
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 08 March 2024
Sec. Pediatric Surgery
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1348789
 Debas Yaregal Melesse*
Debas Yaregal Melesse* Tadesse Teshale Tesema
Tadesse Teshale Tesema Zemenay Ayinie Mekonnen
Zemenay Ayinie Mekonnen Wubie Birlie Chekol
Wubie Birlie Chekol Biruk Adie Admass
Biruk Adie Admass Misganaw Mengie Workie
Misganaw Mengie Workie
Introduction: Postoperative delirium in paediatric patients is a recognised issue. Nevertheless, in low- and middle-income nations, researchers have had luck in determining its extent and predictors. Identifying predictors of postoperative delirium in paediatric patients having general anaesthesia at Tertiary Hospitals in Ethiopia was the aim of this study.
Methods: A multicenter, prospective follow up study was conducted from April 15 to June 15, 2023 at the study settings. During the study period a total of 424 paediatric surgical patients treated under general anaesthesia in all study locations, ranging in age from birth to sixteen were candidates for this study. Charts and direct observation of patient's with assessment tool [Cornell Assessment of Pediatric Delirium (CAPD)] were used from each available patient. Binary logistic regression analysis was performed to determine predictors of postoperative delirium in paediatric patients undergoing surgery under general anaesthesia.
Results: Postoperative delirium occurred in 160 of the 404 paediatric patients who underwent surgery under general anaesthesia. Ophthalmic surgery, corticosteroid use, anticholinergic use, severe postoperative pain, and preoperative anxiety were found to be predictors of postoperative delirium; whereas, sedative medication premedication and paracetamol used for analgesia were found to be protective against postoperative delirium.
Inference and recommendation: The postoperative delirium in paediatric patients undergoing surgery under general anaesthesia was higher compared to developed countries. Ophthalmic surgery, corticosteroids, anticholinergic medications, postoperative pain, and preoperative anxiety were found to be predictors. The impact of postoperative delirium might be lessened by concentrating on its screening and factor control.
Pediatric postoperative delirium is an acute brain dysfunction characterized by various clinical manifestations, including disturbances in awareness, attention, and disorientation (1).
Patients who have had surgery and anaesthesia may experience post-operative delirium (POD), a type of delirium that typically peaks one to three days following the treatment (2). It must be distinguished from emergence delirium, which, particularly in younger patients, happens in 8 to 20% of cases following their awakening from general anaesthesia (3, 4). Postoperative delirium (POD), postoperative cognitive deterioration, and postoperative incident dementia have not yet been completely explained, nor has the potential correlation between them (5).
This condition has a negative impact on children's overall recovery and wellbeing and can affect patients of any age. Its incidence varies depending on the patient's age and is influenced by patient-related risk factors that accumulate differently in each age group. The condition also causes problems, lengthens hospital stays, and increases healthcare costs (1, 6, 7).
In school-age children, postoperative cognitive dysfunction (POCD) may have developed and, in the event that no commensurate remedy was made, may have persisted in 80% of cases for at least one month following the operation (7–9). Preoperative anxiety, postoperative pain, type of anaesthetic agent, young age, specific surgical procedures, opioid exposure, preexisting medical illness, adjunct medication, intraoperative blood loss, infection, and blood transfusion were risk factors linked to the development of postoperative delirium (10–15).
Delirium subtypes were categorised as hyperactive (agitation, restlessness, hypervigilance, and combative behaviour), hypoactive (lethargy, inattention, and decreased responsiveness), and mixed-type delirium (combines elements of both hyperactive and hypoactive delirium) (16). Studies conducted in pediatric patients to assess the long-term impact of postoperative delirium on their development (12, 17–19). Delirium in newborns and infants is difficult to diagnose due to communication limitations (20). In those age groups, symptoms of delirium include non-purposefulness, difficulty in engaging, agitation, restlessness and calming the child (14, 21, 22). Preschool children are more susceptible to developing delirium, which can be attributed to their constant need for stimulation (14). Symptoms of delirium in school children and adolescents are easier to observe and are similar to those in adult patients (12).
The Cornell Assessment of Pediatrics Delirium (CAPD) is an assessment tool used for the rapid screening of delirium in pediatric patients. The CAPD is an observational tool that can be utilized from birth up to 21 years of age (20).
Despite numerous evidence of a negative impact of delirium in pediatric patients, there are no standardized preventive, diagnostic and therapeutic measures to reduce the incidence of delirium among children, shorten a hospital stays, and reduce invasive interventions, improving the quality of life and the patient's condition (9, 17). Preventative measures can be non-pharmacological or pharmacological. Postoperative delirium (POD) is typically a self-limiting condition. If POD occurs, immediate treatment of both causative factors and symptoms has a major impact in reducing its duration (23). Strategies to reduce POD should comprise regular screening for pediatric delirium, aggressive treatment of infections, early removal of catheters and respiratory devices, and, most importantly, as little sedation as possible (1, 24, 25).
This topic has not been the focus of any previous research in the study areas. The purpose of this study was to identify factors that are associated with postoperative delirium in paediatric patients at Amhara Regional State Tertiary Hospitals who are undergoing surgery under general anaesthesia.
The study was conducted in four comprehensive and specialized hospitals (University of Gondar, Debre Tabor, Tibebe Ghion, and Felege Hiwot) which are found in northwest Ethiopia. The prospective observational study was conducted from April 15 to June 15, 2023.
This covered all paediatric surgical patients, aged from birth to sixteen, who were operated under general anaesthesia in all study locations during the study period. Patients having a history of mental impairment, major cognitive dysfunction, coma, deep sedated patient, admission to the critical care unit after surgery, need for artificial breathing after surgery, blindness, or bilateral eye surgery were excluded from the study. The sample size was determined using a single population formula, which produced 424 by taking 50% of the paediatric population, adding a margin of error of 5%, adding a contingency rate of 10%, and calculating a 95% confidence interval (CI). Using a situational analysis of each hospital, the computed sample size was distributed proportionately to all of the hospitals.
“The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies” demonstrated the study participants' eligibility for the final result analysis (26) (Figure 1).
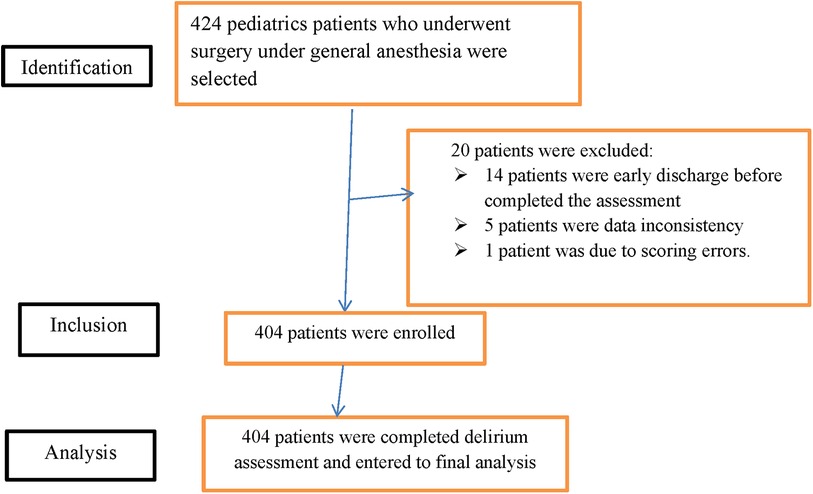
Figure 1. STROBE diagram shows the study participants who were included and excluded in the study (STROBE: strengthening the reporting of observational studies in epidemiology).
Postoperative delirium (yes/no) was the dependent variable whereas age, sex, body mass index (BMI), American Society of Anaesthesiologists (ASA) physical status, premedication, surgical specialty, type of surgery, pre-existing medical conditions, developmental delay, nothing per nose (NPO) time, preoperative anxiety, types of anesthesia, analgesic drug used, induction technique, maintenance of anesthesia, duration of surgery, duration of anesthesia and intraoperative blood loss, post-operative pain, medication exposure, infection, perioperative blood transfusion history, and were independent variables of the study.
Ethical clearance and approval letter was obtained from the School of Medicine ethical review committee, University of Gondar, with the approval number 14/04/524/2023. Additionally, permission to conduct the study was obtained from each hospital. Prior to administering the questionnaire, assent was obtained from the patient's caregivers after providing them with a detailed explanation of the study. Verbal informed consent was obtained from legally authorized representatives before the study. The collected data were used solely for research purposes, and strict measures were taken to ensure confidentiality and anonymity.
It was suggested that the Chinese version of the Corneal Assessment of Paediatric Delirium (CAPD) Scale be used in clinical observation and research to assess paediatric delirium due to its favourable reliability, validity, diagnostic efficacy, and feasibility. We used this tool to diagnose postoperative delirium at the post-anesthesia care unit based on the study's recommendation.
The accuracy and efficacy of CAPD have been validated in pediatric patients booth in an intensive care unit (ICU) and POD with presented excellent performance (sensitivity 96.7% and specificity 93.1%) and high inter-rater agreement (6). With its ability to distinguish between delirium and other causes of altered mental state, CAPD is the preferable tool that has been validated tool for our population. Therefore, the CAPD was used as the postoperative delirium assessment tool for this study, which consists of 8 items, on a Likert scale, scored of 9 or higher was considered as positive delirium assessment (27). A delirium diagnosis is consistent with a total score of more than or equal to nine. The child would open his eyes, make brief eye contact, and momentarily awaken to voice cues before screening began if the Richmond Agitation and Sedation Score (RASS) (28) was greater than −3. Together with the patient's nurse, the same two experienced anesthetists completed each assessment. Twice a day, in the middle of the nurses' morning shifts and at the end of their evening shifts, scoring was conducted. Each patient received six assessments of delirium using CAPD in all over the course of three days in a row. If postoperative delirium developed, the data collectors would speak with the patient's nurse so that it could be treated in accordance with protocol. The data were collected from the selected study population in a study period by using a semi-structured questionnaire consecutively.
Data collection procedures included a review of patients' charts, and direct observation of the patients with an assessment tools. The questionnaire contained socio-demographic data (including age, gender, and BMI), preoperative variables [including ASA classification, developmental delay, premedication, type of surgical specialty, type of surgery, preexisting medical condition, fluid fasting (NPO) time, and preoperative anxiety score using the short form of four domains modified Yale preoperative anxiety scale (mYPAS)], intraoperative variables (including duration of anesthesia, duration of surgery, types of induction agent, types of intraoperative analgesia used, technique of GA used, maintenance anesthesia, and intraoperative blood loss), postoperative variables (including CAPD scores, pain scores (Face, Legs, Activity, Cry, Consolability behavioral tool), and exposure to medications by categories (including narcotics, benzodiazepines, corticosteroids, and anticholinergic) and blood transfusion. During data collection, each questionnaire was revised by the investigator for being complete and appropriate. After data collection, the data were coded, entered, and cleaned prior to statistical analysis.
The Epidata statistical software (version 4.6) was used for data entry, and then exported to the SPSS statistical software (version 23.0) for further processing and analysis. The Shapiro-Wilk test was used. Multicollinearity was assessed for continuous or numeric independent variables in a regression model, with tolerance values below 0.1 or variance inflation factors (VIF) above 10 were removed. The Pearson correlation coefficient was used to determine the relationship between two continuous variables in the data related to postoperative delirium. The chi-square test was used to assess the association between categorical variables in the same dataset. Binary logistic regression analysis was performed to identify predictors of postoperative delirium. The goodness of fit of the model was assessed with the Hosmer–Lemeshow test. Candidate variables for multivariable logistic regression were selected based on a significance level of p-value <0.2 in the bivariable logistic regression. In the multivariable logistic regression, a significance level of p-value <0.05 was taken as statistically significant predictor of postoperative delirium. The strength of the associations was determined by odds ratios (OR) with 95% confidence intervals (CI). The final model presented with the adjusted odds ration (AOR) and 95% CI. Descriptive statistics were presented with text, tables, and graphs.
Out of the study patients, 214 (53%), were males. The study participants' ages were as follows: 155 (38.4%) were between the ages of 7 and 12, 90 (22.3%) were between the ages of 13 and 16, 86 (21.3%) were between the ages of 3 and 6, and 73 (18.1%) were between the ages of birth and 2 years. The study participants' body mass index (BMI) measurements revealed that 45 (11.1%) were underweight (less than the fifth percentile), 235 (58.2%) were in the healthy weight range (5th–85th percentile), 35 (8.7%) were overweight (between the 85th and 95th percentile), 19 (4.7%) were obese (beyond the 95th percentile), and 70 (17.3%) had no BMI measurements because they were younger than two years old. Majority of the study participants, 258 (63.9%) were classified as ASA I. Most of the study participants, 257 (63.6%) were operated in elective basis (Table 1).
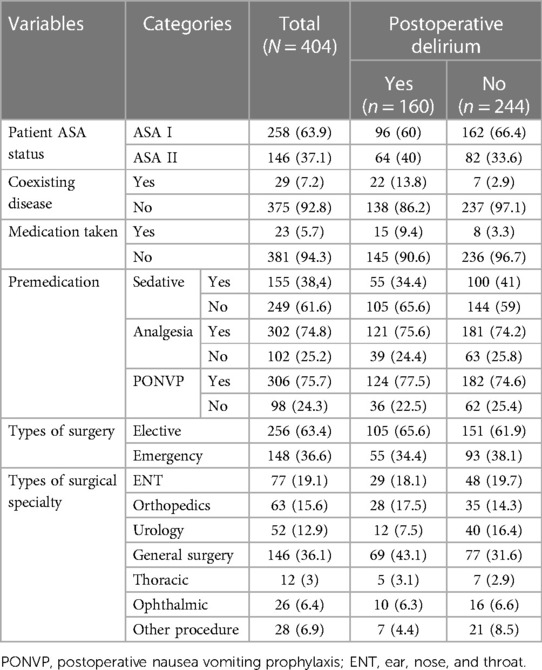
Table 1. Socio-demographic and preoperative variables of pediatrics patients who underwent surgery under general anesthesia, (N = 404).
A total of 399 patients (98.2%) were induced with intravenous anaesthetic medications, whereas 5 patients were induced via inhalational anaesthesia. Over 90% of patients were maintained under inhalational anaesthesia (Table 2). The median duration of surgery was 1 h and 10 min with inter-quartile range (IQR) (1 h to 1 h and 40 min), while the median duration of anesthesia was 1 h and 30 min with IQR (1 h and 10 min–1 h and 50 min). The median intraoperative estimated blood loss was 50 ml with IQR (20 ml−120 ml). Of the patients in the study, 49% did not exhibit any pain signals, 25.2% experienced mild pain, 14.1% had moderate pain, while 11.6% experienced severe pain.
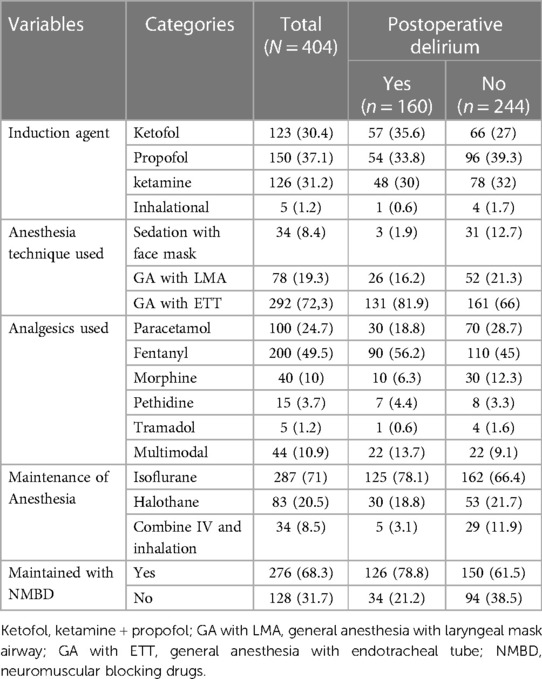
Table 2. Intraoperative and postoperative variables of pediatrics patients who underwent surgery under general anesthesia, (N = 404).
Of the 404 children who were included, 160 (39.6%) at 95%CI: 35–45 were found to have been delirious in at least one evaluation (CAPD ≥ 9) throughout the three-day follow-up. The majority of the patients developed postoperative delirium within 24 h (Figure 2). From the first to the third postoperative day, the daily incidence of postoperative delirium was 77 (19%), 39 (9.7%), and 44 (10.9%), respectively (Figure 3).
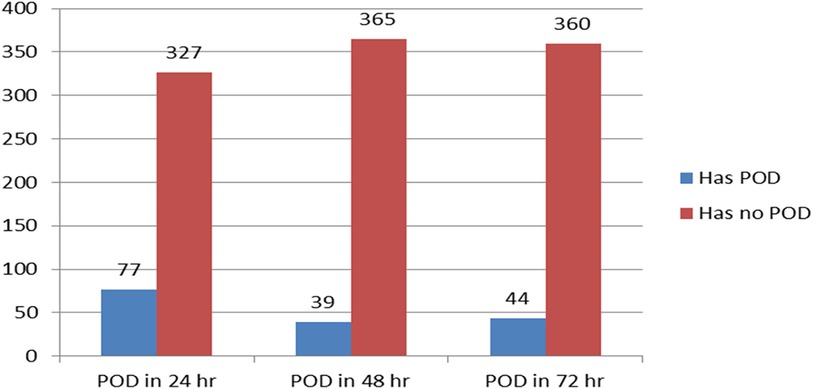
Figure 2. Shows the comparison between patients who experienced postoperative delirium (POD) and those who did not (N = 404). POD, postoperative delirium.
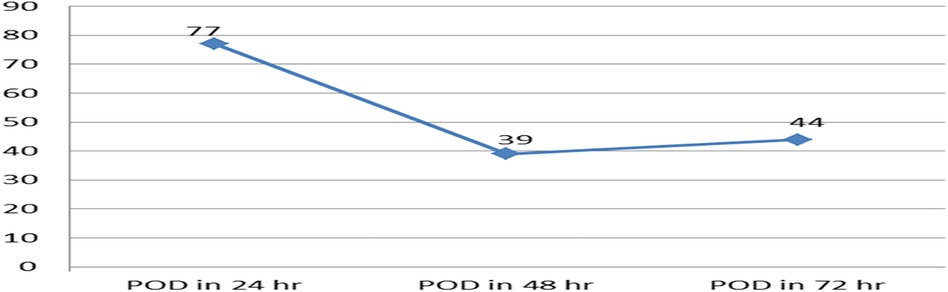
Figure 3. The line graph displays the incidence of postoperative delirium over the course of the three-day follow-up period in pediatric patients who underwent surgery with general anesthesia, (N = 404). POD, postoperative delirium.
The following factors were found to be associated with postoperative delirium at a p-value of less than 0.2: physical status, coexisting diseases, medication taken at the time of preoperative diagnosis for surgery, type of surgical specialty, preoperative anxiety, type of induction agent, technique of anaesthesia used, type of analgesic used, maintenance of anaesthesia, duration of surgery, postoperative pain assessment, medication exposure, and blood transfusion. Multivariable logistic regression revealed that postoperative delirium was significantly correlated with the following factors: ophthalmic surgery, sedative premedication, use of paracetamol as an analgesic, exposure to medications such as corticosteroids and anticholinergics, postoperative pain, and preoperative anxiety (p-value <0.05) (Table 3).
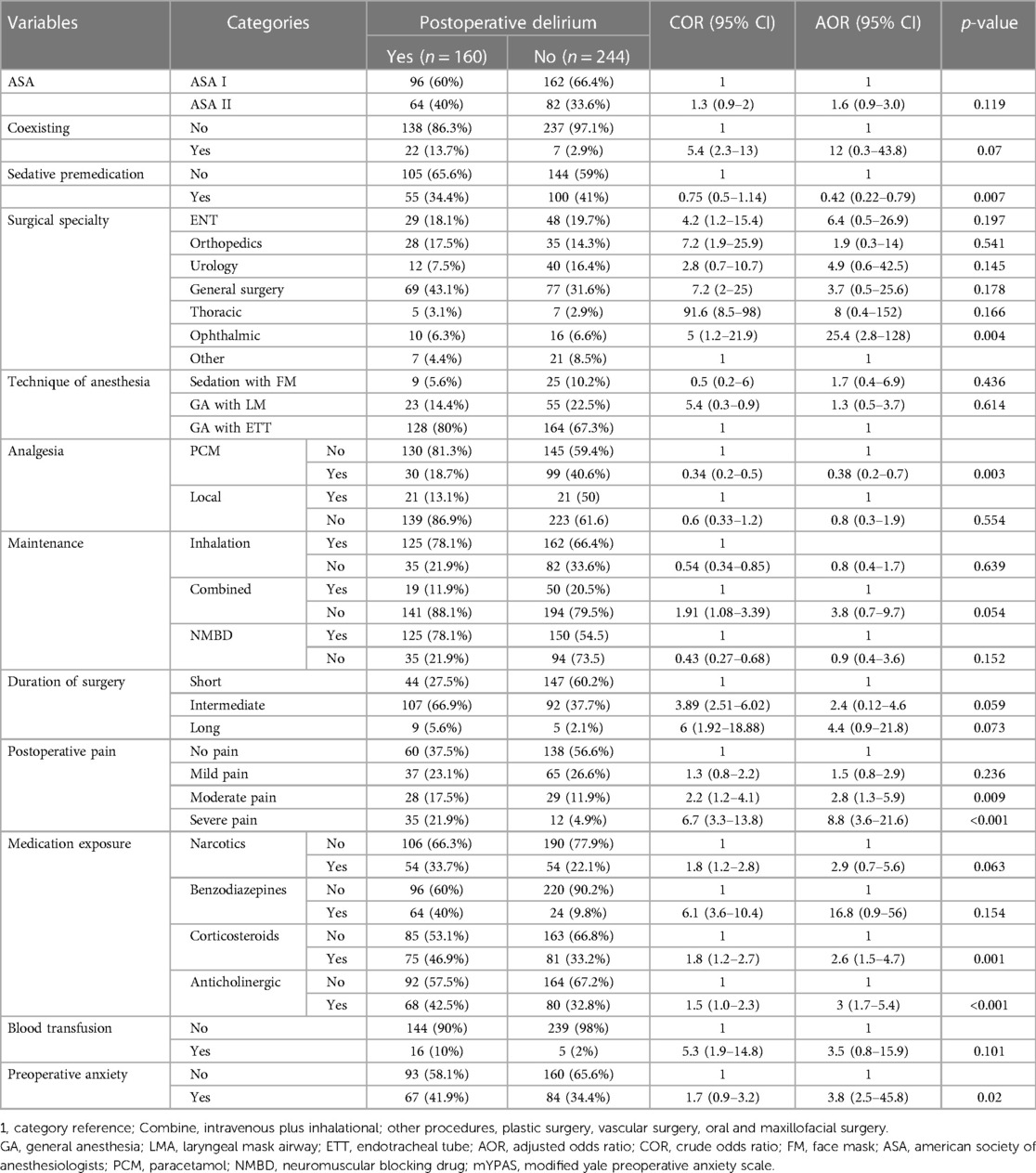
Table 3. Bivariable and multivariable binary logistic regression for predictors of postoperative delirium in pediatric patients who underwent surgery under general anesthesia, (N = 404).
Our study found that 160 (39.6%) of patients experienced postoperative delirium the Corneal Assessment of Paediatric Delirium (CAPD) score ≥9. This study yielded greater results than two studies conducted in Colombia (13.2% and 14.4%) (29, 30), two studies conducted in China (13.5% and 11.1%) (15, 31, 32), and a study conducted in Germany (23%) (33). This variation may be due to the large sample size used in the previous studies, preoperative optimisation differences, or variations in the health care infrastructure.
The results of the current study were lesser than a study done in Germany, where the incidence of post-operative delirium (POD) was 65.9% (33). This discrepancy may be explained by the fact that the study was carried out on children in paediatric intensive care units, where it was anticipated that delirium rates would be higher for critically ill patients than for those in post-anesthesia care units.
Patients in our study who received sedative medication as premedication had a lower risk of developing postoperative delirium; in particular, patients who received sedative medication prior to the induction of general anaesthesia had a 58% lower risk of postoperative delirium than those who did not receive sedative medication. This result was in contrast to previous research conducted in Colombia and a systematic study that did not identify any link between the use of pre-surgical sedatives and a reduction in the incidence of paediatric delirium (29, 34). However, our research was bolstered by two systematic reviews and two meta analyses which demonstrated that patients who took anxiolytics, opioids, and ketofol prior to 10 to 45 min before surgery experienced less postoperative delirium (35–38). The sedative medication helps to calm and relax the patient prior to the procedure, reducing their anxiety and stress levels.
Ophthalmic surgery showed a substantial correlation with postoperative delirium in our study. A study conducted in the United States of America revealed a similar conclusion: ocular surgery was 1.66 times more likely than orthopaedic, urological, and general surgery to result in postoperative delirium (39). The ophthalmic surgery frequently entails manipulating the eye, which some paediatric patients may find stressful or disorienting. According to certain research, these patients' development of postoperative delirium may be influenced by visual abnormalities (23, 37, 40).
According to this study, patients who took paracetamol for analgesia had a 62% lower risk of developing postoperative delirium than patients who did not take the medication. The results of the studies showed that children who received paracetamol had a significantly lower incidence of delirium and pain (19, 41, 42). One possible explanation is that paracetamol, through its effective management of pain, fever, and decreased requirement for opioids, may indirectly reduce the risk of postoperative delirium.
The risk of postoperative delirium increased by three times in individuals taking anticholinergic medications. The outcome of our investigation aligned with a German study that suggested anticholinergic medication use in paediatric patients was substantially linked to a higher risk of postoperative delirium (43). Several studies have discovered that the use of anticholinergic medication during general anaesthesia in paediatric patients was linked to an increased risk of POD (44–47). This might be explained the fact that the action of the neurotransmitter acetylcholine, which is essential for memory, attention, and cognition, may be blocked by anticholinergic drugs (48, 49).
Children on corticosteroids had a 2.6-fold higher chance of postoperative delirium. Our study's findings were consistent with a Colombian study that found patients between the ages of 2 and 10 who took dexamethasone had a 2.39-fold increased risk of postoperative delirium (30).
Our findings were corroborated by additional research done in the USA, Brazil, and a meta-analysis, which showed that children who received corticosteroids had a higher risk of postoperative delirium than children who did not (18, 47, 50). Reasons for this might be: corticosteroids may modify mood, behaviour, and cognitive function. Corticosteroids may also affect glucose metabolism, interact with other medications, increase inflammation, disturb regular sleep patterns, and affect brain function.
The development of POD found to be 2.8 times more likely in patients with moderate pain (with the Face, Legs, Activity, Cry, and Consolability (FLACC) scale of 4–6 and 8.8 times more likely in patients with severe pain (FLACC scale of 7–10). Studies conducted in China and Brazil which evaluated pain using the FLACC scale provided support for our research (15, 51).
Further researches conducted in Brazil and Russia also corroborated this finding, concluding that insufficient analgesia in the preoperative period was strongly associated with the development of delirium (16, 30, 51). The possible explanation for this could be the psychological effects of pain are perceived to be favourable for changes in neurotransmitter systems results in proinflammatory mediators and impair the physiological stress response.
In this study, there was a 3.8-fold increase in the probability of POD development in individuals who experienced preoperative anxiety. This study was supported by a study conducted on Saudi Arabian patients whose anxiety was significantly correlated with delirium (19).
Additional research from the United States, Australia, Brazil, Turkey, and China's systematic review of the literature supports the idea that preoperative anxiety in paediatric patients undergoing general anaesthesia was a significant risk factor for postoperative delirium (52–56). An explanation for this outcome could be preoperative anxiety, through a combination of neurochemical imbalances, impaired cognitive function, sleep disturbance, and increased stress response, may increase the risk of postoperative delirium.
One of the study's limitations is that, since it is outside the purview of this investigation, we do not address medications that were taken during the study period. It is necessary to conduct more research evaluating specific drugs taken and how they affect the development of delirium. The fact that delirium screening was limited to the first three postoperative days is another limitation of this study. Because some children might not have displayed delirium symptoms during the assessment period and because some children may have only been delirious at night, our study may have underestimated the incidence of delirium in children.
The postoperative delirium in paediatric patients undergoing surgery under general anaesthesia was higher compared to developed countries. Ophthalmic surgery, corticosteroids, anticholinergic medications, postoperative pain, and preoperative anxiety were found to be predictors.
In order to develop practical methods for preventing and treating postoperative delirium in paediatric patients receiving general anaesthesia, more research is required. Specifically, randomised controlled trials (RCTs) are needed to examine other factors that were not covered in this study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethical clearance and approval letter was obtained from the School of Medicine ethical review committee, University of Gondar, with the approval number 14/04/524/2023. This study was performed by the Declaration of Helsinki. Additionally, permission to conduct the study was obtained from each hospital. Prior to administering the questionnaire, assent was obtained from the patient's caregivers after providing them with a detailed explanation of the study. The collected data were used solely for research purposes, and strict measures were taken to ensure confidentiality and anonymity. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
DY: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. TT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZA: Supervision, Writing – review & editing. WC: Supervision, Writing – review & editing. BA: Supervision, Writing – review & editing. MM: Supervision, Visualization, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We appreciate the University of Gondar's giving the opportunity to conduct the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. (2017) 34(4):192–214. doi: 10.1097/EJA.0000000000000594
2. Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. (2011) 77(4):448–56.21483389
3. Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anesth. (2010) 57(9):843. doi: 10.1007/s12630-010-9338-9
4. Radtke FM, Franck M, Hagemann L, Seeling M, Wernecke KD, Spies CD. Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol. (2010) 76(6):394–403.20473252
5. Bryson GL. Methods and madness: agitation, delirium, and postoperative cognitive dysfunction. Can J Anaesth. (2010) 57(9):799–803. doi: 10.1007/s12630-010-9339-8
6. Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit Care Med. (2011) 39(1):150–7. doi: 10.1097/CCM.0b013e3181feb489
7. Meyburg J, Dill ML, Traube C, Silver G, von Haken R. Patterns of postoperative delirium in children. Pediatr Crit Care Med. (2017) 18(2):128–33. doi: 10.1097/PCC.0000000000000993
8. Ovezov AM, Lobov MA, Panteleeva MV, Lugovoĭ AV, Miatchin PS, Gus'kov IE. Correction of early cognitive disorders in school-age children operated under total intravenous anaesthesia. Anesteziol Reanimatol. (2012) (3):25–9. PMID: 2299391922993919
9. Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. (2010) (3):CD006632. doi: 10.1002/14651858.CD006632.pub2
10. Behrends M, DePalma G, Sands L, Leung J. Association between intraoperative blood transfusions and early postoperative delirium in older adults. J Am Geriatr Soc. (2013) 61(3):365–70. doi: 10.1111/jgs.12143
11. Nellis ME, Goel R, Feinstein S, Shahbaz S, Kaur S, Traube C. Association between transfusion of red blood cells and subsequent development of delirium in critically ill children. Pediatr Crit Care Med. (2018) 19(10):925. doi: 10.1097/PCC.0000000000001675
12. Hatherill S, Flisher AJ. Delirium in children and adolescents: a systematic review of the literature. J Psychosom Res. (2010) 68(4):337–44. doi: 10.1016/j.jpsychores.2009.10.011
13. Mody K, Kaur S, Mauer EA, Gerber LM, Greenwald BM, Silver G, et al. Benzodiazepines and development of delirium in critically ill children: estimating the causal effect. Crit Care Med. (2018) 46(9):1486. doi: 10.1097/CCM.0000000000003194
14. Silver G, Traube C, Gerber LM, Sun X, Kearney J, Patel A, et al. Pediatric delirium and associated risk factors: a single-center prospective observational study. Pediatr Crit Care Med. (2015) 16(4):303. doi: 10.1097/PCC.0000000000000356
15. Lin N, Liu K, Feng J, Chen R, Ying Y, Lv D, et al. Development and validation of a postoperative delirium prediction model for pediatric patients: a prospective, observational, single-center study. Medicine (Baltimore). (2021) 100(20):e25894. doi: 10.1097/MD.0000000000025894
16. Patel AK, Bell MJ, Traube C. Delirium in pediatric critical care. Clin Pediatr. (2017) 64(5):1117–32. doi: 10.1016/j.pcl.2017.06.009
17. Góźdź A, Bienert A, Bartkowska-Śniatkowska A, Ber JA, Ista E. Delirium in children–new research directions. J Med Sci. (2021) 90(1):e478. doi: 10.20883/medical.e478
18. Flores AE, Oura KH, Rocha PK, Belela-Anacleto AS, Kusahara DM. Incidence and factors associated with delirium in children in a single pediatric intensive care unit in Brazil. J Pediatr Nurs. (2021) 61:e29–34. doi: 10.1016/j.pedn.2021.04.008
19. Aldakhil SK, Salam M, Albelali AA, Alkanhal RM, Alnemer MJ, Alatassi A. The prevalence of emergence delirium and its associated factors among children at a postoperative unit: a retrospective cohort at a Middle Eastern hospital. Saudi J Anaesth. (2020) 14(2):169. doi: 10.4103/sja.SJA_573_19
20. Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell assessment of pediatric delirium: a valid, rapid, observational tool for screening delirium in the PICU. Crit Care Med. (2014) 42(3):656. doi: 10.1097/CCM.0b013e3182a66b76
21. Brahmbhatt K, Whitgob E. Diagnosis and management of delirium in critically ill infants: case report and review. Pediatrics. (2016) 137(3):e20151940. doi: 10.1542/peds.2015-1940
22. Mason K. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. (2017) 118(3):335–43. doi: 10.1093/bja/aew477
23. Grigoriev EV, Ivkin AA. Delirium in children after cardiac surgery: brain resuscitation. In: Shaikh N, Aslanidis T, editors. ICU Management and protocols. Russia: IntechOpen (2022).
24. Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharmacological prevention of sevoflurane-and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth. (2010) 104(2):216–23. doi: 10.1093/bja/aep376
25. Banchs RJ, Lerman J. Preoperative anxiety management, emergence delirium, and postoperative behavior. Anesthesiol Clin. (2014) 32(1):1–23. doi: 10.1016/j.anclin.2013.10.011
26. Cevallos M, Egger M. STROBE (STrengthening the reporting of OBservational studies in epidemiology). In: Moher D, Altman DG, Schulz KF, Simera I, Wager E, editors. Guidelines for reporting health research: A user's Manual. 1st edn. Bern: John Wiley & Sons, Ltd. (2014). p. 169–79.
27. He S, Wang Y, Zuo Z. Clinical application of the Chinese version of cornell assessment of pediatric delirium: a pilot study. Zhonghua Er Ke Za Zhi. (2019) 57(5):344–9. doi: 10.3760/cma.j.issn.0578-1310.2019.05.006
28. Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the richmond agitation-sedation scale (RASS). JAMA. (2003) 289(22):2983–91. doi: 10.1001/jama.289.22.2983
29. Cárdenas VH, Ávila DS, Barajas WJ, Reina MA, Villazón IL, Pulgarín JL, et al. Premedication with midazolam in low-risk surgery in children does not reduce the incidence of postoperative delirium. Cohort study. Colomb J Anesthesiol. (2023) 51(2). doi: 10.5554/22562087.e1055
30. González-Cardenas VH, Munar-González FD, Pinzón-Villazon IL, Cabarique-Serrano SH, Burbano-Paredes CC, Cháves-Rojas N, et al. Study of paediatric postoperative delirium and acute pain in low surgical risk procedures. Colomb J Anesthesiol. (2018) 46(2):126–33. doi: 10.1097/CJ9.0000000000000024
31. Hong H, Guo C, Liu ZH, Wang BJ, Zhou SZ, Mu DL, et al. The diagnostic threshold of Cornell assessment of pediatric delirium in detection of postoperative delirium in pediatric surgical patients. BMC Pediatr. (2021) 21(1):87. doi: 10.1186/s12887-021-02538-x
32. Houben A, Ghamari S, Fischer A, Neumann C, Baehner T, Ellerkmann RK. Pediatric emergence delirium is linked to increased early postoperative negative behavior within two weeks after adenoidectomy: an observational study. Braz J Anesthesiol. (2021). doi: 10.1016/j.bjane.2021.03.008. [Epub ahead of print]33887334
33. Meyburg J, Dill ML, von Haken R, Picardi S, Westhoff JH, Silver G, et al. Risk factors for the development of postoperative delirium in pediatric intensive care patients. Pediatr Crit Care Med. (2018) 19(10):e514–21. doi: 10.1097/PCC.0000000000001681
34. Breschan C, Platzer M, Jost R, Stettner H, Likar R. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth. (2007) 17(4):347–52. doi: 10.1111/j.1460-9592.2006.02101.x
35. Zhang C, Li J, Zhao D, Wang Y. Prophylactic midazolam and clonidine for emergence from agitation in children after emergence from sevoflurane anesthesia: a meta-analysis. Clin Ther. (2013) 35(10):1622–31. doi: 10.1016/j.clinthera.2013.08.016
36. Nair S, Wolf A. Emergence delirium after paediatric anaesthesia: new strategies in avoidance and treatment. BJA Educ. (2018) 18(1):30. doi: 10.1016/j.bjae.2017.07.001
37. Grotmol OG, Nesarajah N, Hansen TG. Postoperative emergence delirium in children: a narrative review of recent publications. Signa Vitae. (2021) 17(3):10–20. doi: 10.22514/sv.2021.057
38. Reduque LL, Verghese ST. Paediatric emergence delirium. Contin Educ Anaesth Crit Care Pain. (2013) 13(2):39–41. doi: 10.1093/bjaceaccp/mks051
39. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. (2003) 96(6):1625–30. doi: 10.1213/01.ANE.0000062522.21048.61
40. She D, Wang ZY, Wu F, Zhang YQ, Ao Q. Meta-analysis of visual pretreatment for the prevention of emergence delirium in children undergoing ophthalmic surgery. J Comp Eff Res. (2022) 11(9):679–88. doi: 10.2217/cer-2022-0037
41. Rezaee M, Soltani ZE, Takzare A, Badripour A, Goudarzi M, Alizade S. Effect of paracetamol on postoperative pain in children undergoing strabismus surgery under Desflurane anesthesia. Perioper Care Oper Room Manag. (2021) 24:100196. doi: 10.1016/j.pcorm.2021.100196
42. Mehrotra S. Postoperative anaesthetic concerns in children: postoperative pain, emergence delirium and postoperative nausea and vomiting. Indian J Anaesth. (2019) 63(9):763–70. doi: 10.4103/ija.IJA_391_19
43. Meyburg J, Ritsert ML, Traube C, Plaschke K, von Haken R. Cholinesterases and anticholinergic medications in postoperative pediatric delirium. Pediatr Crit Care Med. (2020) 21(6):535–42. doi: 10.1097/PCC.0000000000002246
44. Traube C, Silver G, Gerber LM, Kaur S, Mauer EA, Kerson A, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium*. Crit Care Med. (2017) 45(5):891–8. doi: 10.1097/CCM.0000000000002324
45. Madden K, Hussain K, Tasker RC. Anticholinergic medication burden in pediatric prolonged critical illness: a potentially modifiable risk factor for delirium. Pediatr Crit Care Med. (2018) 19(10):917. doi: 10.1097/PCC.0000000000001658
46. Ricardo Ramirez C, Álvarez Gómez ML, Agudelo Vélez CA, Zuluaga Penagos S, Consuegra Peña RA, Uribe Hernández K, et al. Clinical characteristics, prevalence, and factors related to delirium in children of 5 to 14 years of age admitted to intensive care. Med Intensiva (Engl Ed). (2019) 43(3):147–55. doi: 10.1016/j.medine.2019.02.004
47. Wang HY, Chen TY, Li DJ, Lin PY, Su KP, Chiang MH, et al. Association of pharmacological prophylaxis with the risk of pediatric emergence delirium after sevoflurane anesthesia: an updated network meta-analysis. J Clin Anesth. (2021) 75:110488. doi: 10.1016/j.jclinane.2021.110488
48. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium-theory, evidence and practice. Br J Clin Pharmacol. (2016) 81(3):516–24. doi: 10.1111/bcp.12839
49. Maravi P, Mishra DK, Singh A, Niranjan V. Atropine eye-drop-induced acute delirium: a case report. Gen Psychiatry. (2020) 33(3):e100125. doi: 10.1136/gpsych-2019-100125
50. Smith HA, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, et al. The preschool confusion assessment method for the ICU (psCAM-ICU): valid and reliable delirium monitoring for critically ill infants and children. Crit Care Med. (2016) 44(3):592. doi: 10.1097/CCM.0000000000001428
51. Barreto AC, Paschoal AC, Farias CB, Borges PS, Andrade RG, de Orange FA. Risk factors associated with anesthesia emergence delirium in children undergoing outpatient surgery. Braz J Anesthesiol. (2018) 68(2):162–7. doi: 10.1016/j.bjan.2017.11.002
52. Beringer RM, Segar P, Pearson A, Greamspet M, Kilpatrick N. Observational study of perioperative behavior changes in children having teeth extracted under general anesthesia. Pediatr Anesth. (2014) 24(5):499–504. doi: 10.1111/pan.12362
53. Astan S, Kalkan G, Kendirli T, Bayrakci B. Abstract PCCLB-44: SITUATIONAL AWARENESS IN PICU. Pediatr Crit Care Med. (2018) 19(6S):256–7. doi: 10.1097/01.pcc.0000538129.23421.f0
54. Moore AD, Anghelescu DL. Erratum to: emergence delirium in pediatric anesthesia. Pediatr Drugs. (2017) 19(3):267–267. doi: 10.1007/s40272-017-0227-3
55. Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, McClain BC. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. (2006) 118(2):651–8. doi: 10.1542/peds.2005-2920
56. Wu J, Yan J, Zhang L, Chen J, Cheng Y, Wang Y, et al. The effectiveness of distraction as preoperative anxiety management technique in pediatric patients: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud. (2022) 130:104232. doi: 10.1016/j.ijnurstu.2022.104232
Keywords: factors, general anesthesia, incidence, pediatrics, postoperative delirium, predictors
Citation: Yaregal Melesse D, Teshale Tesema T, Ayinie Mekonnen Z, Chekol WB, Admass BA and Mengie Workie M (2024) Predictors of postoperative delirium in paediatric patients undergoing surgery under general anaesthesia at Amhara Regional State Tertiary Hospitals: a multicenter prospective study. Front. Pediatr. 12:1348789. doi: 10.3389/fped.2024.1348789
Received: 5 December 2023; Accepted: 23 February 2024;
Published: 8 March 2024.
Edited by:
Aydin Yagmurlu, Ankara University, Türkiye© 2024 Yaregal Melesse, Teshale Tesema, Ayinie Mekonnen, Chekol, Admass and Mengie Workie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Debas Yaregal Melesse ZGFieXlhcmVnYWw4MkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.