo R, Mappa I, Saccone G, Raffone A, Di Mascio D, et al. Worldwide beliefs among pregnant women on SARS-CoV-2 vaccine: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2022) 268:144–64. doi: 10.1016/j.ejogrb.2021.12.003
11. Nikpour M, Sepidarkish M, Omidvar S, Firouzbakht M. Global prevalence of acceptance of COVID-19 vaccines and associated factors in pregnant women: a systematic review and meta-analysis. Expert Rev Vaccines. (2022) 21(6):843–51. doi: 10.1080/14760584.2022.2053677
12. Poliquin V, Castillo E, Boucoiran I, Wong J, Watson H, Yudin M, Moner D, Van Schalkwyk J, Elwood C. SOGC Statement on COVID-19 Vaccination in Pregnancy. (2020). Available online at: https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf (Accessed January 05, 2022).
13. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. (2021) 3(6):100467. doi: 10.1016/j.ajogmf.2021.100467
14. Pettker CM, Goldberg JD, El-Sayed YY. Committee opinion No 700: methods for estimating the due date. Obstet Gynecol. (2017) 129(5):e150–4. doi: 10.1097/AOG.0000000000002046
15. DeSilva M, Munoz FM, McMillan M, Kawai AT, Marshall H, Macartney KK, et al. Congenital anomalies: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2016) 34(49):6015–26. doi: 10.1016/j.vaccine.2016.03.047
16. Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. (2014) 124(1):16–22. doi: 10.1097/AOG.0000000000000345
17. Prevention, C.f.D.C.a. Update on Overall Prevalence of Major Birth Defects—Atlanta, Georgia, 1978–2005. 2008 January 10, 2008 March 2, 2023]. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm (Accessed January 05, 2022).
18. Kitzmiller JL, Wallerstein R, Correa A, Kwan S. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. (2010) 88(10):791–803. doi: 10.1002/bdra.20734
19. Hosmer DW, Lemeshow S. Applied Logistic Regression. 3rd ed. New York: John Wiley & Sons, Inc. (2013).
20. Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. (2021) 27(10):1693–5. doi: 10.1038/s41591-021-01490-8
21. Villar J, Soto Conti CP, Gunier RB, Ariff S, Craik R, Cavoretto PI, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. (2023) 401(10375):447–57. doi: 10.1016/S0140-6736(22)02467-9
22. Ding C, Liu Y, Pang W, Zhang D, Wang K, Chen Y. Associations of COVID-19 vaccination during pregnancy with adverse neonatal and maternal outcomes: a systematic review and meta-analysis. Front Public Health. (2023) 11:1044031. doi: 10.3389/fpubh.2023.1044031
23. Morgan JA, Biggio JR Jr, Martin JK, Mussarat N, Elmayan A, Chawla HK, et al. Pregnancy outcomes in patients after completion of the mRNA coronavirus disease 2019 (COVID-19) vaccination series compared with unvaccinated patients. Obstet Gynecol. (2023) 141(3):555–62. doi: 10.1097/AOG.0000000000005072
24. Zhang D, Huang T, Chen Z, Zhang L, Gao Q, Liu G, et al. Systematic review and meta-analysis of neonatal outcomes of COVID-19 vaccination in pregnancy. Pediatr Res. (2023) 94(1):34–42. doi: 10.1038/s41390-022-02421-0
25. Prasad S, Kalafat E, Blakeway H, Townsend R, O'Brien P, Morris E, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. (2022) 13(1):2414. doi: 10.1038/s41467-022-30052-w
26. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. (2021) 225(3):303.e1–e17. doi: 10.1016/j.ajog.2021.03.023
27. Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. (2021) 225(2):192–4. doi: 10.1016/j.ajog.2021.03.035
28. Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Boom JA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71(7):264–70. doi: 10.15585/mmwr.mm7107e3
29. Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Pannaraj PS, et al. Maternal vaccination and risk of hospitalization for COVID-19 among infants. N Engl J Med. (2022) 387(2):109–19. doi: 10.1056/NEJMoa2204399
30. Carlsen EO, Magnus MC, Oakley L, Fell DB, Greve-Isdahl M, Kinge JM, et al. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med. (2022) 182(8):825–31. doi: 10.1001/jamainternmed.2022.2442
31. Jorgensen SCJ, Hernandez A, Fell DB, Austin PC, D'Souza R, Guttmann A, et al. Maternal mRNA COVID-19 vaccination during pregnancy and delta or omicron infection or hospital admission in infants: test negative design study. Br Med J. (2023) 380:e074035. doi: 10.1136/bmj-2022-074035
32. Lipschuetz M, Guedalia J, Cohen SM, Sompolinsky Y, Shefer G, Melul E, et al. Maternal third dose of BNT162b2 mRNA vaccine and risk of infant COVID-19 hospitalization. Nat Med. (2023) 29(5):1155–63. doi: 10.1038/s41591-023-02270-2
33. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. (2022) 226(2):236.e1–e14. doi: 10.1016/j.ajog.2021.08.007
34. Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. (2021) 3(6):100464. doi: 10.1016/j.ajogmf.2021.100464
35. Calvert C, Carruthers J, Denny C, Donaghy J, Hopcroft LEM, Hopkins L, et al. A population-based matched cohort study of major congenital anomalies following COVID-19 vaccination and SARS-CoV-2 infection. Nat Commun. (2023) 14(1):107. doi: 10.1038/s41467-022-35771-8
36. Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. (2022) 176(5):470–7. doi: 10.1001/jamapediatrics.2022.0001
37. Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. (2022) 71(1):26–30. doi: 10.15585/mmwr.mm7101e1
38. Ruderman RS, Mormol J, Trawick E, Perry MF, Allen EC, Millan D, et al. Association of COVID-19 vaccination during early pregnancy with risk of congenital fetal anomalies. JAMA Pediatr. (2022) 176(7):717–9. doi: 10.1001/jamapediatrics.2022.0164
39. Prabhu M, Riley LE. Coronavirus disease 2019 (COVID-19) vaccination in pregnancy. Obstet Gynecol. (2023) 141(3):473–82. doi: 10.1097/AOG.0000000000005100
40. Norman M, Magnus MC, Soderling J, Juliusson PB, Naver L, Ortqvist AK, et al. Neonatal outcomes after COVID-19 vaccination in pregnancy. JAMA. (2024) 331(5):396–407. doi: 10.1001/jama.2023.26945
Keywords: neonatal, abnormalities, birth defects, neonatal outcomes, COVID-19, pregnancy, vaccination
Citation: Santos J, Miller M, Branda ME, Mehta RA and Theiler RN (2024) Maternal COVID-19 vaccination status and association with neonatal congenital anomalies. Front. Pediatr. 12:1355502. doi: 10.3389/fped.2024.1355502
Received: 14 December 2023; Accepted: 2 April 2024;
Published: 19 April 2024.
Edited by:
Maurizio Aricò, Azienda Sanitaria Locale, ItalyReviewed by:
Eleftheria Hatzidaki, University of Crete, Greece© 2024 Santos, Miller, Branda, Mehta and Theiler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regan N. Theiler dGhlaWxlci5yZWdhbkBtYXlvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.