
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 15 March 2024
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1328506
This article is part of the Research Topic Insights in Pediatric Gastroenterology, Hepatology & Nutrition View all 6 articles
 Jessica V. Baran1*†
Jessica V. Baran1*† Jerry M. Brown1,†
Jerry M. Brown1,† Andrew A. Farrar2
Andrew A. Farrar2 Lea V. Oliveros3
Lea V. Oliveros3 Jesse Beacker1
Jesse Beacker1 Luke Lamos1
Luke Lamos1 Jared Florio1
Jared Florio1 Abigail L. Fabbrini2,‡
Abigail L. Fabbrini2,‡ Michael J. Wilsey4,‡
Michael J. Wilsey4,‡
Background: In 2022, the United States experienced a national shortage of infant formula due to a global supply chain crisis and a large-scale domestic formula recall. The existing literature on healthcare providers' (HCPs) clinical decision-making during formula shortages is limited. This study aims to analyze the factors influencing pediatric HCP clinical decision-making when switching between amino acid formulas (AAF) for managing cow's milk protein allergy (CMPA) in infants under 24 months of age during an unprecedented national formula shortage.
Methods: The study included pediatric HCPs with experience managing CMPA in infants and toddlers under 24 months during the formula shortage from January 2022 to November 2022. A de-identified survey comprising 26 questions examining driving factors used in clinical decision-making was administered to pediatric HCPs using a real-time mobile data collection tool.
Results: Among the surveyed pediatric HCPs (n = 75), the factors most frequently considered as “extremely important” when switching to another AAF included safety (85%), tolerability (73%), and efficacy (83%). No statistically significant differences were found in HCP ratings among the listed examined factors of the four AAFs. The availability of specific formulas was the only factor that exhibited a statistically significant difference in perceived performance among pediatric HCPs when comparing the four AAFs (p < 0.05).
Discussion: This study elucidates the crucial aspects that influenced pediatric HCPs' selection of AAFs for CMPA management during the 2022 formula shortage. The findings highlight the significance of safety, tolerability, efficacy, and availability in the pediatric HCP decision-making processes.
The United States experienced a shortage of infant formula starting in 2020 due to import restrictions caused by the COVID-19 pandemic (1). This shortage worsened in 2021 due to a global supply chain crisis, reaching a critical peak in February 2022 due to a large-scale product recall from a company supplying 40% of infant formula in the United States (2–4). This shortage significantly impacted the availability of infant formulas across various retail channels, including grocery stores, pharmacies, and online stores (1, 2, 4). Such shortages have profound implications for infants reliant on formula feeding, often leaving parents uncertain about safely feeding their infants (4).
Infant formulas are used for various reasons, including parental choice, breastfeeding supplementation, convenience, or addressing health conditions such as cow's milk protein allergy (CMPA) (1, 5). CMPA, triggered by an immunological reaction to cow's milk protein, affects a significant proportion of children, with symptoms ranging from mild gastrointestinal discomfort to severe anaphylaxis (6–13). Hypoallergenic formulas, such as amino acid formulas (AAF) and extensively hydrolyzed formulas, are the mainstay of CMPA management in formula-fed infants, reducing exposure to cow's milk protein and symptom severity (6, 14). AAFs composed of amino acids and extensively hydrolyzed formulas containing short peptides and amino acids, are well-tolerated in most infants (9, 15–23). However, in cases where infants fail to respond adequately to eHF or exhibit severe allergic reactions, AAFs may be considered as an alternative due to their hypoallergenic nature and ability to meet the nutritional needs of infants with complex medical conditions (23). Factors influencing the choice between these formulas include the severity of CMA, cost, availability, and patient tolerance. Despite being more expensive, AAFs ensure optimal growth while minimizing allergic reactions (18–23).
During the 2022 national formula shortage, pediatric healthcare providers (HCPs) in the United States faced challenges managing CMPA due to the limited availability of hypoallergenic formulas. This study investigated the clinical decision-making process of pediatric HCPs managing infants with CMPA during a national formula shortage crisis. This included an examination of the use of different AAFs as part of CMPA management strategies. Understanding the availability and utilization of various AAFs is crucial for optimizing patient care during formula shortages. Based on the observed increased availability of AAF-1 during the crisis, we hypothesized that HCPs would prioritize the most readily available AAF during the formula shortage and were more likely to continue recommending that formula after the shortage was over.
This study aims to conduct a cross-sectional analysis of de-identified survey data collected from pediatric HCPs in the United States. The focus is on understanding the key attributes considered by HCPs when switching between AAFs to manage CMPA during a national formula shortage crisis. The study population comprises pediatric HCPs responsible for managing infants with CMPA during the shortage. Through the survey, we aim to assess the clinical decision-making process regarding the utilization of different AAFs and identify factors influencing their choice among pediatric HCPs.
Inclusion criteria for pediatric HCPs included: greater than 2 years of experience in a clinic-based, pediatric practice setting; specialization in general pediatrics, pediatric gastroenterology, or pediatric allergy/immunology; seeing at least seven newly diagnosed CMPA patients in the past month; managing CMPA in infants aged 0–24 months; and having switched infants' formulas from either AAF-2, AAF-3 or AAF-4 to AAF-1 during the national formula shortage period from January 2022 to November 2022 (Table 1). Exclusion criteria for pediatric HCPs included incomplete data collection. This study received exempt status from the Institutional Review Board (IRB), indicating that it met the criteria for exemption from full IRB review.
The survey was designed to capture pediatric HCP demographic information, formula switch details, availability of the preferred formula, ease of obtaining the new formula, impact of the formula switch on infant health, and the financial burden of the formula switch.
The survey was administered to pediatric HCPs in the United States in English via a mobile-based, data collection application (ZSMoments, by ZS Associates) that allows for the rapid, secure documentation of real-time patient data accurately and freely (19, 20). The survey consisted of 26 questions and took approximately 10–15 min to complete. Survey questions were developed based on a literature review and discussions with pediatric HCPs who manage infants with CMPA. The questionnaire was comprised of yes/no questions and questions rated on a scale from 1 to 10. Scores were categorized into low (1–3), moderate (4–7), and high/extremely (8–10).
Descriptive statistics were used to summarize the data, and chi-square tests were conducted to determine the association between demographic variables and formula switch details. Logistic regression analysis was performed to identify factors associated with difficulty obtaining the new formula and increased financial burden. Statistical significance was set at p < 0.05. All analyses were carried out using SPSS® analytics software (IBM®, Armon, NY, USA).
A total of 75 pediatric HCPs were included in data analysis. Pediatric HCP specialties included general pediatrics (64%, n = 48), pediatric allergy/immunology (27%, n = 20), and pediatric gastroenterology (9%, n = 7).
Among the 75 pediatric HCPs surveyed, the importance of certain factors when choosing an infant formula was assessed (Table 2). The findings indicate that before and after the formula shortages, the pediatric HCPs most frequently rated safety (88% and 93%, respectively) as the most important factor when deciding on a formula for managing CMPA. In addition, before the formula shortage, pediatric HCPs most frequently rated efficacy (87%) and availability (87%) as the tied second-most important factors when deciding on a formula for managing CMPA. However, after the 2022 formula shortage, pediatric HCPs most frequently rated efficacy (92%) as the second most important factor.
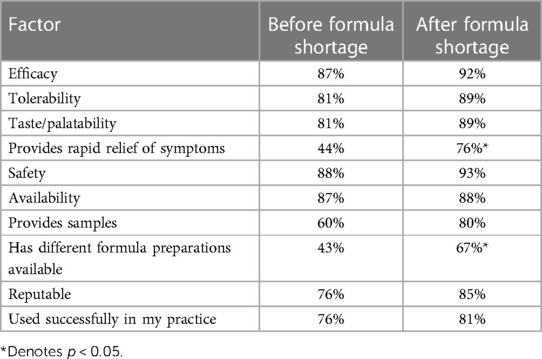
Table 2. Comparison of perception of importance of attributes among pediatric health care providers.
Of the 75 pediatric HCPs that switched formula to AAF-1 for patients during the shortage, 53% (n = 40) were switched from AAF-2, 20% (n = 15) from AAF-3, and 17% (n = 13) from AAF-4 to (Figure 1).
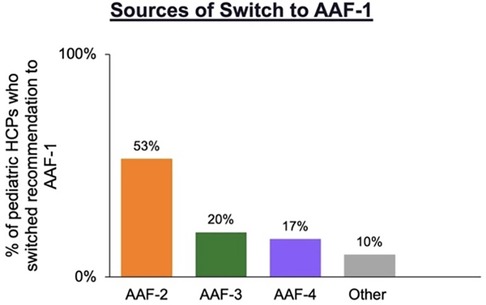
Figure 1. Pediatric HCPs who switched recommendation to AAF-1 (n = 75) during the formula shortage by previous AAF recommended.
When Pediatric HCPs compared quality attributes (Figure 2) of previously recommended formulas to AAF-1 use, AAF-1 was “highly” available significantly more frequently than AAF-2 (p < 0.05). No attributes for AAF-1 vs. AAF-3 or vs. AAF-4 were rated as performing significantly better. The percentage of pediatric HCPs that rated each attribute as “extremely important” is listed in Supplementary Table S1.
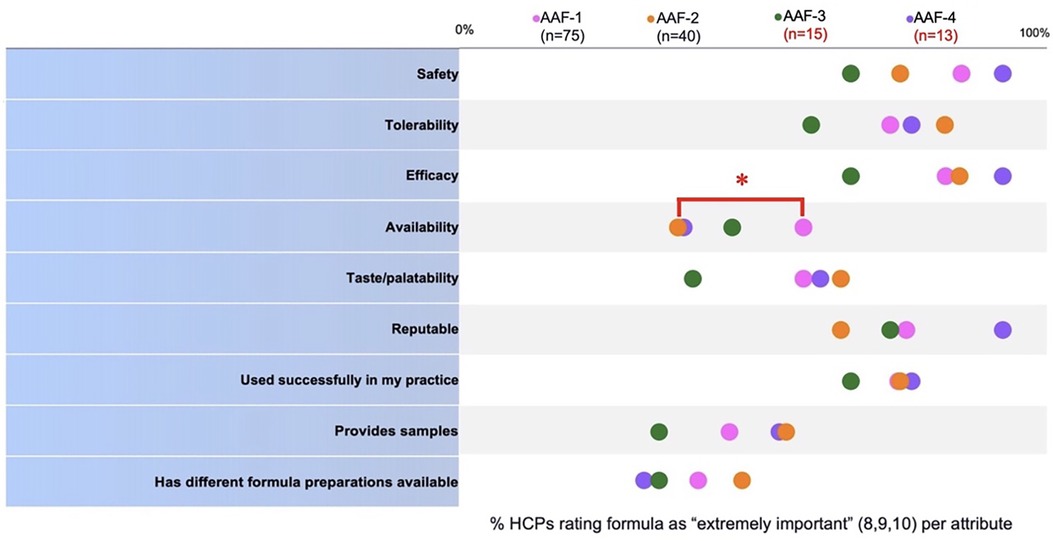
Figure 2. Comparison of pediatric HCP perception of performance of the formulas they used prior to switching (AAF-2, 3, or 4) and pediatric HCP perception of performance of the formula they switched to (AAF-1). *Denotes p < 0.05.
Pediatric HCP satisfaction ratings (Figure 3A) and future preference after switching to AAF-1 (Figure 3B) were collected. Of 98% (n = 39) pediatric HCPs who switched from AAF-2, 70% (n = 28) were “highly” satisfied with AAF-1% and 78% (n = 31) had a “high” future preference to AAF-1. Of 13 pediatric HCPs that switch from initially recommended AAF-4, 77% (n = 10) were “highly” satisfied with AAF-1 (Figure 3) and 62% (n = 9) had a “high” future preference to AAF-1. Of 15) who switched from AAF-3, 80% were “highly” satisfied with AAF-1 (Figure 3) and 67% (n = 10) rated “high” future preference.
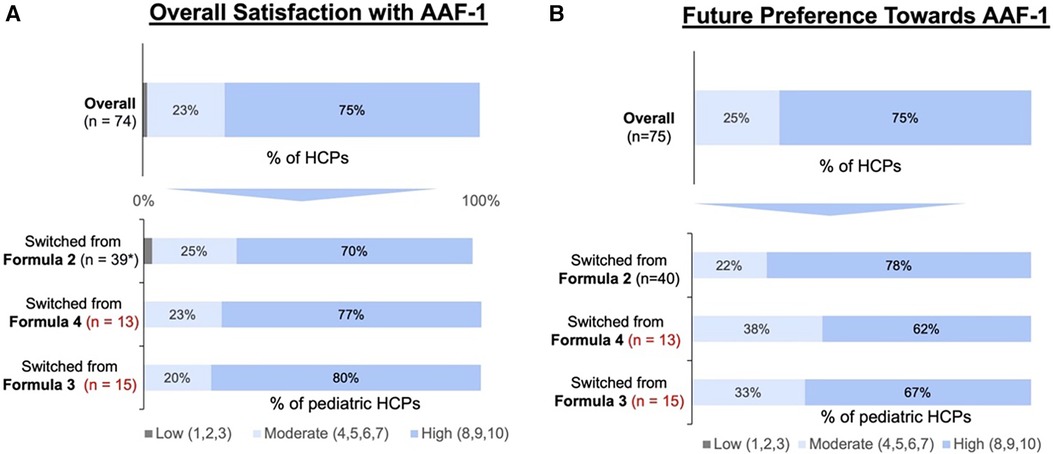
Figure 3. Pediatric HCP rating for: (A) AAF-1 overall quality perception after switching formulas during the shortage and (B) AAF-1 future preference after resolution of the shortage. (*Forty pediatric HCPs initially recommended AAF-2 and then switched to AAF-1. However, only 39 of the 40 pediatric HCP's felt as if they has enough experience with AAF-1 to rate their satisfaction. Therefore, only 74 pediatric HCPs were included in the overall sample).
Specific beliefs and benefits of AAF-1 were rated by pediatric HCPs who switched to AAF-1 during the shortage (Figure 4A). Of the 75 pediatric HCPs included in the study overall, 75% (n = 56) would continue to recommend AAF-1 following resolution of the shortage. AAF-1 was reported as having “high” tolerability in 73% (n = 55) and 59% (n = 44) considered AAF-1 to have “high” taste/palatability. In addition, 83% (n = 62) considered AAF-1 to have “high” efficacy and 85% (n = 64) considered AAF-1 to have “high” safety. Figure 4B depicts the attributes pediatric HCPs rated for continued preference of AAF-1.
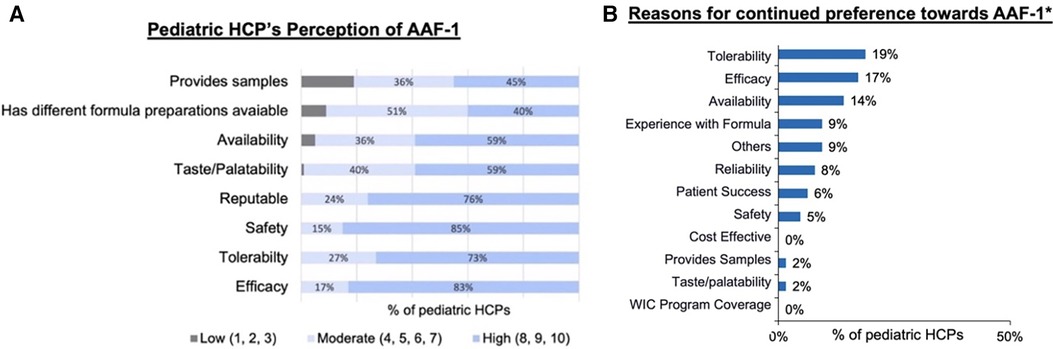
Figure 4. (A) Pediatric HCP rating of perception of AAF-1 performance. (B) Reason pediatric HCPs who rated these attributes as extremely important continued preference towards AAF-1. *Includes pediatric HCPs that rated these attributes at extremely important (8–10).
In this cross-sectional analysis, we evaluated the driving factors utilized by pediatric HCPs in clinical decision making when switching between AAFs to manage CMPA in infants ≤24 months before and after the 2022 formula shortage. Here, we report a cohort of 75 pediatric HCPs that switched their formula recommendation from AAF-2-4 to AAF-1 during the shortage.
Understanding what influences clinical decision-making among pediatric HCPs managing infants with CMPA is crucial. Once a diagnosis is established, pediatric HCPs need to develop an individualized treatment plan for infants based on symptom severity and available resources. Clinical decision-making involved evaluating various management options, considering patient specific needs and circumstances, and make informed decisions about the most suitable management strategies. This process can optimize the effectiveness and safety of management strategies in pediatric patients. Understanding clinical decision making is especially important in times of formula shortage. This understanding allows pediatric HCPs to be better prepared for management dilemmas, more aware of the various treatment options and their respective benefits, and overall, more successful in managing infants with CMPA using hypoallergenic formulas.
Considering the formula shortage crisis, it's crucial to examine how different amino acid formulas (AAF) were affected and their implications for managing infants with cow's milk protein allergy (CMPA). The shortage significantly disrupted the supply chain, particularly impacting the availability of AAFs, which are essential for infants with severe CMPA. This scarcity emphasized the importance of understanding the availability and accessibility of various AAFs during crises. It also highlighted the need for proactive measures to ensure an adequate supply and distribution of specialized formulas to mitigate potential risks associated with shortages. Collaborative efforts among stakeholders are essential to address these challenges and develop strategies to safeguard the availability of essential formulas for vulnerable populations during crises (24, 25).
Our findings from this survey indicate that pediatric HCPs during the national formula crisis in the US rated availability significantly higher when comparing AAF-1 to AAF-3, with availability driving most switches to AAF-1 during the shortage. Furthermore, post-shortage, most pediatric HCPs expressed intent to continue recommending AAF-1, highlighting its sustained preference.
Safety remained the most important factor when choosing a formula both before and after the formula shortage. Interestingly, availability shifted from being tied-second most important before the shortage to efficacy becoming second most important after the shortage. During the formula shortage, the increased availability of AAF-1 overshadowed other formula attributes, significantly impacting clinical decision-making among pediatric HCPs. This insight into decision-making during and after formula shortages can inform improvements in CMPA patient treatment and management.
After shifting to AAF1, most pediatricians expressed high satisfaction with AAF-1 and demonstrated a strong future preference for AAF-1. This finding underscores the importance of considering pediatricians' satisfaction and preferences when evaluating the effectiveness and acceptability of hypoallergenic formulas. Future studies should delve deeper into the reasons behind pediatricians' satisfaction with AAF-1 and explore potential factors influencing formula preference.
Limitations include the study's focus on specific pediatric HCPs in the United States, potentially limiting generalizability (19). These findings may not be applicable to HCPs in other countries, potentially limiting the generalizability of the results. In addition, the data collected from pediatric HCPs were based on self-reporting through a survey. This method relies on participants' recall and subjective responses, which can introduce biases such as recall bias or social desirability bias. The accuracy and reliability of the data depend on the participant's ability to recall information. Further, this study utilizes a cross-sectional design, which captures data at a specific point in time, limiting the ability to establish causal relationships or assess changes over time. Future research should explore potential confounding variables like symptom severity or socioeconomic factors influencing pediatric HCP perceptions.
Our study, the first survey of its kind, highlights the impact of formula availability on clinical decision-making among pediatric HCPs managing CMPA infants during a national formula shortage. AAF-1’s increased availability drove most switches, underscoring its importance amidst formula shortages. However, our findings suggest that safety, tolerability, and efficacy also influenced decision-making, albeit to a lesser extent than availability. This emphasizes the multifaceted nature of pediatric HCP decision-making. Further research is warranted to validate these findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The requirement of ethical approval was waived by Advarra Institutional Review Board for the studies involving humans. The studies wereconducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JVB: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. JMB: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. AAF: Data curation, Formal Analysis, Writing – review & editing. LVO: Data curation, Formal Analysis, Writing – review & editing. JB: Data curation, Formal Analysis, Writing – review & editing. LL: Data curation, Formal Analysis, Writing – review & editing. JF: Data curation, Formal Analysis, Writing – review & editing. ALF: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. MJW: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
A grant (RB1463001528431842) from Reckitt supported article processing and presentation fees for this research. Additionally, Reckitt provided funding for data collection through ZS Associates.
We would like to thank Heather Evans, MS, RDN, LDN, CNSC, CLC and Jennifer Wampler, PhD. for their critical review of the manuscript.
MW has served as a consultant for Reckitt.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1328506/full#supplementary-material
1. Abrams SA, Duggan CP. Infant and child formula shortages: now is the time to prevent recurrences. Am J Clin Nutr. (2022) 116:289–92. doi: 10.1093/ajcn/nqac149
2. Kalaitzandonakes M, Ellison B, Coppess J. Coping with the 2022 infant formula shortage. Prev Med Rep (2023) 32:102123. doi: 10.1016/j.pmedr.2023.102123
3. Asiodu IV. Infant formula shortage: this should not be our reality. J Perinat Neonatal Nurs. (2022) 36:340–3. doi: 10.1097/JPN.0000000000000690
4. Jung J, Widmar NO, Ellison B. The curious case of baby formula in the United States in 2022: cries for urgent action months after silence in the midst of alarm bells. Food Ethics. (2023) 8:4. doi: 10.1007/s41055-022-00115-1
6. Vandenplas Y, Brough HA, Fiocchi A, Miqdady M, Munasir Z, Salvatore S, et al. Current guidelines and future strategies for the management of cow’s milk allergy. J Asthma Allergy. (2021) 14:1243–56. doi: 10.2147/JAA.S276992
7. Vitaliti G, Cimino C, Coco A, Pratico AD, Lionetti E. The immunopathogenesis of cow’s milk protein allergy (CMPA). Ital J Pediatr. (2012) 38:35. doi: 10.1186/1824-7288-38-71
8. Flom JD, Sicherer SH. Epidemiology of cow’s milk allergy. Nutrients. (2019) 11. doi: 10.3390/nu11051051
9. Meyer R, Groetch M, Venter C. When should infants with cow’s milk protein allergy use an amino acid formula? A practical guide. J Allergy Clin Immunol Pract. (2018) 6:383–99. doi: 10.1016/j.jaip.2017.09.003
10. Lozinsky AC, Meyer R, Anagnostou K, Dziubak R, Reeve K, Godwin H, et al. Cow’s milk protein allergy from diagnosis to management: a very different journey for general practitioners and parents. Children (Basel). (2015) 2:317–29. doi: 10.3390/children2030317
11. Caffarelli C, Baldi F, Bendandi B, Calzone L, Marani M, Pasquinelli P, et al. Cow’s milk protein allergy in children: a practical guide. Ital J Pediatr. (2010) 36:5. doi: 10.1186/1824-7288-36-5
12. Walsh J, O’Flynn N. Diagnosis and assessment of food allergy in children and young people in primary care and community settings: NICE clinical guideline. Br J Gen Pract. (2011) 61:473–5. doi: 10.3399/bjgp11X583498
13. Vandenplas Y, De Greef E, Devreker T. Treatment of cow’s milk protein allergy. Pediatr Gastroenterol Hepatol Nutr. (2014) 17:1–5. doi: 10.5223/pghn.2014.17.1.1
14. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J Pediatr Gastroenterol Nutr. (2012) 55:221–9. doi: 10.1097/MPG.0b013e31825c9482
15. Host A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European society for paediatric allergology and clinical immunology (ESPACI) committee on hypoallergenic formulas and the European society for paediatric gastroenterology, hepatology and nutrition (ESPGHAN) committee on nutrition. Arch Dis Child. (1999) 81:80–4. doi: 10.1136/adc.81.1.80
16. Beacker J, Brown JM, Florio J, Baran JV, Lamos L, Oliveros L, et al. Clinician experience with using hypoallergenic formulas to treat infants with suspected cow’s milk protein allergy: a secondary analysis of a prospective survey cohort. Pediatr Gastroenterol Hepatol Nutr. (2023) 26(5):277–83. doi: 10.5223/pghn.2023.26.5.277
17. Fabbrini AL, Farrar AA, Brown JM, Oliveros LV, Florio J, Beacker J, et al. Navigating formula shortages: associations of parental perspectives on transitioning to alternative infant formulas for cow’s milk protein allergy during the 2022 national formula shortage. Front Allergy. (2024) 4:1333570. doi: 10.3389/falgy.2023.1333570
18. Ludman S, Shah N, Fox AT. Managing cows’ milk allergy in children. Br Med J. (2013) 347:f5424. doi: 10.1136/bmj.f5424
19. Wilsey MJ, Florio J, Beacker J, Lamos L, Baran JV, Oliveros L, et al. Extensively hydrolyzed formula improves allergic symptoms in the short term in infants with suspected cow’s milk protein allergy. Nutrients. (2023) 15. doi: 10.3390/nu15071677
20. Wilsey MJ, Baran JV, Lamos L, Beacker J, Florio J, Oliveros L, et al. Short-term symptom improvement in infants with suspected cow’s milk protein allergy using amino acid formula: a prospective cohort analysis. Front Nutr. (2023) 10:1208334. doi: 10.3389/fnut.2023.1208334
21. Meyer R, Smith C, Sealy L, Mancell S, Marino LV. The use of extensively hydrolysed and amino acid feeds beyond cow’s milk allergy: a national survey. J Hum Nutr Diet. (2021) 34:13–23. doi: 10.1111/jhn.12794
22. Nowak-Wegrzyn A, Czerkies LA, Collins B, Saavedra JM. Evaluation of hypoallergenicity of a new, amino acid-based formula. Clin Pediatr (Phila). (2015) 54:264–72. doi: 10.1177/0009922814557785
23. D'Auria E, Salvatore S, Acunzo M, Peroni D, Pendezza E, Di Profio E, et al. Hydrolysed formulas in the management of cow’s milk allergy: new insights, pitfalls and tips. Nutrients. (2021) 13(8):2762. doi: 10.3390/nu13082762
24. Doherty T, Coutsoudis A, McCoy D, Lake L, Pereira-Kotze C, Goldhagen J, et al. Is the US infant formula shortage an avoidable crisis? Lancet. (2022) 400(10346):83–4. doi: 10.1016/S0140-6736(22)00984-9
Keywords: amino acid formula, formula shortage, formula recall, infant nutrition, cow’s milk protein allergy, ZSMoments
Citation: Baran JV, Brown JM, Farrar AA, Oliveros LV, Beacker J, Lamos L, Florio J, Fabbrini AL and Wilsey MJ (2024) Impact of the 2022 national formula shortage on clinical decision-making of healthcare providers in switching amino acid formulas for infants with cow’s milk protein allergy: a survey-based study. Front. Pediatr. 12:1328506. doi: 10.3389/fped.2024.1328506
Received: 26 October 2023; Accepted: 29 February 2024;
Published: 15 March 2024.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Enza D’Auria, Vittore Buzzi Children’s’ Hospital, Italy© 2024 Baran, Brown, Farrar, Oliveros, Beacker, Lamos, Florio, Fabbrini and Wilsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica V. Baran amVzc2ljYXZiYXJhbkBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.