- 1The First Affiliated Hospital of Chengdu Medical College Clinical Medical College, Chengdu, Sichuan, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Department of Otolaryngology, Chengdu First People’s Hospital, Chengdu, Sichuan, China
Background and aim: Recent studies have demonstrated the anti-allergic effects of probiotics in humans. However, their role in preventing and treating pediatric allergic rhinitis has not been thoroughly investigated. This study aimed to systematically review the efficacy and preventive effects of probiotics on pediatric allergic rhinitis.
Methods: We systematically searched PubMed, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science databases for all relevant studies on probiotics and pediatric allergic rhinitis. Studies meeting the inclusion criteria were included, data were extracted, and meta-analyses were performed.
Results: A total of 28 studies with 4,765 participants were included in this study. The pooled results showed that the use of probiotics was associated with a significant improvement in total nose symptom scores (SMD, −2.27; 95% CI, −3.26 to −1.29; P < 0.00001), itchy nose scores (SMD, −0.44; 95% CI, −0.80 to −0.07; P = 0.02), sneezing scores (SMD, −0.47; 95% CI, −0.84 to −0.10; P = 0.01), eye symptoms (SMD, −3.77; 95% CI, −5.47 to −2.07; P < 0.00001), and Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (SMD, −2.52; 95% CI, −4.12 to −0.92; P < 00001). However, the use of probiotics was not associated with the incidence of allergic rhinitis (RR, 0.9; 95% CI, 0.74–1.08; P = 0.26).
Conclusions: The present study demonstrated that probiotics were effective and safe for improving pediatric allergic rhinitis symptoms and quality of life. However, probiotics could not prevent pediatric allergic rhinitis.
1 Introduction
Allergic rhinitis (AR) is a common disease in children, characterized by nasal congestion, nasal itching, sneezing, and rhinorrhea (1). The prevalence of AR in children continues to increase (2). Based on the International Study of Asthma and Allergies in Childhood, involving 98 countries, up to 45% of children have symptoms of AR (3). About 90% of children with AR symptoms continue to experience them into adulthood (4). Although AR is not life-threatening, it can severely impact the quality of life in children and is associated with sleep-disordered breathing, learning impairment, activity limitations, and emotional disturbances (5). The current management options include allergen avoidance, antihistamines, and intranasal corticosteroids. However, achieving complete symptom resolution of AR is challenging. A survey showed that a large number of patients were dissatisfied with their medication, and up to 60% of patients were interested in finding new allergy treatments (6).
Studies have revealed that gut dysbiosis is associated with allergic diseases such as asthma, eczema, and food allergies (7–9). The allergic diseases are correlated with reduced microbial diversity before the onset of clinical symptoms, further proving the critical role of gut microbiota in these conditions (7, 8). Therefore, probiotic supplementation is considered potentially beneficial in preventing or alleviating allergic diseases. Probiotics are live microorganisms that offer immunological protection to the host by regulating, stimulating, and modulating immune responses. Some meta-analyses have demonstrated the preventive and therapeutic effects of probiotics in children with allergic diseases, such as atopic dermatitis and eczema (9, 10). However, other meta-analyses have failed to prove their preventive effect in developing asthma or wheezing in children (11). No conclusive evidence exists regarding the effects of probiotics on children with AR. The present study aimed to include more high-quality trials to evaluate the role of probiotics in the prevention and treatment of children with AR.
2 Materials and methods
The present meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (12). The clinical trials were searched in the following databases: Medline, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science, with a deadline of August 2022. The following keywords were used: “rhinitis, allergic,” “allergic rhinitis,” “allergic rhinitides, seasonal,” “pollen allergy,” “pollinosis,” “probiotics,” “prebiotics,” “children,” “childhood,” “infant,” “teenagers,” “adolescents,” “randomized,” and “trial.” The search was limited to studies in English. In addition, the references of studies or reviews on similar topics were also reviewed to avoid missing potentially relevant studies.
2.1 Inclusion and exclusion criteria
The inclusion criteria for preventive studies of AR in children were as follows: (1) infants born in families with a history of allergic disease, infants with food allergies considered at high risk of developing atopy, or healthy children, (2) in the probiotic group, the mother during pregnancy and/or the infant took probiotics, (3) in the control group, the participants received the same therapy except for probiotics, and (4) the outcome reported the incidence of AR.
The inclusion criteria for treatment studies of AR in children were as follows: (1) children diagnosed with AR, based on clinical examination, skin-prick tests, and serum allergen-specific immunoglobulin E (IgE) (2) in the probiotic group, children took probiotics, including all types of probiotic strains, (3) in the control group, children received the same therapy as the probiotic group, except for probiotics, and (4) the primary outcome included nose symptoms of AR and the secondary outcome included eye symptoms, Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ), and immunological parameters.
The exclusion criteria were as follows: (1) non-human studies; (2) non-comparative studies; (3) non-randomized controlled trials (RCTs); (4) full text not available; (5) data used in more than one study; in such cases, we included only one study and excluded the others; (6) repeatedly published trials; (7) case reports, comments, letters, reviews, and retrospective studies; (8) ongoing trials without results; and (9) no relevant outcomes.
2.2 Study selection, data extraction, and quality assessment
Two independent investigators assessed the titles abstracts, and full-text articles based on the inclusion and exclusion criteria. Any disagreements were resolved through discussion or by consulting a third investigator. Two investigators independently extracted data from each eligible study, including the name of the first author, year of publication, study design, the regimen of intervention in the probiotic group (including the probiotic dose, strain of probiotics, and treatment course), study duration, outcomes, and adverse events. When a study compared more than one probiotic group with one control group, the number of participants in the control group was divided by the number of probiotic groups. When the outcomes were reported at different time points, the data were extracted from the last time point. The study quality was assessed using the Cochrane risk-of-bias tool, which included selection bias, performance and detection bias, attrition bias, reporting bias, and other sources of bias. Two independent investigators performed the assessment, and any discrepancies were resolved by a third author.
2.3 Data synthesis and analysis
Odds ratio (OR) was used to assess the incidence of AR. Weighted mean difference (WMD) or standardized mean difference (SMD) was used to assess the AR symptoms and cytokines. When outcomes of the included studies were reported using different measurement scales, SMD was used to assess the pooled effect. The fixed-effects model was used to assess the pooled effect when low heterogeneity was considered; otherwise, the random-effects model was used. The heterogeneity among studies was assessed using the inconsistency index (I2). We considered I2 ≤ 25% as low heterogeneity, between 25% and 50% as moderate heterogeneity, and >50% as significant heterogeneity (13).
2.4 Publication bias and sensitivity analysis
Potential publication bias was assessed using a funnel plot. The sensitivity analysis was performed by deleting one study at a time to assess the stability of the pooled results. The data were analyzed using Review Manager, version 5.3 (Oxford, UK) or Stata 15.
3 Results
3.1 Literature selection and study characteristics
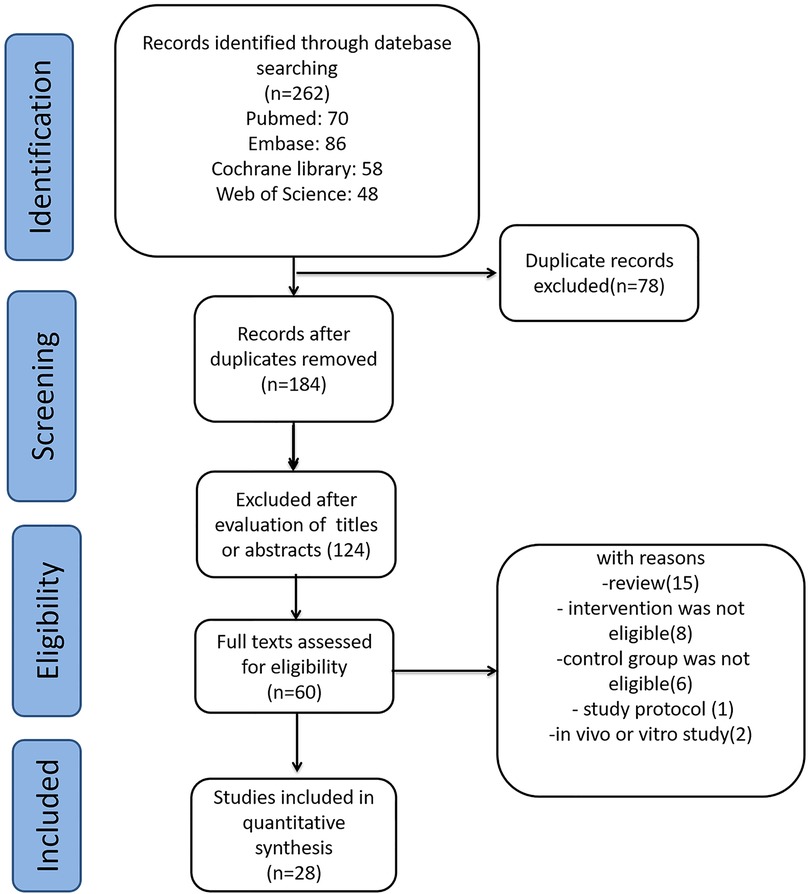
We identified 262 relevant publications from the databases. After removing 78 duplicate publications using Endnote, 184 studies were excluded based on titles and abstracts. Further, 156 studies were excluded based on the inclusion and exclusion criteria. Finally, 28 studies met the criteria and were included in this meta-analysis. The other studies were excluded for reasons such as being reviews, in vivo studies, study protocols, or having ineligible intervention or control groups. A flow diagram depicting the selection of studies is shown in Figure 1.
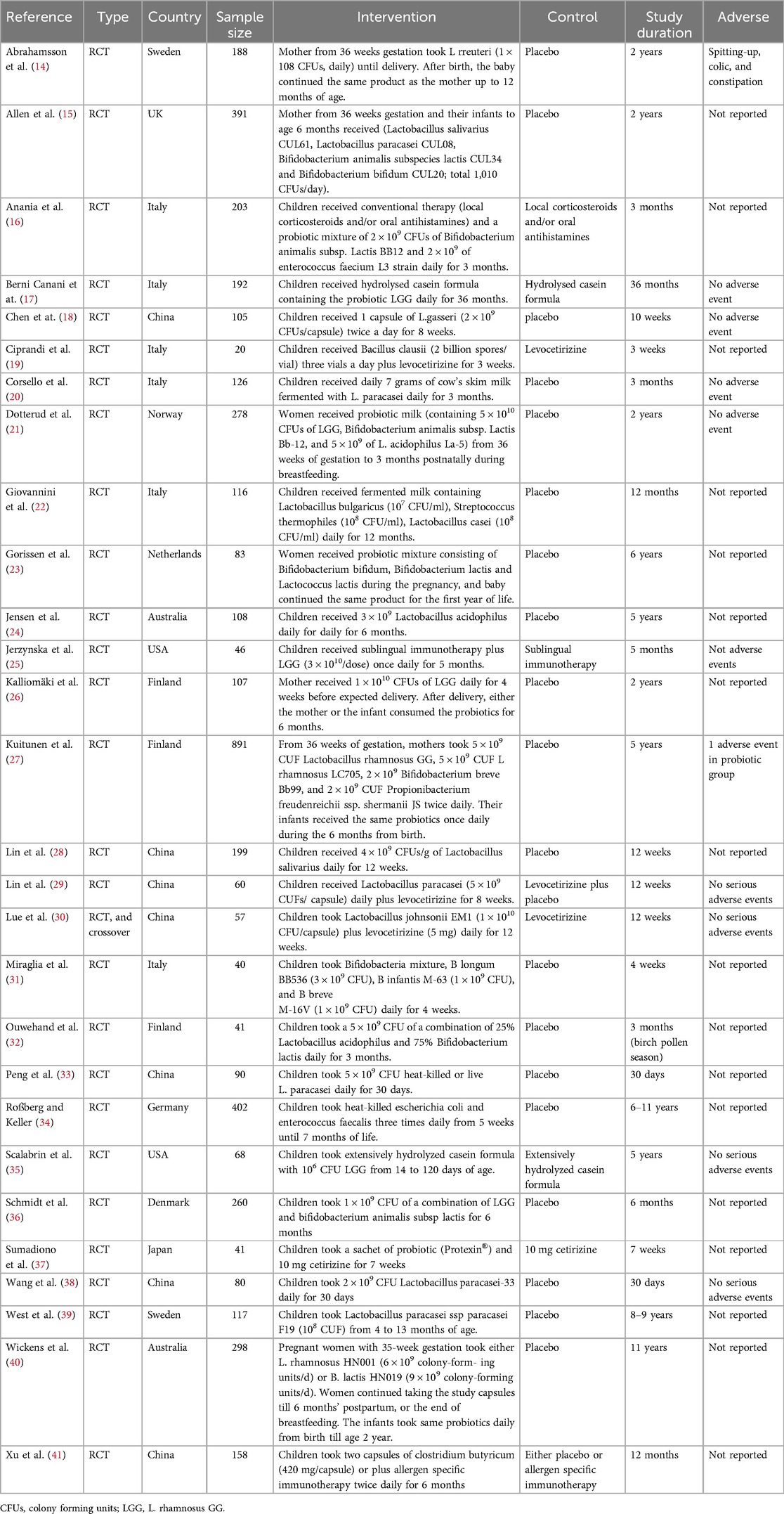
The characteristics of the included studies are presented in Table 1. A total of 28 trials with 4,765 participants were included in this systematic review and meta-analysis (17–44). Among these, 14 trials assessed the preventive effects of probiotics (17, 18, 20, 23, 24, 26, 27, 29, 30, 37–39, 42, 43), whereas the other 14 trials assessed the treatment effects of probiotics (19, 21, 22, 25, 28, 31–36, 40, 41, 44). In the studies assessing the preventive effects of probiotics, seven trials included pregnant mothers and their children (14, 15, 21, 23, 26, 27, 40). Probiotics included Bifidobacterium, Lactobacillus, Enterococcus, Escherichia, and Clostridium butyricum strains. The treatment duration of probiotics ranged from 3 weeks to 39 months.
3.2 Risk of bias
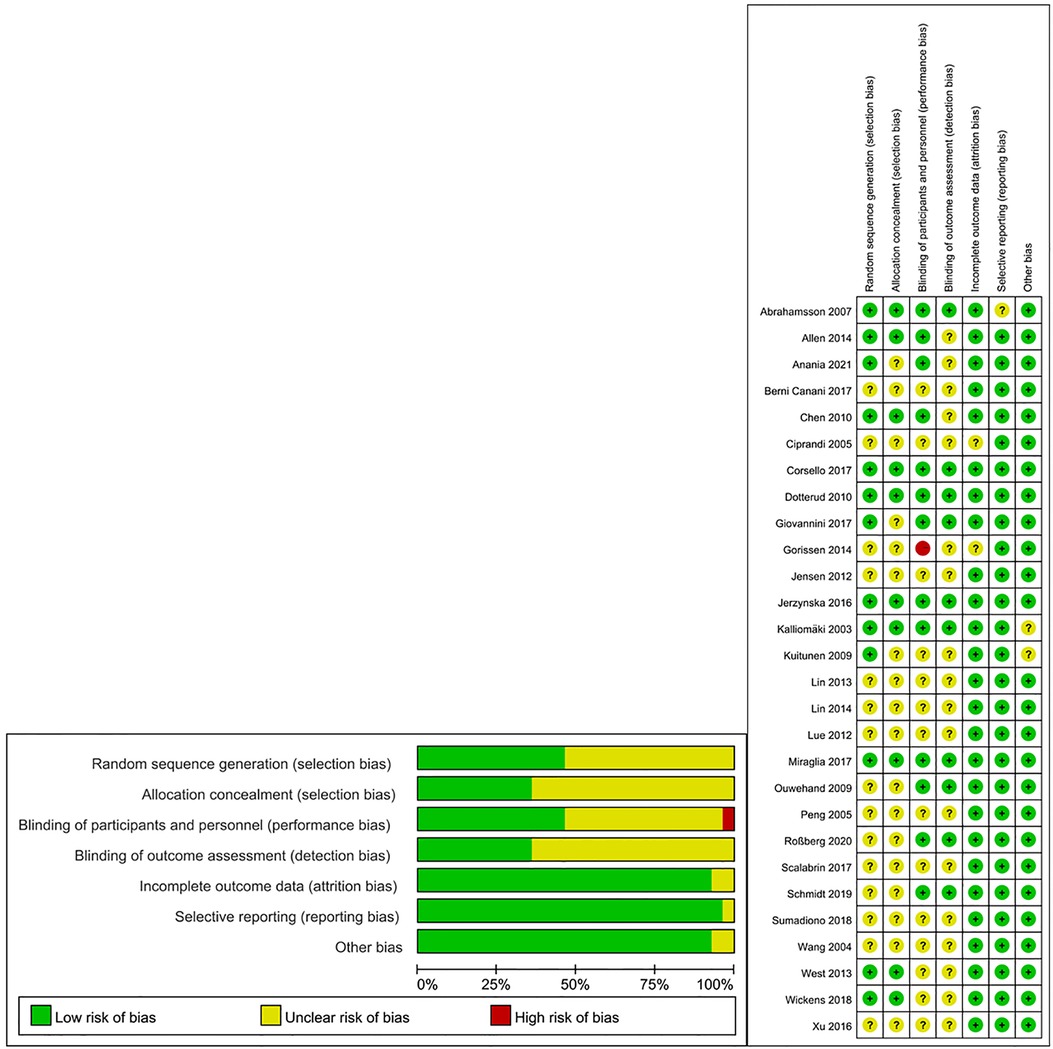
The summary of the risk of bias in the present meta-analysis is shown in Figure 2. All studies reported that they were randomized trials, with concrete methods of randomization reported in several studies (17, 18, 19, 21, 23–25, 28, 31, 34). However, insufficient information was available to judge the masking method as “low risk” or “high risk” for most of the studies. One trial was a single-blinded study and was considered “high risk” for performance bias (23).
3.3 Probiotics for preventing allergic diseases
Fourteen trials assessed the preventive role of probiotics for children with AR (17, 18, 20, 23, 24, 26, 27, 29, 30, 37–39, 42, 43). The pooled effect of the meta-analysis showed that the use of probiotics was not associated with the incidence of AR (OR, 0.90; 95% CI, 0.74–1.08; P = 0.26; I2 = 31%) (Figure 3). The subgroup analysis based on pregnant mothers taking probiotics (pregnant mother group) or only children taking probiotics (children group) revealed no significant difference compared with the control group within either the pregnant mother group (OR, 1.02; 95% CI, 0.80–1.31; P = 0.86; I2 = 21%) or the children group (OR, 0.75; 95% CI, 0.56–1.00; P = 0.05; I2 = 37%) (Supplementary Figure S1). Another subgroup analysis based on children with high risk or non-high-risk of allergy showed no significant differences in the incidence of AR between the probiotic and control groups in children with high risk (OR, 0.89; 95% CI, 0.73–1.09; P = 0.26; I2 = 50%) or non-high risk (OR, 0.95; 95% CI, 0.54–1.66; P = 0.84; I2 = 0%) (Supplementary Figure S2).
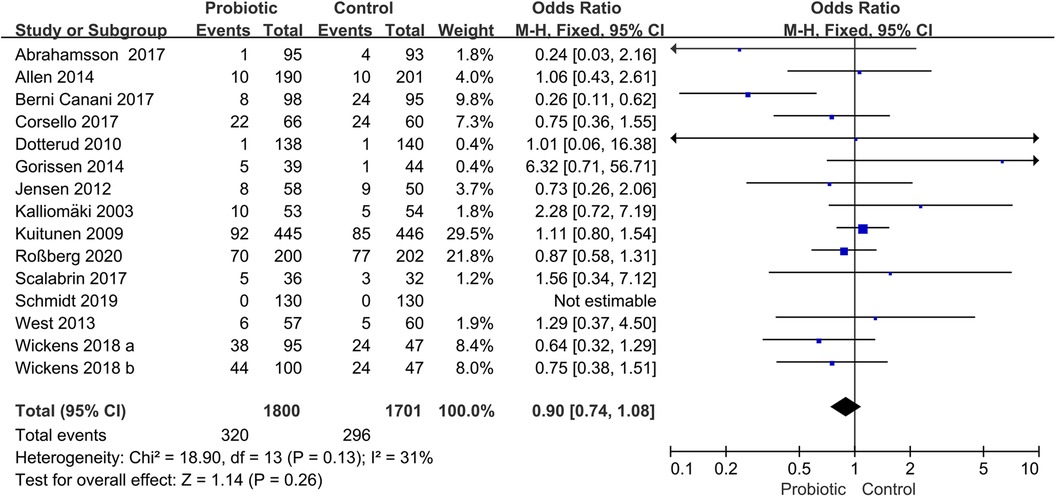
Figure 3. Forest plot for the effects of probiotics on the incidence of children with AR. AR, Allergic rhinitis.
3.4 Probiotics for symptom scores of AR
Six trials reported the total symptom score (TSS), including assessments of nasal congestion, sneezing, nasal itching, and rhinorrhea (16, 19, 28, 33, 38, 41). The pooled results showed that the use of probiotics was associated with a significant improvement in TSS (SMD, −2.27; 95% CI, −3.26 to −1.29; P < 0.00001; I2 = 96%) (Figure 4). The subgroup analysis based on children receiving probiotics and other therapies such as corticosteroids or antihistamines (combination group) or only probiotics intervention (monotherapy group). The subgroup analysis revealed no improvement in TSS in the combination group (SMD, −2.94; 95% CI, −5.90 to 0.01; P = 0.05; I2 = 98%). Another subgroup analysis revealed no significant improvement in the probiotic group compared with the placebo group (SMD, −1.93; 95% CI, −3.09 to −0.76; P = 0.001; I2 = 95%) (Supplementary Figure S3).
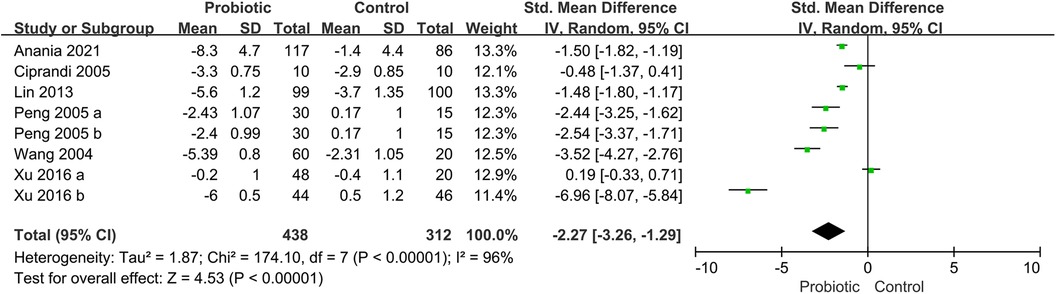
Figure 4. Forest plot for the effects of probiotics on TSS in children with AR. AR, Allergic rhinitis; TSS, total symptom score.
Two trials reported the scores of itchy nose and sneezing (29, 30). The pooled results showed that the use of probiotic was associated with a significant improvement of itchy nose scores (SMD, −0.44; CI, −0.80, −0.07; P = 0.02; I2 = 0%), Supplementary Figure S4, and sneezing scores (SMD, −0.47; CI, −0.84, −0.10; P = 0.01; I2 = 41%), Supplementary Figure S5.
Three trials reported the eye symptoms (28, 33, 38). The pooled results showed that the use of probiotic was associated with a significant improvement of eye symptoms (SMD, −3.77; CI, −5.47, −2.07; P < 0.00001), with significant heterogeneity (I2 = 95%), Supplementary Figure S6.
3.5 Probiotics for PRQLQ
Five trials reported the PRQLQ (32–34, 36, 41), including nasal symptoms, ocular symptoms, practical problems, activity limitations, and other symptoms (42). The pooled results showed that the use of probiotics was associated with a significant improvement in PRQLQ (SMD, −2.52; 95% CI, −4.12 to −0.92; P < 0.00001, I2 = 96%) (Supplementary Figure S7).
3.6 Probiotics for immunological parameters
Five trials reported the effects of probiotics on IgE (18, 22, 28, 30, 41). The pooled results showed no significant difference between the two groups (SMD, −0.77; 95% CI, −1.53 to −0.01; P = 0.05; I2 = 95%) (Supplementary Figure S8). Four trials reported the effects of probiotics on interleukin (IL)-10 levels. The pooled results showed no significant difference between the two groups (SMD, −0.15; 95% CI, −0.43 to 0.12; P = 0.28; I2 = 0%) (Supplementary Figure S9).
3.7 Adverse events
Sixteen trials did not report adverse events during the study (18, 19, 25–27, 29, 31, 34–37, 39, 40, 42–44). Five trials reported no adverse events in the probiotic and control groups (17, 18, 20, 21, 25). Four trials reported no serious adverse events in the probiotic and control groups (29, 30, 35, 38). One trial reported one adverse event in the probiotic group (27). One trial reported mild adverse events, including spitting up, abdominal colic, and constipation, with no significant difference between the two groups (14).
3.8 Publication bias and sensitivity analysis
The funnel plot analysis showed symmetry for the events of AR in children (Supplementary Figure S10). Similarly, Egger's test did not detect a significant publication bias (P > 0.05). The sensitivity analysis was assessed by leave-one-out analysis for events of AR in children, and the pooled results and heterogeneity did not significantly change.
4 Discussion
The present meta-analysis included 28 trials with 4,765 participants. The results revealed that probiotic supplementation alleviated the symptoms of AR and improved the PRQLQ in children.
However, it could not prevent the development of AR in children and had no significant impact on regulating IgE and IL-10 levels in children with AR. Additionally, probiotics were shown to be safe and not associated with an increased risk of side effects.
Probiotics have been widely explored for preventing allergic diseases, and evidence has been established for their supplementation in reducing the development of certain allergic diseases. Children who received Lactobacillus or Bifidobacterium supplementation were associated with a reduced prevalence of eczema and wheezing (40, 43, 44). However, whether probiotics can effectively prevent AR remains unclear; some studies have even demonstrated that probiotics may increase the incidence of AR (45). The present study provided reliable evidence that probiotics were not associated with increasing or reducing the incidence of AR. Interestingly, both atopic eczema and AR are allergic diseases, and AR often co-occurs with eczema, suggesting a shared pathogenesis or mechanism (46). However, probiotics have a different impact on the incidence of eczema and AR in children, and the underlying mechanisms remain unclear. Another aspect to consider is the development of gut microbes in infants. The transmission of maternal microbes during delivery plays a vital role in colonizing the infant's gut (47). Some studies even suggest that the existence of bacteria in infants begins prenatally (48), indicating that microbes may influence the immune system before birth. However, no evidence shows the role of probiotics in preventing allergic diseases in adults, indicating that earlier probiotic supplementation might have a better effect in preventing AR. Therefore, the beginning of probiotic supplementation in pregnant women or infants might have a different effect on AR. However, the pooled results showed that the initiation of probiotic supplementation by either pregnant women or infants was not associated with the incidence of AR. Finally, we found that probiotic supplementation was not associated with the incidence of AR in children with a high or non-high risk for allergy.
Regarding the effects of probiotics on children with AR, most of the included studies showed that probiotics improved the severity of AR symptoms; two studies found that probiotics reduced the occurrence of AR symptoms (18, 37). The mechanism of probiotics in improving the symptoms of AR has not yet been completely explained, but some possible mechanisms have been suggested. It is suggested that the regulation of T helper (Th) cells may be involved in the protective effect of probiotics. The cells are classified into two subsets, Th1 and Th2, and maintaining the balance of Th1/Th2 cells is crucial for regulating the adaptive immune response. A Th2-dominant condition has been shown to increase the risk of allergic diseases. Probiotics have been found to promote the function of Th1 cells while inhibiting Th2 responses, which helps control the overproduction of IgE and pro-inflammatory cytokines (49, 50). In addition, other evidence suggests that probiotics increase the number of regulatory T cells by changing the composition of intestinal microflora and modifying antigen-specific serum IgE levels in animal models (51). Probiotics have been shown to improve the barrier function of the intestinal mucosa, reducing the leakage of antigens through the mucosa and improving the local immune system by enhancing the immunoglobulin A response (52, 53). However, further studies are needed to explore how probiotics can improve AR symptoms but not prevent the development of AR in children.
Based on the results of this study, we do not recommend probiotic supplementation for preventing AR in children. However, we recommend probiotic supplementation for alleviating AR symptoms in children. Several advantages of probiotics in alleviating children with AR exist. First, probiotics can be mixed with milk or yogurt, making it easier for children or infants to take. Second, probiotics are generally safe and have few or only mild side effects. Third, probiotics also can improve gastrointestinal dysfunctions, such as diarrhea, constipation, and indigestion. However, probiotics cannot replace anti-AR drugs, such as antihistamines or steroids, during acute episodes of AR.
5 Strengths and limitations
This was not the first meta-analysis to assess the effect of probiotics in AR. However, it had several strengths compared with other meta-analyses. First, in our meta-analysis, we studied both the preventive and therapeutic effects of probiotics in AR, whereas other studies only studied the preventive or therapeutic effects of probiotics. Second, we included a large number of trials and participants, which allowed us to obtain more stable results. Third, this meta-analysis included only children as study participants, reducing heterogeneity between children and adults.
This study had several limitations as well. First, high heterogeneity was observed in some pooled results, including TSS and PRQLQ and IgE levels. The heterogeneity might have originated from different probiotic strains, treatment duration, and symptom severity. However, due to the low number of trials, we did not perform subgroup analyses to explore the cause of heterogeneity. Second, we did not explore the effect of different probiotic strains on AR, and this was also due to the low number of trials. Third, our search was restricted to publications in English; hence, some studies in other languages might have been missed.
6 Conclusions
The present study demonstrated that probiotics effectively and safely improved pediatric AR symptoms and PRQLQ. However, probiotics could not prevent AR in children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XL: Writing – original draft. HW: Writing – original draft. HL: Writing – original draft. YC: Writing – original draft. LT: Writing – original draft, Writing – review & editing. QJ: Writing – original draft, Writing – review & editing. DX: Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by Chengdu Medical College High-level Talent Research Start-up Fund (CYFY-GQ56).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1352879/full#supplementary-material
Abbreviations
AR, allergic rhinitis; OR, odds ratio; PRQLQ, Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; RCTs, randomized controlled trials; SMD, standardized mean difference; Th, T helper; TSS, total nose symptoms; WMD, weighted mean difference.
References
1. Hoyte FCL, Nelson HS. Recent advances in allergic rhinitis. F1000Res. (2018) 7. doi: 10.12688/f1000research.15367.1
2. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
3. Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) phase three. Allergy. (2009) 64(1):123–48. doi: 10.1111/j.1398-9995.2008.01884.x
4. Linna O, Kokkonen J, Lukin M. A 10-year prognosis for childhood allergic rhinitis. Acta Paediatr. (1992) 81(2):100–2. doi: 10.1111/j.1651-2227.1992.tb12182.x
5. Izquierdo-Domínguez A, Valero AL, Mullol J. Comparative analysis of allergic rhinitis in children and adults. Curr Allergy Asthma Rep. (2013) 13(2):142–51. doi: 10.1007/s11882-012-0331-y
6. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. (2007) 28(1):3–9. doi: 10.2500/aap.2007.28.2934
7. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. (2011) 128(3):646–52. e1–5. doi: 10.1016/j.jaci.2011.04.060
8. Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. (2012) 129(2):434–40, 440.e1–2. doi: 10.1016/j.jaci.2011.10.025
9. Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. (2014) 44(6):842–50. doi: 10.1111/cea.12253
10. Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2015) 136(4):952–61. doi: 10.1016/j.jaci.2015.04.031
11. Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Br Med J. (2013) 347:f6471. doi: 10.1136/bmj.f6471
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
14. Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. (2007) 119(5):1174–80. doi: 10.1016/j.jaci.2007.01.007
15. Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor MB, Garaiova I, et al. Probiotics in the prevention of eczema: a randomised controlled trial. Arch Dis Child. (2014) 99(11):1014–9. doi: 10.1136/archdischild-2013-305799
16. Anania C, Di Marino VP, Olivero F, De Canditiis D, Brindisi G, Iannilli F, et al. Treatment with a probiotic mixture containing Bifidobacterium animalis subsp. lactis BB12 and Enterococcus faecium L3 for the prevention of allergic rhinitis symptoms in children: a randomized controlled trial. Nutrients. (2021) 13(4). doi: 10.3390/nu13041315
17. Berni Canani R, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. (2017) 139(6):1906–1913.e4. doi: 10.1016/j.jaci.2016.10.050
18. Chen YS, Jan RL, Lin YL, Chen HH, Wang JY. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. (2010) 45(11):1111–20. doi: 10.1002/ppul.21296
19. Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii effects in children with allergic rhinitis. Allergy. (2005) 60(5):702–3. doi: 10.1111/j.1398-9995.2005.00722.x
20. Corsello G, Carta M, Marinello R, Picca M, De Marco G, Micillo M, et al. Preventive effect of cow’s milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: a multicenter randomized controlled trial. Nutrients. (2017) 9:7. doi: 10.3390/nu9070669
21. Dotterud CK, Storrø O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. (2010) 163(3):616–23. doi: 10.1111/j.1365-2133.2010.09889.x
22. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. (2007) 62(2):215–20. doi: 10.1203/PDR.0b013e3180a76d94
23. Gorissen DM, Rutten NB, Oostermeijer CM, Niers LE, Hoekstra MO, Rijkers GT, et al. Preventive effects of selected probiotic strains on the development of asthma and allergic rhinitis in childhood. The Panda study. Clin Exp Allergy. (2014) 44(11):1431–3. doi: 10.1111/cea.12413
24. Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol. (2012) 130(5):1209–1211.e5. doi: 10.1016/j.jaci.2012.07.018
25. Jerzynska J, Stelmach W, Balcerak J, Woicka-Kolejwa K, Rychlik B, Blauz A, et al. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. (2016) 37(4):324–34. doi: 10.2500/aap.2016.37.3958
26. Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. (2003) 361(9372):1869–71. doi: 10.1016/s0140-6736(03)13490-3
27. Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. (2009) 123(2):335–41. doi: 10.1016/j.jaci.2008.11.019
28. Lin TY, Chen CJ, Chen LK, Wen SH, Jan RH. Effect of probiotics on allergic rhinitis in df, dp or dust-sensitive children: a randomized double blind controlled trial. Indian Pediatr. (2013) 50(2):209–13. doi: 10.1007/s13312-013-0068-2
29. Lin WY, Fu LS, Lin HK, Shen CY, Chen YJ. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6–13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. (2014) 55(3):181–8. doi: 10.1016/j.pedneo.2013.10.001
30. Lue KH, Sun HL, Lu KH, Ku MS, Sheu JN, Chan CH, et al. A trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7–12 years. Int J Pediatr Otorhinolaryngol. (2012) 76(7):994–1001. doi: 10.1016/j.ijporl.2012.03.018
31. Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. (2017) 43(1):25. doi: 10.1186/s13052-017-0340-5
32. Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol. (2009) 15(26):3261–8. doi: 10.3748/wjg.15.3261
33. Peng GC, Hsu CH. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol. (2005) 16(5):433–8. doi: 10.1111/j.1399-3038.2005.00284.x
34. Roßberg S, Keller T. Orally applied bacterial lysate in infants at risk for atopy does not prevent atopic dermatitis, allergic rhinitis, asthma or allergic sensitization at school age: follow-up of a randomized trial. Allergy. (2020) 75(8):2020–5. doi: 10.1111/all.14247
35. Scalabrin D, Harris C, Johnston WH, Berseth CL. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: a 5-year follow-up. Eur J Pediatr. (2017) 176(2):217–24. doi: 10.1007/s00431-016-2825-4
36. Schmidt RM, Pilmann Laursen R, Bruun S, Larnkjaer A, Mølgaard C, Michaelsen KF, et al. Probiotics in late infancy reduce the incidence of eczema: a randomized controlled trial. Pediatr Allergy Immunol. (2019) 30(3):335–40. doi: 10.1111/pai.13018
37. Sumadiono S, Satria C, Mardhiah N, Susanti G. Immunotherapy and probiotic treatment for allergic rhinitis in children. Paediatr Indones. (2018) 58:280–5. doi: 10.14238/pi58.6.2018.280-5
38. Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol. (2004) 15(2):152–8. doi: 10.1111/j.1399-3038.2004.00156.x
39. West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease–follow-up at 8–9 years of age. Allergy. (2013) 68(8):1015–20. doi: 10.1111/all.12191
40. Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, et al. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol. (2018) 29(8):808–14. doi: 10.1111/pai.12982
41. Xu LZ, Yang LT, Qiu SQ, Yang G, Luo XQ, Miao BP, et al. Combination of specific allergen and probiotics induces specific regulatory B cells and enhances specific immunotherapy effect on allergic rhinitis. Oncotarget. (2016) 7(34):54360–9. doi: 10.18632/oncotarget.10946
42. Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. (1998) 101(2 Pt 1):163–70. doi: 10.1016/s0091-6749(98)70380-x
43. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. (2001) 357(9262):1076–9. doi: 10.1016/S0140-6736(00)04259-8
44. Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol. (2010) 21(2 Pt 2):e386–93. doi: 10.1111/j.1399-3038.2009.00958.x
45. Peldan P, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5–10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy. (2017) 47(7):975–9. doi: 10.1111/cea.12924
46. Steiner UC, Bachmann LM, Soyka MB, Regenass S, Steinegger L, Probst E. Relationship between rhinitis, asthma, and eczema and the presence of sensitization in young Swiss adults. Allergy Rhinol (Providence). (2018) 9:2152656718773606. doi: 10.1177/2152656718773606
47. Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. (2016) 6:23129. doi: 10.1038/srep23129
48. Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes. (2017) 12(Suppl 1):3–17. doi: 10.1111/ijpo.12217
49. Winkler P, Ghadimi D, Schrezenmeir J, Kraehenbuhl JP. Molecular and cellular basis of microflora-host interactions. J Nutr. (2007) 137(3 Suppl 2):756s–72. doi: 10.1093/jn/137.3.756S
50. Masuda S, Yamaguchi H, Kurokawa T, Shirakami T, Tsuji RF, Nishimura I. Immunomodulatory effect of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi grown in high-salt medium. Int J Food Microbiol. (2008) 121(3):245–52. doi: 10.1016/j.ijfoodmicro.2007.10.011
51. Torii A, Torii S, Fujiwara S, Tanaka H, Inagaki N, Nagai H. Lactobacillus acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. (2007) 56(3):293–301. doi: 10.2332/allergolint.O-06-459
52. Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. (1992) 32(2):141–4. doi: 10.1203/00006450-199208000-00002
Keywords: meta-analysis, pediatric allergy rhinitis, probiotics, prevention, treatment
Citation: Luo X, Wang H, Liu H, Chen Y, Tian L, Ji Q and Xie D (2024) Effects of probiotics on the prevention and treatment of children with allergic rhinitis: a meta-analysis of randomized controlled trials. Front. Pediatr. 12:1352879. doi: 10.3389/fped.2024.1352879
Received: 14 December 2023; Accepted: 30 July 2024;
Published: 3 October 2024.
Edited by:
Aleksandra Szczepankiewicz, Poznan University of Medical Sciences, PolandReviewed by:
Zorica Momcilo Zivkovic, University Hospital Center Dr Dragiša Mišović, SerbiaOzge Yilmaz, Manisa Celal Bayar University, Türkiye
Copyright: © 2024 Luo, Wang, Liu, Chen, Tian, Ji and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Ji, cWh5ODIwOTE1QDE2My5jb20=; Dengpiao Xie, Mjg3NjUyMjI1M0BxcS5jb20=
 Xinyi Luo1
Xinyi Luo1 Li Tian
Li Tian Qing Ji
Qing Ji Dengpiao Xie
Dengpiao Xie