
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 08 February 2024
Sec. Pediatric Infectious Diseases
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1336589
 Kentaro Okunushi
Kentaro Okunushi Hironobu Kobayashi
Hironobu Kobayashi Yuri Yoh
Yuri Yoh Masaya Kunimatsu
Masaya Kunimatsu Tadashi Shiohama
Tadashi Shiohama Tomozumi Takatani
Tomozumi Takatani Hiromichi Hamada*†
Hiromichi Hamada*†
We encountered a pediatric case of pulmonary hypertension triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A 14-year-old girl was brought to the emergency department of our hospital with fever, respiratory distress, and impaired consciousness. She tested positive for SARS-CoV-2 upon a polymerase chain reaction examination and had prolonged hypoxemia without pneumonia. An echocardiography revealed elevated right ventricular pressure. She was diagnosed with pilocytic astrocytoma at the age of 10 years and underwent a resection of a pituitary tumor. Hormone replacement therapy was administered postoperatively, but her growth hormones were not activated because of concerns about tumor recurrence. Echocardiography at the age of 13 years showed normal right ventricular pressure. On admission, she had an abnormal liver function, elevated liver fibrosis markers, a decreased platelet count, and hepatosplenomegaly, suggesting pulmonary and portal hypertension. The diagnosis was pulmonary hypertension associated with SARS-CoV-2 infection. The mechanism of the pulmonary hypertension was thought to be portal hypertension owing to growth hormone deficiency and SARS-CoV-2 infection. The patient's symptoms improved with oxygenation and bed rest without additional targeted pulmonary hypertension therapy, and her right ventricular pressure decreased. This case demonstrates that a pediatric patient with subclinical pulmonary hypertension may develop pulmonary hypertension triggered by SARS-CoV-2 infection.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been the subject of a considerable number of clinical reports, and a detailed picture of the disease has emerged, especially in the acute phase. The pathogenesis of SARS-CoV-2 infection in children is also becoming better understood. The clinical course has been reported to be milder in children than in the elderly; however, there have been deaths in children, the majority of which were caused by neurological and cardiovascular complications (1, 2).
There have been reports of pulmonary hypertension as a complication of SARS-CoV-2 infection, but most have concerned adult cases (3). This report describes the case of a pediatric patient who was admitted to hospital in the acute phase of SARS-CoV-2 infection with fever, respiratory distress, and impaired consciousness and was diagnosed as having pulmonary hypertension as a complication of SARS-CoV-2 infection.
The patient was a 14-year-old girl who was brought to the emergency department with fever, respiratory distress, and impaired consciousness. She developed fever on the first day of illness and became aware of respiratory distress while walking on the second day. Later in the day, she was observed to have episodes of loss of consciousness and was taken to the emergency department of another hospital. On arrival, she had a Glasgow Coma Scale score of E2V3M5, SpO2 of 92%, and blood glucose level of 41 mg/dL. Her level of consciousness gradually improved with the administration of hydrocortisone and glucose, but she continued to have respiratory distress and hypoxemia. An echocardiography performed on day 3 of illness revealed elevated right ventricular pressure, and the patient was transferred to our hospital.
At the age of 10 years, she was diagnosed with pilocytic astrocytoma and underwent an endoscopic transnasal resection of a pituitary tumor. She was subsequently diagnosed with panhypopituitarism and was recommended levothyroxine, hydrocortisone, and desmopressin acetate hydrate. At the age of 13 years, a physical examination at her school led to a diagnosis of long QT syndrome. An echocardiography at that time showed normal right ventricular pressure. She had no history of syncope.
The patient had a height of 143.8 cm (−2.4 SD), weight of 43.0 kg (−0.9 SD), body mass index of 20.8, temperature of 36.7°C, heart rate of 83 beats/min, blood pressure of 149/109 mmHg, respiratory rate of 26 breaths/min, oxygen saturation of 90%–91% on room air, and oxygen saturation of 97% on oxygen at 1 L/min. She was conscious but had orthopnea and was using her shoulder muscles for accessory breathing. The color of her skin was good. Lung sounds were clear, and respiratory sounds were not diminished. Her heart rhythm was regular with a mild hypertonicity of tone II. Tone III was inaudible, and no heart murmur was noted. The abdomen was flat and soft with no hepatosplenomegaly. There was no peripheral coldness of the extremities, but mild edema was noted.
A chest x-ray showed no cardiomegaly. A chest computed tomography scan showed no abnormalities in the lung fields or pulmonary vessels. An echocardiography revealed an enlargement of the right atrium and ventricle, as well as elevated right ventricular pressure, with an estimated tricuspid regurgitation pressure gradient (TRPG) of 64 mmHg and an estimated maximum pulmonary valve regurgitation pressure gradient of 47 mmHg (Figure 1). The electrocardiography did not show pulmonary P waves or right ventricular hypertrophy (Figure 1). Negative T waves were observed in leads V1–V5 at the age of 13 years after a resection of a pituitary tumor (Figure 2), but they were not detected on the electrocardiogram recorded during the current admission at 14 years of age. The corrected QT time was 0.331 s. Blood tests revealed thrombocytopenia and elevated fibrin–fibrinogen degradation, aspartate aminotransferase, and B-type natriuretic peptide levels. Elevated total bile acids and liver fibrosis markers (i.e., hyaluronic acid and type 4 collagen) were also found. She tested negative for all autoantibodies examined for collagen disease. However, the result of a polymerase chain reaction test for SARS-CoV-2 was positive (Table 1).

Figure 1. Cardiac screening data before the onset of severe acute respiratory syndrome coronavirus 2 infection. (A) Electrocardiograms recorded at the age of 10 years before surgery for a brain tumor and at the age of 13 years at the time of a physical examination in junior high school. The electrocardiogram obtained at the age of 13 years shows negative T waves in leads V1–V5. The QTc interval was 433 ms. (B) Echocardiographic images obtained at the age of 13 years after abnormal electrocardiographic findings. There is no evidence of elevated right ventricular pressure in either the four-chamber view or the short-axis view.
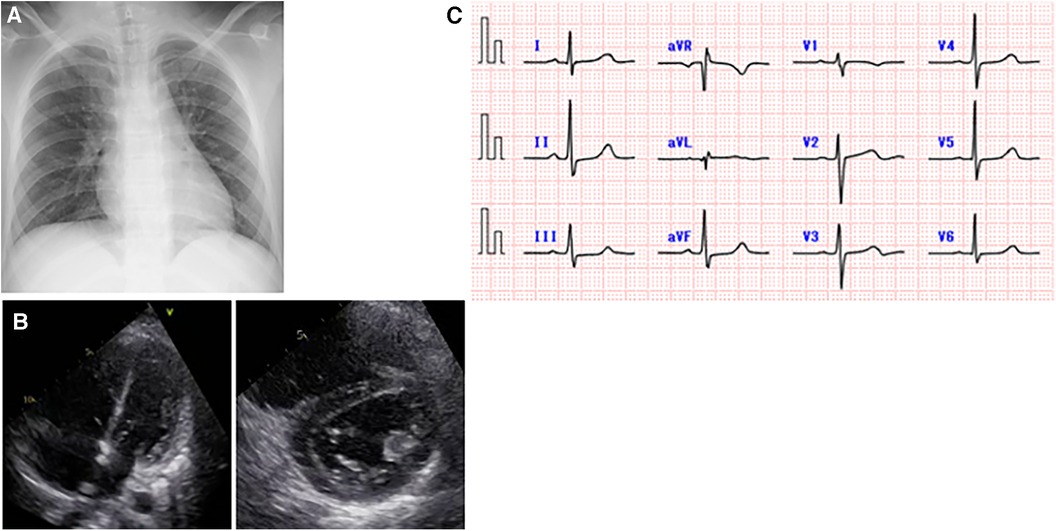
Figure 2. Cardiac examination data after the onset of severe acute respiratory syndrome coronavirus 2 infection. (A) A chest x-ray on day 2 of illness. There was no cardiomegaly or pulmonary congestion. There were no obvious radiographic findings suggestive of pneumonia that would explain the hypoxia. (B) Echocardiographic images on day 3 of illness. Elevated right ventricular pressure was found in the four-chamber and short-axis views, with an estimated tricuspid regurgitation pressure gradient of 64 mmHg and a maximum estimated pulmonary valve regurgitation pressure gradient of 47 mmHg. (C) An electrocardiogram obtained on day 3 of illness showed no abnormal T waves.
Contrast-enhanced computed tomography scans of the abdomen showed hepatosplenomegaly, and an abdominal echocardiography showed a fatty liver (Figure 3). There was no obvious portal vein circulatory shunt.

Figure 3. Other imaging data obtained after the onset of severe acute respiratory syndrome coronavirus 2 infection. (A) A contrast-enhanced abdominal computed tomography scan showing hepatomegaly and splenomegaly. (B) An abdominal echocardiographic image showing increased echoluminance of the liver, which indicates a fatty liver.
The above findings indicated SARS-CoV-2 infection, potentially complicated by pulmonary hypertension and liver dysfunction.
The patient was diagnosed as having non-alcoholic fatty liver disease and portal hypertension on a background of panhypopituitarism with subclinical pulmonary hypertension caused by the portal hypertension that manifested clinically during SARS-CoV-2 infection.
The clinical course after admission is shown in Figure 4. Oxygen administration was continued and the patient was placed on bed rest in isolation. She did not require targeted pulmonary hypertension therapy or positive pressure respiratory support. Her complaints of respiratory distress gradually resolved, and an echocardiography on day 12 of illness showed an almost normal ventricular septal morphology and improvement in the TRPG to 31 mmHg. The right ventricular pressure did not increase again after oxygen was stopped and the patient was allowed to mobilize. She was discharged after 19 days in hospital. At the first postdischarge outpatient visit (day 27 after the onset of illness), her TRPG had increased again to 42 mmHg and the ventricular septum appeared to be pushed slightly toward the left ventricle. However, these abnormalities subsequently normalized. Thereafter, the patient has shown no further symptoms and has returned to her normal everyday life.
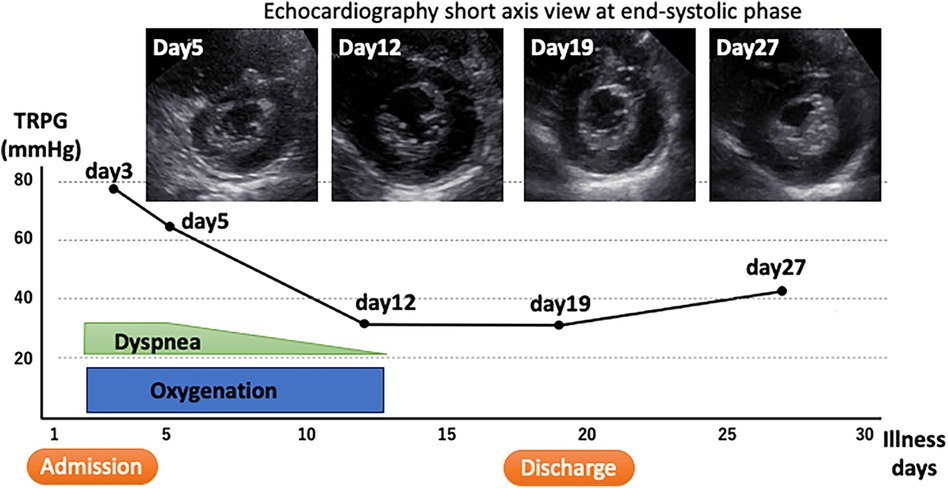
Figure 4. Clinical course. The tricuspid regurgitation pressure gradient, which is a measure of right ventricular pressure, is shown on the vertical axis along with the time course of treatment. The upper image is a short-axis echocardiographic view that is shown with its time course.
There have been some previous reports of pulmonary hypertension in pediatric patients with SARS-CoV-2 infection (4–6). Olfe et al. reported a 16-old-year patient with pulmonary hypertension and underlying mitral valve disease (4). Morales-Demori et al. reported on the status of COVID-19 morbidity in a pediatric pulmonary hypertension center (6). They found that of 23 children with COVID-19, 8 were hospitalized and that 3 of these patients required an intensification of targeted pulmonary hypertension therapy. These reports suggest that COVID-19 can lead to overt pulmonary hypertension in children with subclinical pulmonary hypertension. We consider that the pulmonary hypertension in our patient was a complication caused by a combination of postoperative hypopituitarism and SARS-CoV-2 infection.
An electrocardiogram recorded when the patient was 13 years old (1 year before SARS-CoV-2 infection) showed flat T waves in leads II, III, and aVf, negative T waves in leads V1–V5, and flat T waves in V6, all of which were abnormal findings. An abnormal electrocardiogram is often associated with intracranial abnormalities, possibly because of increased catecholamines in response to a hyperactive sympathetic nervous system (7). At the age of 13 years, she had an abnormal electrocardiogram during screening at school. A subsequent echocardiography revealed normal right ventricular pressure. Therefore, it is unlikely that she had pulmonary hypertension before the onset of SARS-CoV-2 infection. During the present admission, there were no findings suggestive of right ventricular hypertrophy on the electrocardiogram. The short-axis echocardiographic image and TRPG value also suggested that the patient did not have pulmonary hypertension to the extent that it exceeded her systemic blood pressure.
The growth hormone (GH) and insulin-like growth factor-1 (the secretion of which is stimulated by the GH) are important factors in terms of reducing fat accumulation and fibrosis in the liver. An inadequate secretion of the GH can lead to liver disease, including non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, and even a cirrhosis of the liver (8). Our patient was consuming levothyroxine, hydrocortisone, and desmopressin acetate hydrate for panhypopituitarism after a resection of a pituitary tumor, but she was not under any medication for activating her GHs because of concerns about tumor recurrence. Mildly elevated alanine aminotransferase and elevated liver fibrosis markers suggested liver damage associated with GH deficiency.
Previous reports indicate that portal hypertension is present in approximately 30% of patients with non-alcoholic fatty liver disease, and that even in the absence of significant fibrosis, portal hypertension is correlated with the severity of fatty liver disease (9). Splenomegaly and thrombocytopenia, which indicate hypersplenism, were also observed in our patient. Elevated liver fibrosis markers were also detected. These findings suggest the possibility of portal hypertension.
Although the pathogenesis of pulmonary hypertension associated with portal hypertension has not been well understood, it is thought to involve increased pulmonary vascular shear stress caused by increased cardiac output and inflammatory cytokines and endotoxins that do not pass through the liver (10).
SARS-CoV-2 infection is established when spike proteins on the surface of SARS-CoV-2 bind to angiotensin 2 receptors, which are highly expressed in alveolar epithelial cells and the vascular endothelium. The viral infection is thought to induce pulmonary hypertension in response to diffuse lung injury, thrombosis, and cytokine storm (11). In the case of SARS-CoV-2, the virus is internalized along with angiotensin 2 receptors by endocytosis, resulting in a loss of function of these receptors in the cell membrane and functional impairment; this is thought to cause vasoconstriction and induce pulmonary hypertension by increasing the amount of angiotensin 2 that is not degraded and facilitating its binding to the type 1 angiotensin receptor (12). This pathophysiology could indicate that SARS-CoV-2 infection exacerbates pulmonary hypertension, although symptoms of pneumonia were not prominent in our patient.
In conclusion, we described the case of a pediatric patient with pulmonary hypertension as a complication of SARS-CoV-2 infection. We consider that she had a pre-existing subclinical pulmonary hypertensive condition as a result of underlying disease that manifested clinically as pulmonary hypertension upon SARS-CoV-2 infection.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by Chiba University ethical committee review board for the studies involving humans because ethical approval was waived in case report at the Chiba University ethical committee review board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KO: Data curation, Investigation, Writing – original draft. HK: Investigation, Validation, Writing – original draft. YY: Investigation, Validation, Writing – review & editing. MK: Investigation, Validation, Writing – review & editing. TS: Supervision, Writing – review & editing. TT: Investigation, Methodology, Supervision, Writing – review & editing. HH: Funding acquisition, Supervision, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work is funded by Future Medicine Funds at Chiba University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. UNICEF data. Child Mortality and COVID-19. (2023). Available online at: https://data.unicef.org/topic/child-survival/covid-19/ (accessed January 24, 2024).
2. National Institute of Infectious Disease Japan. A Prospective Epidemiological Study of Deaths among Persons under 20 Years of Age after Infection with Novel Coronaviruses (Report 2). (2022). (Japanese). Available online at: https://www.niid.go.jp/niid/ja/2019-ncov/2559-cfeir/11727-20.html (accessed January 24, 2024).
3. Majeed H, Cannon HR, Raj K, Nasrullah A, Majeed S, Gangu K, et al. COVID-19 Patients with pulmonary hypertension hospitalized in the United States during the early pandemic: analysis of in-hospital mortality, clinical outcomes, and racial disparities. Curr Probl Cardiol. (2023) 48:101933. doi: 10.1016/j.cpcardiol.2023.101933
4. Olfe J, Grafmann M, Kozlik-Feldman R. A teenager with a congenital heart defect and COVID-19. Cardiol Young. (2020) 30:1358–9. doi: 10.1017/S1047951120002127
5. Rodriguez-Gonzalez M, Rodriguez-Campy P, Sanchez-Codez M, Guiterrez-Rosa I, Castellano-Martinez A, Rodriguez-Benitez A. New-onset right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol Young. (2020) 30:1346–9. doi: 10.1017/S1047951120001857
6. Morales-Demori R, Mallory GB, Do CC, Coleman R, Ruiz F, Villafranco N, et al. Outcomes of COVID-19 infection in pediatric pulmonary hypertension: a single-center experience. Pediatr Pulmonol. (2021) 56:3960–5. doi: 10.1002/ppul.25650
7. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res. (2017) 121:451–68. doi: 10.1161/CIRCRESAHA.117.311170
8. Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. (2004) 39:909–14. doi: 10.1002/hep.20140
9. Ropoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. (2007) 133:481–8. doi: 10.1053/j.gastro.2007.05.024
10. Gracia-Sancho J, Marrone G, Fernandez-Iglesias AF. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. (2019) 16:221–34. doi: 10.1038/s41575-018-0097-3
11. Georgieva E, Ananiev J, Yovchev Y, Arabadzhiev G, Abrashev H, Abrasheva D, et al. COVID-19 complications: oxidative stress, inflammation, and mitochondrial and endothelial dysfunction. Int J Mol Sci. (2023) 24:14876. doi: 10.3390/ijms241914876
Keywords: SARS-CoV-2, pulmonary hypertension, children, panhypopituitarism, growth hormone, portal hypertension
Citation: Okunushi K, Kobayashi H, Yoh Y, Kunimatsu M, Shiohama T, Takatani T and Hamada H (2024) A case report of a child with pulmonary hypertension associated with SARS-CoV-2 infection. Front. Pediatr. 12:1336589. doi: 10.3389/fped.2024.1336589
Received: 11 November 2023; Accepted: 23 January 2024;
Published: 8 February 2024.
Edited by:
Cristina Tudoran, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Sachin Gajanan Damke, Dr Rajendra Gode Medical College, India© 2024 Okunushi, Kobayashi, Yoh, Kunimatsu, Shiohama, Takatani and Hamada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiromichi Hamada aGlyb21pY2hpLmhhbWFkYUBnbWFpbC5jb20=
†ORCID Hiromichi Hamada orcid.org/0000-0002-8990-5265
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.