- 1Department of Medical Ultrasound, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Pathology, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
Introduction: It has been reported that the cartilaginous roof of the acetabulum is thicker in infants with developmental dysplasia of the hip (DDH) than in those with healthy hips. However, there is limited research on the changes in the thickness of acetabular cartilage after follow-up or treatment of DDH. This study aims to report the thickness of acetabular cartilage before and after treatment of DDH.
Materials and methods: In this prospective study, infants with clinical suspicion of DDH were enrolled in the pediatric outpatient service in our hospital from January 2022 to August 2023. The thickness of acetabular cartilage was measured in the standard coronal plane. Borderline hips (Graf IIa type) were monitored with monthly ultrasound examination until they were classified as normal hips (Graf I type), while dysplastic hips (Graf IIb type or worse) were treated with the Pavlik harness until they were also classified as normal hips in the final ultrasound examination.
Results: A total of 592 children [median age, 96 days (interquartile range, 70–142 days); 197 boys] were enrolled in the study. The thickness of acetabular cartilage in dysplastic hips (4.3 ± 1.6 mm) was greater than that in normal hips (3.0 ± .39 mm, P < 0.001) and borderline hips (3.1 ± .57 mm, P < 0.001). In borderline hips, the thickness of acetabular cartilage decreased from 3.1 ± .57 mm in the initial evaluation to 2.9 ± .53 mm in the final follow-up scan (P = 0.01). In dysplastic hips, the thickness of acetabular cartilage decreased from 4.3 ± 1.6 mm in the initial evaluation to 3.5 ± .51 mm after treatment (P = 0.003). The thickness of acetabular cartilage in dysplastic hips after treatment remained greater than that in normal hips (P < 0.0001).
Conclusion: The thickness of acetabular cartilage decreased after follow-up or treatment of DDH. Further research is required to determine whether cartilage that remain thicker in dysplastic hips than that in normal hips after treatment should be considered an early indicator of residual acetabular dysplasia.
Introduction
Developmental dysplasia of the hip (DDH) includes a broad spectrum of disorders affecting the developing hip, ranging from subtle dysplasia detected by ultrasound and/or radiography without any clinical findings to a dislocated hip that can or cannot be reduced (1). DDH is a major cause of hip osteoarthritis, which can lead to severe disability in young adults (2). Clinical examination alone is not sensitive enough to identify every child with DDH. The sensitivity has been reported to be as low as 50% (1). Ultrasound has been widely used in screening DDH in infants younger than 6 months. The most commonly known ultrasound techniques used worldwide are the Graf (3) technique and the Harcke technique (4), which are recommended by clinical practice guidelines from several medical academies or institutions (1, 5, 6). With both ultrasound techniques, it was proven that hip sonography could detect abnormality not detected by clinical examination or radiograph (6, 7). However, universal screening by ultrasound may cause initial overtreatment without reducing the prevalence of surgical treatment (8, 9). According to the Graf method, treatment is required for any hip classified as type IIa minus or worse, i.e., with an alpha value of <60° by the end of the 12th week of life (3, 10). However, whether a hip with an alpha angle of slightly <60° should be treated is controversial (11). A study showed that many mild forms of DDH resolve without treatment (12). What's more, the interrater variability of Graf's alpha angle is problematically high (13), which may potentially alter the final diagnosis in 50%–75% of infants if scanned by a nonexpert (14).
If diagnosed and treated early, most cases of DDH are potentially reversible. Splints and braces, such as the Pavlik harness, to maintain abduction and flexing of the hips, are considered the gold standard for DDH treatment under 6 months of age with a reducible hip (15). Closed reduction and spica casts are the first-line treatment for a late-diagnosed dislocated hip (at >6 months of age). Open reduction is indicated when closed methods fail (16).
In infants with DDH, there is a hypertrophied ridge of acetabular articular cartilage and labrum in the superolateral aspect of the acetabulum (17, 18). Damage to the epiphyseal acetabular cartilage may hinder hip growth and development (18). Graf (3) considered that in a dysplastic hip, the wide as-yet unossified cartilage roof must finally ossify to become a normal joint. This makes acetabular cartilage thickness a potential indicator, in addition to the alpha angle, to describe pathological changes in DDH. Arthrographic indices (19), such as the cartilaginous acetabular index, enable assessment of the lateral labral margin. A cartilaginous acetabular index of >10◦ at the age of 5 years can predict poor acetabular development, but this modality is invasive (20). Ultrasound has been used to assess the cartilaginous roof of the acetabulum (21, 22). However, there is limited research on the changes in the thickness of acetabular cartilage after follow-up or treatment of DDH. This study aims to report the thickness of acetabular cartilage before and after treatment of DDH.
Materials and methods
Patients
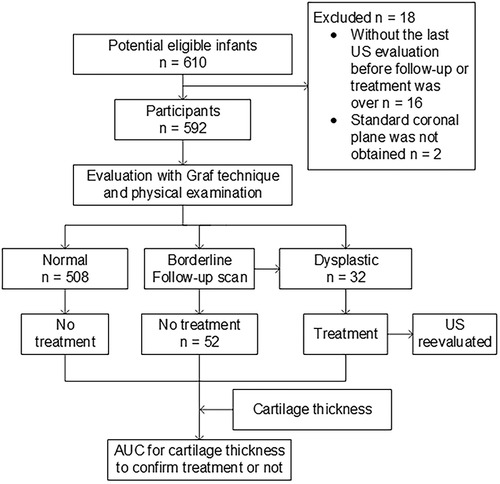
The study was approved by the institutional review board of our hospital and registered on the Chinese clinical trial registry website (http://www.chictr.org.cn, number ChiCTR2000040953). The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. The participants consisted of a consecutive sample of infants who met the predetermined inclusion criteria. Written informed consent was obtained from the parent(s) or guardian(s) of the infants. The infants were enrolled in the pediatric outpatient service in our hospital from January 2021 to August 2023. The inclusion criteria were infants with clinical suspicion of DDH, usually because of breech presentation, asymmetric folds, family history of developmental dysplasia, and a hip click. Borderline or dysplastic hips were followed up or treated by the Pavlik harness until they were classified as normal based on monthly ultrasound evaluation and physical examination. Infants were excluded if a standard coronal plane was not achieved in the ultrasound examination or if the last ultrasound evaluation of hips was not undertaken before the follow-up or treatment was over (Figure 1).
Ultrasound imaging
Ultrasound of the hip was performed with an ultrasonic machine (Logiq E9, GE Medical Systems, or Philips EPIQ 7 system, Philips) equipped with a 7 or 10 MHz transducer.
Ultrasound examination of all infants was performed and interpreted by one radiologist (C.Z., with 10 years of experience in DDH evaluation). Part of the archived images of infants were reevaluated by another two radiologists (K.H. and Y.Z., with 5 and 2 years of experience in DDH evaluation, respectively) to test the interrater agreement of the measurement of the thickness of acetabular cartilage.
The ultrasound assessment of the hips was performed with the Graf technique (3, 9). The thickness of the acetabular cartilage was measured in the standard coronal plane. The intersection of the wing of the ilium and the bony roof of the acetabulum was considered the starting point for measurement. The thickness was measured along the baseline from the starting point to the boundary of the acetabular cartilage. Slight flexion and extension movement of the hip of the infant during ultrasound examination assisted by the investigator or guardian could make the boundary of the acetabular cartilage adjacent to the femoral head get clearer display (Figure 2, Supplementary Video 1).
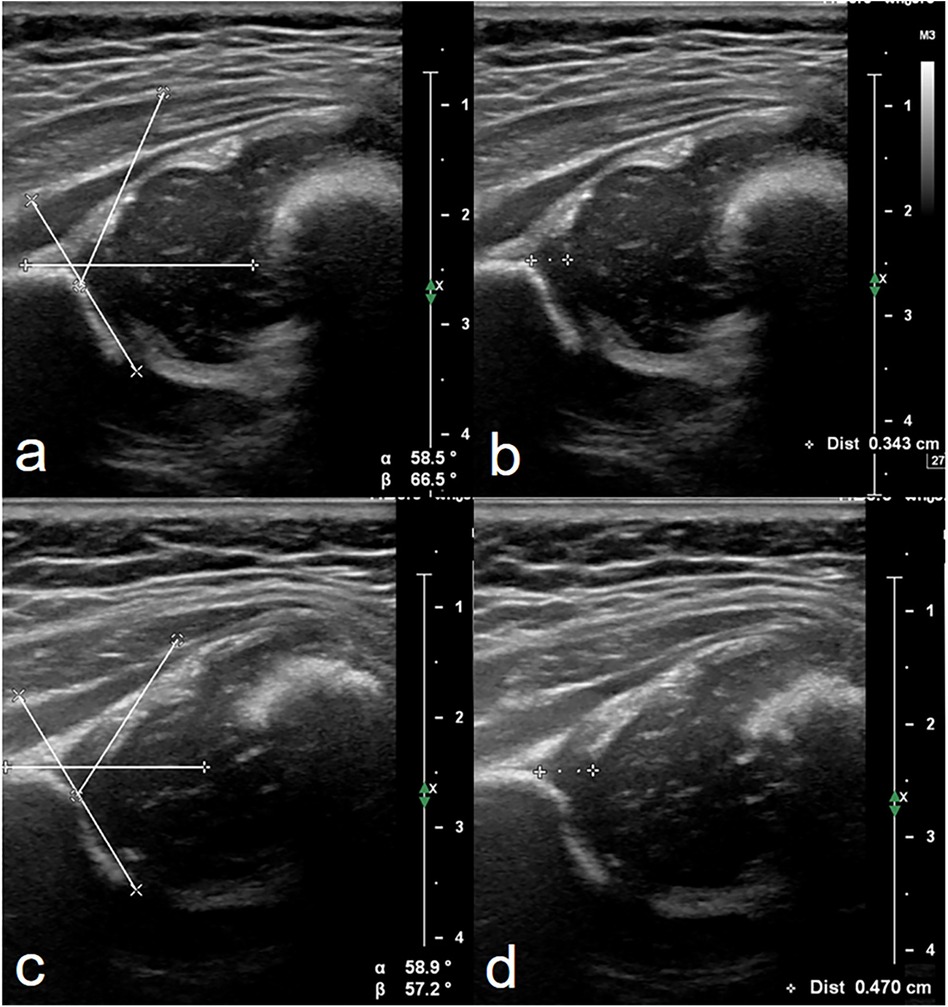
Figure 2. Measurement of alpha angle and the thickness of acetabular cartilage of hips in ultrasound images. Both were evaluated in the standard coronal plane described by Graf (10). Images (a,b) were from a 46-day-old female infant identified to be normal after a follow-up scan of hips 1 month later. Images (c,d) were from a 118-day-old female infant accepting treatment with the Pavlik harness for 1 month with a history of breech presentation. The same values of alpha angle (59°) were evaluated in these two patients. The thickness of acetabular cartilage in d was thicker than that in b. This provided more information to pediatric orthopedists in addition to alpha angle.
Thickness of acetabular cartilage
Relationships of the thickness of acetabular cartilage with age, length, and weight of infants were described. The thickness of acetabular cartilage in different types of hips was compared. Correlations between the thickness of acetabular cartilage and alpha angle were analyzed. Changes in the thickness of acetabular cartilage after follow-up scan in borderline hips or after treatment in dysplastic hips were described. Test performance characteristics by using the thickness of acetabular cartilage from the first scan to help detect DDH that required treatment were determined and compared with clinical reference standard diagnosis.
Interrater reliability was calculated (intraclass correlation coefficient) to confirm the reliability of the assessment of the thickness of acetabular cartilage.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM) and Prism 8.0 (GraphPad). The differences in the thickness of acetabular cartilage in different sex or age groups were tested with an independent-sample t-test, considering data were from the two independent groups. The differences in the thickness of acetabular cartilage from multiple groups, that is, the groups of normal hips, borderline hips, and dysplastic hips, were tested with ANOVA. Pearson correlation analysis was used for the assessment of the correlation between the thickness of acetabular cartilage and length, weight, or alpha angle. The changes in cartilage thickness before and after follow-up scan or treatment were analyzed by a paired-sample t-test, considering the data were from the same group of samples. P < 0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 592 infants [median age, 96 days (interquartile range, 70–142 days); 197 boys] were enrolled in the study. The cohort included those with normal hips (n = 508), borderline hips (Graf IIa, later normalizing spontaneously; n = 52), or dysplastic hips (Graf IIb or worse, n = 32) clinically diagnosed after more than 6 months of follow-up (Figure 1).
Relationships of cartilage thickness with sex, age, length, and weight in infants with normal hips
There was no difference in the thickness of acetabular cartilage between the boys (2.9 ± .39 mm, n = 114) and the girls (3.0 ± .38 mm, n = 394) (P = 0.31). Infants with normal hips were divided into two groups according to age less than or more than 90 days, which was considered the age for hips to reach maturity (1, 10). There was no difference between the thickness of acetabular cartilage in younger infants (3.1 ± .41 mm, n = 110) and that in elder infants (3.0 ± .39 mm, n = 398) (P = 0.12). No correlation between the thickness of acetabular cartilage with length (Pearson r = 0.11, P =0 .21) or weight (Pearson r = .12, P = 0.16) of infants was found.
Thickness of acetabular cartilage in different types of hips
There was no difference in the thickness of acetabular cartilage between normal hips (3.0 ± .39 mm) and borderline hips (3.1 ± .57 mm, P = 0.09). The thickness of acetabular cartilage in dysplastic hips (4.3 ± 1.6 mm) was greater than that in normal hips (P < 0.001) and borderline hips (P < 0.001) (Figure 3).
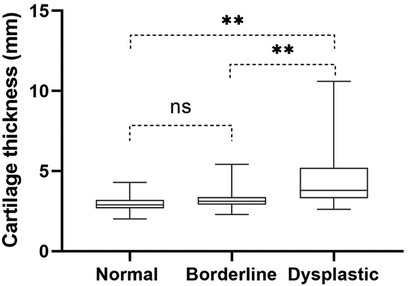
Figure 3. The thickness of acetabular cartilage in normal hips, borderline hips, and dysplastic hips. ns, no significant differences, **, P < 0.001.
Correlation between the thickness of acetabular cartilage and alpha angle
No correlation between the thickness of acetabular cartilage and alpha angle was found in normal hips (P = 0.50, Figure 4a) or borderline hips (P = 0.58, Figure 4b). In dysplastic hips, the thickness of acetabular cartilage increased with the decrease in alpha angle (P < 0.001, Figure 4c).
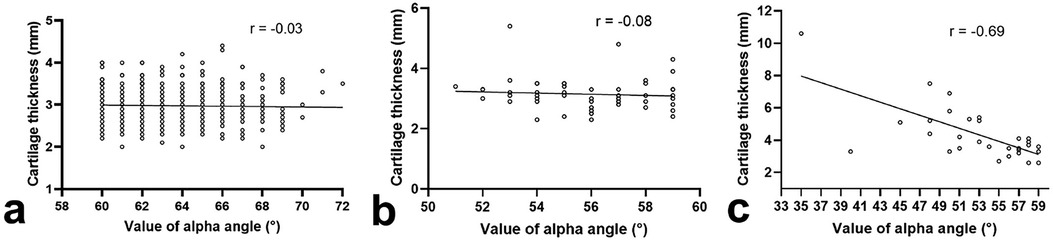
Figure 4. Correlation between the thickness of acetabular cartilage and alpha angle in normal hips (a), borderline hips (b), or dysplastic hips (c).
Thickness of acetabular cartilage before and after follow-up or treatment
The thickness of acetabular cartilage evaluated in the last ultrasound examination was compared with that in the first examination for hips that were followed up or treated. In borderline hips, the thickness of acetabular cartilage decreased from 3.1 ± .57 mm in the first evaluation to 2.9 ± .53 mm in the last follow-up scan (P = 0.01). In dysplastic hips, the thickness of acetabular cartilage decreased from 4.3 ± 1.6 mm in the initial evaluation to 3.5 ± .51 mm after treatment (P = 0.003) (Figure 5). The thickness of acetabular cartilage in dysplastic hips after treatment remained greater than that in normal hips (P < 0.0001).
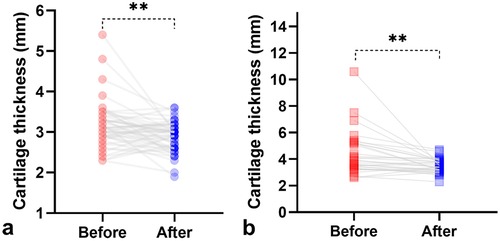
Figure 5. Comparison of the thickness of acetabular cartilage between the primary and the last ultrasound evaluation. The thickness of acetabular cartilage deceased after follow-up in borderline hips (A) or after treatment in dysplastic hips (B). **P < 0.01.
AUC of cartilage thickness to determine treatment or not
Receiver operating characteristic curves were generated (Figure 6) for the thickness of acetabular cartilage from the first scan to help detect dysplastic hips that required treatment. The area under the receiver operating characteristic curve was 0.85 (95% CI, 0.77–0.93, P < 0.0001). With a cutoff value of 3.3 mm (the value with the highest Youden index), the thickness of acetabular cartilage can detect dysplastic hips with a sensitivity of 81.3% and a specificity of 73.9%.
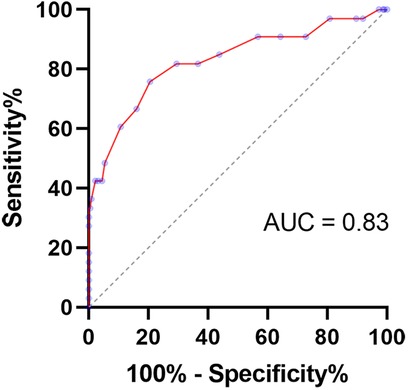
Figure 6. Receiver operating characteristic curve for the thickness of acetabular cartilage to detect hips that required treatment.
Interrater reliability
Ultrasound images from 50 hips were randomly selected for reevaluation of the thickness of acetabular cartilage. The interrater repeatability of the intraclass correlation coefficient was 0.91 [(95% CI: 0.86–0.95), P < 0.001].
Discussion
The thickness of the acetabular cartilage of infants was evaluated in this study. We confirmed that the thickness of acetabular cartilage in dysplastic hips was greater than that in normal hips. The thickness of acetabular cartilage in borderline hips and dysplastic hips decreased after follow-up or treatment.
The mean thickness of acetabular cartilage in normal hips we measured (3.0 mm) is greater than that of the cartilage measured by Soboleski and Babyn (2.6 mm) (21). This is due to the different measuring methods used in the two studies. Soboleski and Babyn used the apex of the alpha angle as the starting point for measuring the thickness of cartilage (21). This may underestimate the thickness of acetabular cartilage in a considerable part of immature hips (e.g., Figure 2c).
In our study, we confirmed that the more severe the dysplasia, the greater the thickness of acetabular cartilage. Tréguier et al. (22) reported 15 irreducible neonatal dislocated hips. The thickness of acetabular cartilage was between 5 and 7 mm, which was greater than the mean thickness (4.3 mm) of dysplastic hips in our study. This is because 97% (31/32) of dysplastic hips in our study are non-dislocated hips, with relatively mild dysplasia compared to dislocated hips. In dysplastic hips, articular hyaline cartilage may also contribute to the thicker cartilage, which was confirmed by Nishii et al. (23) using MRI in patients aged 16–44 years, considering the physis and epiphysis in the adult patients should have already been ossified.
The thickness of acetabular cartilage decreased after follow-up in borderline hips or treatment in dysplastic hips. This change in cartilage thickness reflects the gradual ossification of the cartilage over time, which is promoted by harness treatment in a direction conducive to the normalization of acetabulum morphology. The cartilage thickness in dysplastic hips after treatment (3.5 ± .51 mm) was still greater than that in normal hips (3.0 ± .42 mm). Some cartilaginous indicators based on MRI or hip arthrography have been proposed to be used as early warning indicators of residual acetabular dysplasia (24). The residual acetabular dysplasia may require acetabuloplasty to protect the hip in the later stage (24) or total hip arthroplasty in adulthood (25). Whether the still thicker cartilage measured by ultrasound should be considered as an indicator of residual acetabular dysplasia needs to be further studied.
There are some issues that need to be addressed. First, the acquisition of ultrasound images was performed by a single operator, and the interexaminer reliability still needs to be clarified. In future studies, acetabular cartilage should be imaged by multiple ultrasound operators to determine the consistency between different examiners. Second, the thickness of acetabular cartilage in our study was measured in the superior lateral part of the cartilaginous roof of the acetabulum, which was evaluated in the standard coronal plane. However, in some dislocated hips, the standard coronal plane may not be achievable by ultrasound. In such cases, the thickness of the acetabular cartilage could be measured after the hips are reduced.
Conclusions
The thickness of acetabular cartilage decreased after follow-up or treatment of DDH. Further research is required to determine whether cartilage that remain thicker in dysplastic hips than that in normal hips after treatment should be considered an early indicator of residual acetabular dysplasia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
KH: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. JW: Data curation, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. YZ: Investigation, Methodology, Writing – original draft. CZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (Grant No. 82171959).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1351296/full#supplementary-material
Supplementary Video 1 | Slight movement of the infant's thigh during ultrasound imaging of the hip may facilitate recognition of the boundary of acetabular cartilage.
References
1. O'Beirne JG, Chlapoutakis K, Alshryda S, Aydingoz U, Baumann T, Casini C, et al. International interdisciplinary consensus meeting on the evaluation of developmental dysplasia of the hip. Ultraschall in Med. (2019) 40(4):454–64. doi: 10.1055/a-0924-5491
2. Engesaeter I, Lie SA, Lehmann TG, Furnes O, Vollset SE, Engesaeter LB. Neonatal hip instability and risk of total hip replacement in young adulthood: follow-up of 2,218,596 newborns from the Medical Birth Registry of Norway in the Norwegian Arthroplasty Register. Acta Orthop. (2008) 79(3):321–6. doi: 10.1080/17453670710015201
3. Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic combound treatment. Arch Orthop Trauma Surg. (1980) 97(2):117–33. doi: 10.1007/BF00450934
4. Morin C, Harcke HT, MacEwen GD. The infant hip: real-time US assessment of acetabular development. Radiology. (1985) 157(3):673–7. doi: 10.1148/radiology.157.3.3903854
5. American Institute of Ultrasound in Medicine. AIUM-ACR-SPR-SRU practice parameter for the performance of an ultrasound examination for detection and assessment of developmental dysplasia of the hip. J Ultrasound Med. (2018) 37(11):E1–e5. doi: 10.1002/jum.14829
6. Rosendahl K, Toma P. Ultrasound in the diagnosis of developmental dysplasia of the hip. The European approach. A review of methods, accuracy and clinical validity. Eur Radiol. (2007) 17(8):1960–7. doi: 10.1007/s00330-006-0557-y
7. Harcke HT. Imaging methods used for children with hip dysplasia. Clin Orthop Relat Res. (2005) 434:71–7. doi: 10.1097/01.blo.0000162411.55355.c8
8. Kuitunen I, Uimonen MM, Haapanen M, Sund R, Helenius I, Ponkilainen VT. Incidence of neonatal developmental dysplasia of the hip and late detection rates based on screening strategy: a systematic review and meta-analysis. JAMA network Open. (2022) 5(8):e2227638. doi: 10.1001/jamanetworkopen.2022.27638
9. Cheok T, Smith T, Wills K. Universal screening may reduce the incidence of late diagnosis of developmental dysplasia of the hip: a systematic review and meta-analysis. Bone Joint J. (2023) 105-B(2):198–208. doi: 10.1302/0301-620X.105B2.BJJ-2022-0896.R1
10. Graf R. Hip sonography: background; technique and common mistakes; results; debate and politics; challenges. Hip Int. (2017) 27(3):215–9. doi: 10.5301/hipint.5000514
11. Shaw BA, Segal LS. Evaluation and referral for developmental dysplasia of the hip in infants. Pediatrics. (2016) 138(6):e20163107. doi: 10.1542/peds.2016-3107
12. Sakkers R, Pollet V. The natural history of abnormal ultrasound findings in hips of infants under six months of age. J Child Orthop. (2018) 12(4):302–7. doi: 10.1302/1863-2548.12.180056
13. Quader N, Schaeffer EK, Hodgson AJ, Abugharbieh R, Mulpuri K. A systematic review and meta-analysis on the reproducibility of ultrasound-based metrics for assessing developmental dysplasia of the hip. J Pediatr Orthop. (2018) 38(6):e305–11. doi: 10.1097/BPO.0000000000001179
14. Morin C, Zouaoui S, Delvalle-Fayada A, Delforge PM, Leclet H. Ultrasound assessment of the acetabulum in the infant hip. Acta Orthop Belg. (1999) 65(3):261–5.10546348
15. Pavone V, de Cristo C, Vescio A, Lucenti L, Sapienza M, Sessa G, et al. Dynamic and static splinting for treatment of developmental dysplasia of the hip: a systematic review. Children (Basel). (2021) 8(2):104. doi: 10.3390/children8020104
16. Scott EJ, Dolan LA, Weinstein SL. Closed vs. Open reduction/salter innominate osteotomy for developmental hip dislocation after age 18 months: comparative survival at 45-year follow-up. J Bone Joint Surg Am. (2020) 102(15):1351–7. doi: 10.2106/jbjs.19.01278
17. Bohaček I, Plečko M, Duvančić T, Smoljanović T, Vukasović Barišić A, Delimar D. Current knowledge on the genetic background of developmental dysplasia of the hip and the histomorphological status of the cartilage. Croat Med J. (2020) 61(3):260–70. doi: 10.3325/cmj.2020.61.260
18. Landa J, Benke M, Feldman DS. The limbus and the neolimbus in developmental dysplasia of the hip. Clin Orthop Relat Res. (2008) 466(4):776–81. doi: 10.1007/s11999-008-0158-yS
19. Miyake T, Tetsunaga T, Endo H, Yamada K, Sanki T, Fujiwara K, et al. Predicting acetabular growth in developmental dysplasia of the hip following open reduction after walking age. J Orthop Sci. (2019) 24(2):326–31. doi: 10.1016/j.jos.2018.09.015
20. Canavese F, Castañeda P, Hui J, Li L, Li Y, Roposch A. Developmental dysplasia of the hip: promoting global exchanges to enable understanding the disease and improve patient care. OTSR. (2020) 106(7):1243–4. doi: 10.1016/j.otsr.2020.09.004
21. Soboleski DA, Babyn P. Sonographic diagnosis of developmental dysplasia of the hip: importance of increased thickness of acetabular cartilage. AJR. (1993) 161(4):839–42. doi: 10.2214/ajr.161.4.8372771
22. Tréguier C, Baud C, Ferry M, Ferran JL, Darnault P, Chapuis M, et al. Irreducible developmental dysplasia of the hip due to acetabular roof cartilage hypertrophy. Diagnostic sonography in 15 hips. Orthop Traumatol Surg Res. (2011) 97(6):629–33. doi: 10.1016/j.otsr.2011.03.023
23. Nishii T, Sugano N, Sato Y, Tanaka H, Miki H, Yoshikawa H. Three-dimensional distribution of acetabular cartilage thickness in patients with hip dysplasia: a fully automated computational analysis of MR imaging. Osteoarthritis Cartilage. (2004) 12(8):650–7. doi: 10.1016/j.joca.2004.04.009
24. Yang S, Su F, Jia HR, Liu CX, Lu QD, Yang YT, et al. Cartilaginous predictors of residual acetabular dysplasia (RAD) in developmental dysplasia of the hip following closed or open reduction: a systematic review and meta-analysis. Front Pediatr. (2023) 11:1124123. doi: 10.3389/fped.2023.1124123
Keywords: developmental dysplasia of the hip, ultrasound, acetabular, cartilage, hip
Citation: Hong K, Wan J, Zhao Y and Zhang C (2024) Evaluation of the thickness of acetabular cartilage by ultrasound in developmental dysplasia of the hip. Front. Pediatr. 12:1351296. doi: 10.3389/fped.2024.1351296
Received: 6 December 2023; Accepted: 27 August 2024;
Published: 10 September 2024.
Edited by:
Federico Canavese, Centre Hospitalier Regional et Universitaire de Lille, FranceReviewed by:
Marco Sapienza, University of Catania, ItalyHouping Chen, Guiyang Children’s Hospital, China
Copyright: © 2024 Hong, Wan, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zhang, emhhbmdjaGFvX3RqQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Kai Hong
Kai Hong Jie Wan2,†
Jie Wan2,† Chao Zhang
Chao Zhang