- 1Department of Rheumatology and Immunology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Pediatric Cardiology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3Diagnostic Imaging Center, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Libman-Sacks endocarditis (LSE) is a cardiac condition characterized by the growth of verrucous vegetation. Although relatively rare in children, LSE is nevertheless a known cardiac manifestation of autoimmune diseases, including systemic lupus erythematosus (SLE). The mitral valve is the most commonly affected region, followed by the aortic valve, while the tricuspid and pulmonary valves are rarely affected. The management of established Libman-Sacks vegetation poses significant challenges, often necessitating surgical interventions, although surgery is not the primary treatment modality. Herein, we present the case of a 14-year-old Chinese female patient whose initial lupus manifestation included LSE, among other symptoms and signs that provided insights into the final diagnosis of SLE. After early comprehensive pharmacological treatment, tricuspid regurgitation and vegetation disappeared within 28 days without necessitating cardiac surgery, indicating that the resolution of LSE vegetation in this patient was achieved through a combination of immunosuppressive and anticoagulant therapy. These findings suggest the potential of this treatment approach as a viable model for the management of LSE in young patients.
Introduction
Libman-Sacks endocarditis (LSE), also known as non-bacterial thrombotic endocarditis (NBTE), was first described by Libman and Sacks in 1924. It is a typical cardiac manifestation of SLE and/or antiphospholipid syndrome (APS). Pediatric LSE can manifest with various clinical features, including cardiac murmurs, signs of heart failure, embolic events such as stroke or peripheral artery occlusion, as well as constitutional symptoms like fatigue and weight loss (1). Diagnosis of LSE in children requires a high index of suspicion, as symptoms may be subtle or nonspecific. A literature search revealed only 7 reported casesof LSE in children over the past 20 years. The mitral valve is the most frequently affected site in LSE, followed by the aortic valve, while the tricuspid and pulmonary valves are seldom affected (2). Unfortunately, LSE is commonly misdiagnosed as bacterial endocarditis. Herein, we report a case of LSE in a child with a tricuspid valve treated in our department. In this report, we describe the patient's successful treatment course and provide a review of the relevant literature to improve our understanding of the disease, avoid misdiagnosis, and provide evidence for its clinical treatment and prognosis.
Case presentation
A 14-year-old Chinese female patient experienced hair loss for more than a year. The patient was 160 cm (in the 54th percentile) tall and weighed 49.3 kg (in the 45.8th percentile) (BMI = 19.26). Before being hospitalized at our center, she had experienced a fever of over 38°C, accompanied by persistent cough, for 8 days. In addition, she had experienced chest pain for 6 days and shortness of breath for 3 days. She further experienced chest and back pain, chest tightness, weakness, and an inability to lie down. In addition, the patient experienced pain in the left index finger joint without discoloration and was unable to straighten it. She reported experiencing no night sweats, hemoptysis, or contact history of tuberculosis. Moreover, she had no significant history of high-risk sexual encounters, travel, medical illnesses, or family illnesses. She had been diagnosed with SLE combined with infection at another hospital and was treated with intravenous cefotiam, azithromycin, and piperacillin for 3 days, the exact dosage of which were unknown; however, her symptoms did not improve. No cultures were performed at the other hospital. The patient was subsequently referred to our hospital.
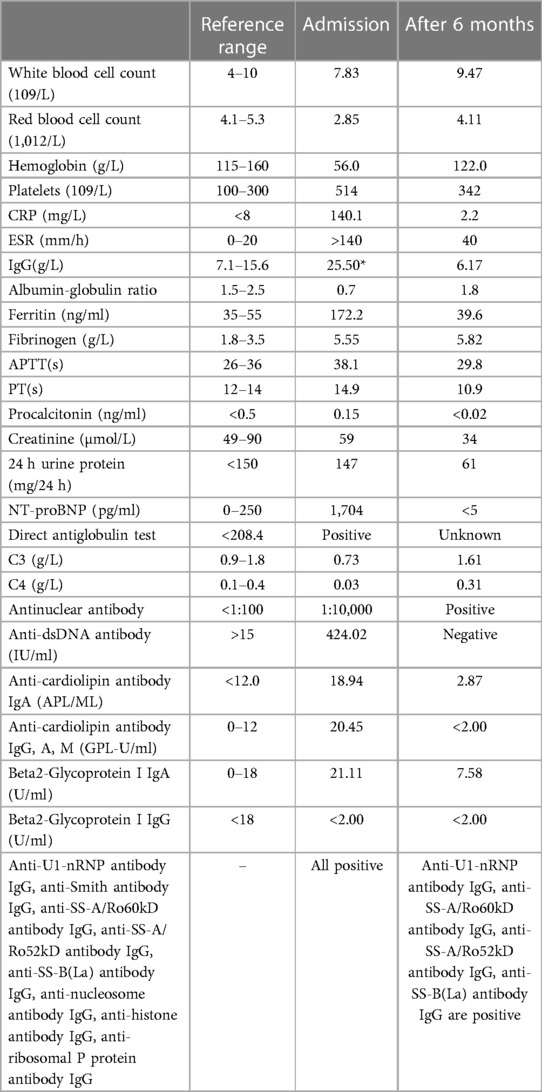
Laboratory tests and imaging were performed upon admission at our hospital. The laboratory data at admission and 6 months after therapy are summarized in Table 1.
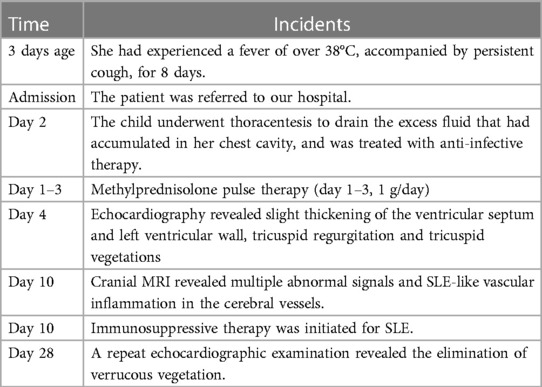
Plain chest computed tomography (CT) revealed bilateral pleural effusions with atelectasis and pericardial effusion, consistent with lung infection. Echocardiography revealed the presence of a small amount of pericardial effusion, pulmonary hypertension, slight thickening of the ventricular septum and left ventricular wall, tricuspid regurgitation, and two moderately echogenic masses attached at the level of the tricuspid chordae tendineae, which oscillated in synchronization with the cardiac cycle and measured approximately 0.82 cm × 0.62 cm, 0.92 cm × 0.86 cm in size (Figure 1). Two blood cultures, one urine culture, one pleural fluid culture, and one respiratory specimen culture were all confirmed to be free of pathogens and fungal growth on day 3. Next-generation sequencing (NGS) of the pleural fluid on day 3 revealed Enterococcus faecalis infection. The patient was finally diagnosed with LSE. Cranial magnetic resonance imaging (MRI) revealed multiple abnormal signals in the right frontal and posterior horns of the bilateral ventricles on both sides and bilateral semi-oval centers, SLE-like vascular inflammation in the cerebral vessels on day 10. Peripheral blood smears occasionally showed helmet-shaped red blood cells. The SLEDAI-2 K score 24 h after admission was 22 (6 for serositis and acute pericarditis, 4 for low complement, 4 for autoimmune hemolytic anemia, 6 for anti-Smith antibody positivity, and 2 for alopecia). The patient was in the active stage of the disease and experienced a period of severe disease activity.
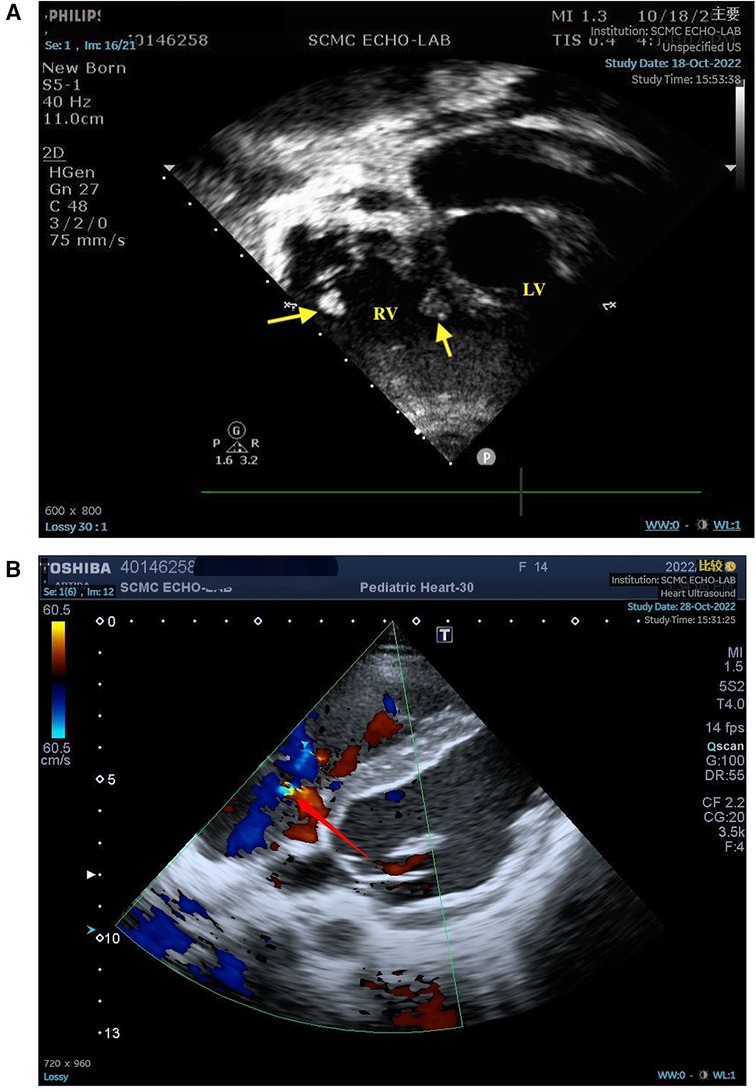
Figure 1. (A) Two moderately echogenic masses attached at the level of the tricuspid chordae tendineae measured in atypical apical four-chamber (4C) view. (B) The yellow part of the arrow indicates turbulence, indicating tricuspid regurgitation. LV, left ventricle; RV, right ventricle; arrow, masses.
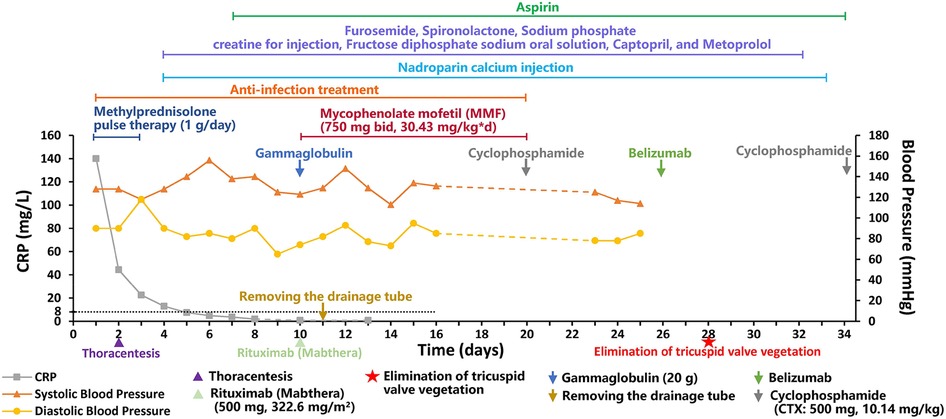
The patient was finally diagnosed with SLE, lupus encephalopathy, LSE, community-acquired pneumonia (severe), pleural effusion, pericardial effusion (non-inflammatory), severe anemia, and cardiolipin antibody positivity. Anti-infection treatment was administered from day 1 to day 20 of hospitalization in our center. Gammaglobulin (20 g) was also administered on day 10. Fresh frozen plasma was provided as supportive care. The child underwent thoracentesis on day 2 to drain the excess fluid that had accumulated in her chest cavity, and the tube was withdrawn 10 days after drainage. To treat SLE, methylprednisolone pulse therapy was administered for the first 3 days (day 1–3, 1 g/day) of admission, while methylprednisolone (2 mg/kg) was administered from the fourth day. From day 4 to day 32, the patient received furosemide and spironolactone for diuresis, sodium phosphate creatine for injection, fructose diphosphate sodium oral solution for myocardial nutrition, captopril for blood pressure reduction, and metoprolol for heart rate control. On day 10, rituximab (Mabthera) (500 mg, 322.6 mg/m2) injection was administered along with mycophenolate mofetil (MMF) (750 mg bid, 30.43 mg/kg*d, day 10-day 20). On day 10, the child's blood pressure was found to be high (148/93 mmHg), and captopril was administered to lower the blood pressure. On day 22, the child's heart rate fluctuated between 128 and 108 bpm during the day, and metoprolol was administered to control the heart rate. The patient was administered low-dose cyclophosphamide (CTX: 500 mg, 10.14 mg/kg) on days 20 and 34 of treatment due to an observed decline in mathematical ability, while cranial MRI revealed SLE-like vascular inflammatory alterations in the cerebral vasculature. On day 26, the patient was administered belizumab (480 mg, 9.74 mg/kg). On day 28, echocardiogram was repeated, showing slight thickening in parts of the tricuspid tendon cords, along with localized echogenicity and elimination of verrucous vegetation (Figure 2). The patient was discharged with a prescription for oral prednisone (60 mg/day) and aspirin (100 mg/day), followed by monthly CTX (1 g, 20.28 mg/kg), and belizumab (480 mg, 9.74 mg/kg) treatment. We created a timeline (Table 2) and medication diagram (Figure 3) depicting the treatment course. A repeat cranial MRI performed 3 months after admission revealed fewer and smaller aberrant signals in the brain compared to the initial MRI. Five months after initial admission, repeat cardiac MRI showed no significant abnormal signals in the cardiac chambers and no significant abnormal myocardial signal, and the SLEDAI-2K score was 0.
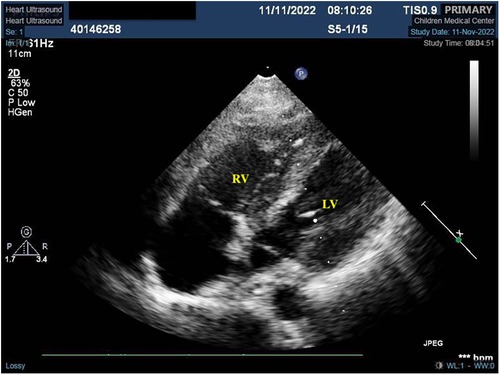
Figure 2. Localized mass disappeared after treatment. *This is an atypical section (that is, not standardized, as it is not standardized because of the need to see the redundant organisms) of the four-chambered heart.
Discussion
LSE is rare in children. Patients with antiphospholipid, anti-ro/SS-A, and anti-la/SS-B antibody-positive SLE are more likely to have valve involvement than those without (3, 4). Patients with LSE are typically asymptomatic unless the lesions progress to more severe valvular dysfunction or embolic events, which may be related to highly variable SLE activity (alternating between exacerbations and remissions) and standard anti-inflammatory and/or anti-thrombotic therapy (1). Life-threatening manifestations, such as cardiac tamponade and cardiogenic shock, may present as the initial symptoms (5). The pathogenesis of LSE involves the formation of fibrin-platelet thrombi due to inflammation, which leads to fibrosis and scarring, with subsequent valve dysfunction developing over time (6). LSE is commonly misdiagnosed as infective endocarditis (7). Approximately 10% of patients with infective endocarditis do not exhibit growth in blood cultures, thereby complicating the diagnosis process (8). The vegetations that develop in LSE are more fragile than those that develop in infective endocarditis because of the comparatively diminished in situ inflammation, which leads to a greater risk of partial vegetation detachment with resultant embolic phenomena (9).
In our patient, infective endocarditis could not be ruled out because of the persistent fever and increased CRP levels. Multiple pathogen tests returned to normal, and only the presence of enterococcal infection was revealed through NGS analysis in the pleural fluid; however, the fever persisted even after antibiotic therapy in different hospitals. Combined with the patient's laboratory data, we surmised that the persistent fever and elevated CRP levels may be due to SLE activity. In one prior study, LSE was identified as an independent risk factor for neuropsychiatric lupus, which increases the risk of cerebral thrombosis, stroke, transient ischemia, and neuropsychiatric symptoms in patients with LSE (10). However, our patient presented with no embolic manifestations other than SLE-like vascular inflammatory changes on cranial MRI with diminished in mathematical ability. This indicates that the vegetation was lysed following treatment.
In this case, MMF, rituximab, CTX, and belizumab were administered as immunosuppressants. According to the “2019 EULAR Recommendations for the Management of Systemic Lupus Erythematosus" (11), MMF is a potent immunosuppressant that has demonstrated efficacy in both renal and non-renal lupus, although it may not be effective in treating neuropsychiatric manifestations of the disease. In cases of persistent or recurrent extrarenal disease activity, the addition of belimumab should be considered. In situations where the disease poses a threat to vital organs and is refractory to other treatments, rituximab may be a viable option. It is crucial to initiate high-intensity immunosuppressive therapy during the induction phase to effectively manage disease activity in severe cases of SLE. So, in this case, rituximab and MMF were administered together on day 10. Belimumab is a recombinant fully humanized IgG1λ monoclonal antibody that targets and inhibits B-cell activating factor (BAFF), also known as B lymphocyte stimulator (BLyS) (12). It reduces the differentiation of B-cells into plasma cells that produce immunoglobulins, decreases serum autoantibodies, and continues to reduce immune complex deposition, thereby delaying organ damage and overall improving lupus outcomes.
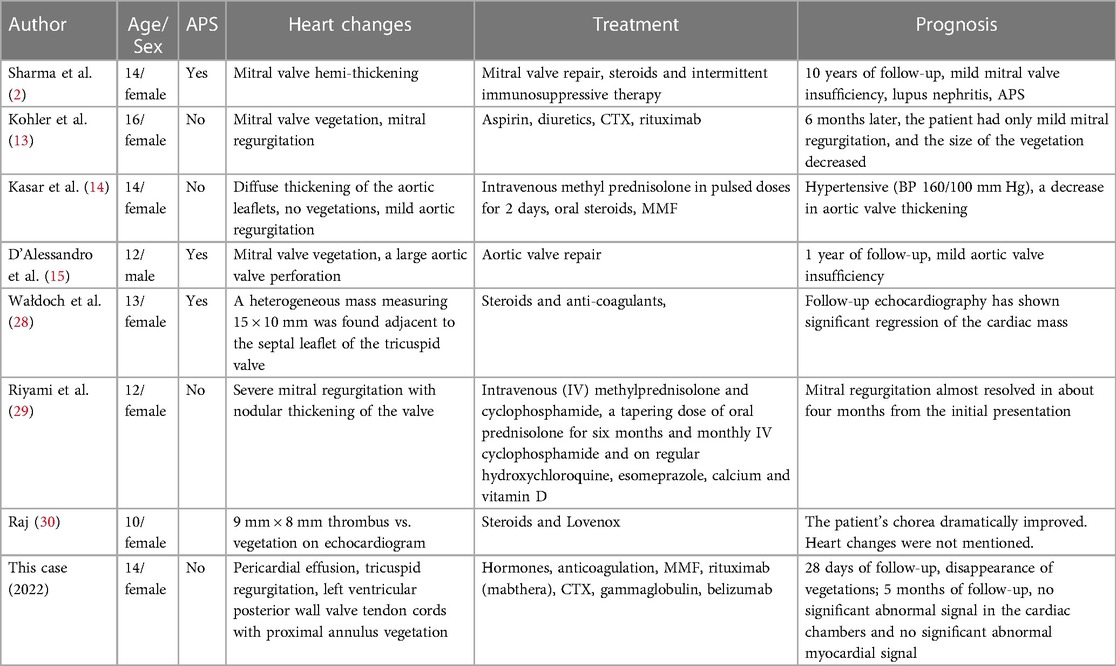
In the past 20 years, only 7 cases of pediatric LSE have been documented. The clinical data of these cases and our case, totaling 8 children, are summarized in Table 3.
Diagnosing LSE can be challenging, especially in the absence of cardiac symptoms. Therefore, it is recommended to implement regular echocardiography screening for patients with SLE, even in the absence of cardiac symptoms (1, 16). Transthoracic echocardiography (TTE) is the first imaging modality of choice for the evaluation of patients with suspected LSE. However, transesophageal echocardiography (TEE) has demonstrated improved sensitivity compared to TTE, for the diagnosis of NBTE. In a recent 20-year cohort study, compared to TTE, TEE was associated with a higher sensitivity for the diagnosis of LSE (17).
The management of LSE in the pediatric age group has not been extensively described. However, there is some evidence available from studies conducted in the adult population. For instance, although corticosteroids can reduce the inflammatory response induced by LSE in adults, they can also increase tissue scarring and fibrosis, which can worsen valve damage (18). However, a case report involving a pregnant woman revealed that following hormone treatment (80 mg/day), the patient experienced a decrease in vegetations and an improvement in ejection fraction (19). Anti-inflammatory and anti-thrombotic therapies have been shown to reduce the activity of SLE, resolve or significantly improve Libman-Sacks vegetation and valve regurgitation, and cause a pathological shift from an active to a mixed or healed type of Libman-Sacks vegetation, which lowers the risk of embolization and may prevent the requirement for high-risk valve surgery (3). According to the 2015 European Society of Cardiology guidelines for the management of infective endocarditis, anticoagulation therapy should be pursued in patients with NBTE if there are no contraindications (20). However, the incidence of thrombotic events in children with antiphospholipid antibody positivity is lower than that in adults, which may be related to the relative difference between the level of blood coagulation protein and development of the vascular endothelium. This suggests that the management and therapeutic strategies for children with LSE and antiphospholipid antibody positivity differ from those in adult patients (21). Furthermore, several adult case studies have previously demonstrated that LSE can be resolved or improved by the administration of immunosuppressive medications in conjunction with anti-thrombotic and hydroxychloroquine therapy (3, 22–26). However, the specific time range during which LSE vegetation can disappear after early active treatment, which requires future multicenter clinical validation, has not yet been documented in the literature.
For severe valve regurgitation, surgery may be considered in patients who show recurrent embolic events despite anticoagulation, large vegetation > 10 mm, and prosthetic valve involvement, depending on the situation (27). In young patients, mitral valve repair, vegetation removal, and ring-shaped plasty are preferred to avoid long-term anticoagulation and promote body growth (2). Artificial valve replacement should be performed when the valve is thickened and tissues are damaged (18).
Conclusion
In conclusion, LSE is rare in children with SLE. In the present case, resolution of the LSE vegetation and improvement of cardiac function may have been achieved by the early introduction of steroids in conjunction with immunosuppression, anticoagulation, gamma globulin, and plasma support. However, whether this approach can be applied to similar cases with reliable outcomes remains unclear, and further research is necessary to investigate thi aspect of LSE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Declaration of Helsinki and the Research Ethics Committee of Shanghai Children's Medical Center (SCMC) (SCMCIRBK2022068). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Validation. SB: Formal Analysis, Investigation, Validation, Writing – review & editing. XX: Formal Analysis, Investigation, Validation, Writing – review & editing. YJ: Formal Analysis, Investigation, Validation, Writing – review & editing. CL: Data curation, Formal Analysis, Investigation, Writing – review & editing. YZ: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. QW: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. YJ: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by grants from the Pediatric SLE Clinical Research Center [ELYZX202102].
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roldan CA, Tolstrup K, Macias L, Qualls CR, Maynard D, Charlton G, et al. Libman-Sacks endocarditis: detection, characterization, and clinical correlates by three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. (2015) 28(7):770–9. doi: 10.1016/j.echo.2015.02.011
2. Sharma J, Lasic Z, Bornstein A, Cooper R, Chen J. Libman-Sacks endocarditis as the first manifestation of systemic lupus erythematosus in an adolescent, with a review of the literature. Cardiol Young. (2013) 23(1):1–6. doi: 10.1017/s1047951112001023
3. Roldan CA, Sibbitt WL Jr, Greene ER, Qualls CR, Jung RE. Libman-Sacks endocarditis and associated cerebrovascular disease: the role of medical therapy. PLoS One. (2021) 16(2):e0247052. doi: 10.1371/journal.pone.0247052
4. Shahin AA, Shahin HA, Hamid MA, Amin MA. Cardiac involvement in patients with systemic lupus erythematosus and correlation of valvular lesions with anti-ro/ss-a and anti-la/ss-B antibody levels. Mod Rheumatol. (2004) 14(2):117–22. doi: 10.1007/s10165-004-0277-6
5. Unic D, Planinc M, Baric D, Rudez I, Blazekovic R, Senjug P, et al. Isolated tricuspid valve Libman-Sacks endocarditis in systemic lupus erythematosus with secondary antiphospholipid syndrome. Tex Heart Inst J. (2017) 44(2):147–9. doi: 10.14503/thij-16-5856
6. Chartash EK, Lans DM, Paget SA, Qamar T, Lockshin MD. Aortic insufficiency and mitral regurgitation in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Med. (1989) 86(4):407–12. doi: 10.1016/0002-9343(89)90337-9
7. Bussani R, DE-Giorgio F, Pesel G, Zandonà L, Sinagra G, Grassi S, et al. Overview and comparison of infectious endocarditis and non-infectious endocarditis: a review of 814 autoptic cases. In Vivo. (2019) 33(5):1565–72. doi: 10.21873/invivo.11638
8. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. (2016) 387(10021):882–93. doi: 10.1016/s0140-6736(15)00067-7
9. Zmaili M, Alzubi J, Lo Presti Vega S, Ababneh E, Xu B. Non-bacterial thrombotic endocarditis: a state-of-the-art contemporary review. Prog Cardiovasc Dis. (2022) 74:99–110. doi: 10.1016/j.pcad.2022.10.009
10. Roldan CA, Sibbitt WL Jr, Qualls CR, Jung RE, Greene ER, Gasparovic CM, et al. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging. (2013) 6(9):973–83. doi: 10.1016/j.jcmg.2013.04.012
11. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the eular recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. (2019) 78(6):736–45. doi: 10.1136/annrheumdis-2019-215089
12. Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. (2008) 58(8):2453–9. doi: 10.1002/art.23678
13. Kohler JA Sr, Ellis AR. Libman-Sacks endocarditis in pediatric patient with systemic lupus erythematosus. Pediatr Cardiol. (2012) 33(8):1466–8. doi: 10.1007/s00246-012-0421-6
14. Kasar PA, Mathew M, Abraham G, Kumar RS. Occult systemic lupus erythematosus with active lupus nephritis presenting as Libman-Sacks endocarditis. Ann Pediatr Cardiol. (2012) 5(1):85–8. doi: 10.4103/0974-2069.93720
15. D'Alessandro LC, Paridon SM, Gaynor JW. Successful repair of aortic valve perforation in pediatric Libman-Sacks endocarditis. J Thorac Cardiovasc Surg. (2012) 144(6):e151–3. doi: 10.1016/j.jtcvs.2012.09.034
16. Chang JC, Knight AM, Xiao R, Mercer-Rosa LM, Weiss PF. Use of echocardiography at diagnosis and detection of acute cardiac disease in youth with systemic lupus erythematosus. Lupus. (2018) 27(8):1348–57. doi: 10.1177/0961203318772022
17. Zmaili MA, Alzubi JM, Kocyigit D, Bansal A, Samra GS, Grimm R, et al. A contemporary 20-year Cleveland clinic experience of nonbacterial thrombotic endocarditis: etiology, echocardiographic imaging, management, and outcomes. Am J Med. (2021) 134(3):361–9. doi: 10.1016/j.amjmed.2020.06.047
18. Akhlaq A, Ali TA, Fatimi SH. Mitral valve replacement in systemic lupus erythematosus associated Libman-Sacks endocarditis. J Saudi Heart Assoc. (2016) 28(2):124–6. doi: 10.1016/j.jsha.2015.09.003
19. Liu Y, Tang H, Xu Z, Liu Y. Libman-Sacks endocarditis in a puerpera with systemic lupus erythematosus. Ann Thorac Surg. (2019) 107(3):e169–e70. doi: 10.1016/j.athoracsur.2018.07.064
20. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 Esc guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (esc). endorsed by: european association for cardio-thoracic surgery (eacts), the European association of nuclear medicine (eanm). Eur Heart J. (2015) 36(44):3075–128. doi: 10.1093/eurheartj/ehv319
21. Myones BL. Update on antiphospholipid syndrome in children. Curr Rheumatol Rep. (2011) 13(1):86–9. doi: 10.1007/s11926-010-0148-4
22. Omarjee L, Jaquinandi V, Camarzana A, Rouleau F, Mezdad TH, Le Tourneau T, et al. Improvement of Libman-Sacks endocarditis with combined hydroxychloroquine-vitamin K antagonist therapy in a primary antiphospholipid syndrome patient. Circ J. (2018) 82(9):2380–2. doi: 10.1253/circj.CJ-17-1131
23. Sonsöz MR, Tekin RD, Gül A, Buğra Z, Atılgan D. Treatment of Libman-Sacks endocarditis by combination of warfarin and immunosuppressive therapy. Turk Kardiyol Dern Ars. (2019) 47(8):687–90. doi: 10.5543/tkda.2019.29213
24. Ishizu K, Isotani A, Yamaji K, Ando K. Immunosuppressive therapy to reduce mitral regurgitation in Libman-Sacks endocarditis: a case report. Eur Heart J Case Rep. (2019) 3(3):1–5. doi: 10.1093/ehjcr/ytz133
25. Senesael E, Plein D, La Meir M, Droogmans S. A case report of an unusual cause of mitral stenosis in a young woman. Eur Heart J Case Rep. (2018) 2(4):yty118. doi: 10.1093/ehjcr/yty118
26. Lampert J, Halista M, Pujadas E, Alexander S, Bier B, Hadley M, et al. Cardiogenic shock and mitral valve chord rupture: a rare presentation of Libman-Sacks endocarditis. JACC Case Rep. (2020) 2(12):1988–91. doi: 10.1016/j.jaccas.2020.06.044
27. Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev. (2016) 24(5):244–7. doi: 10.1097/crd.0000000000000106
28. Wałdoch A, Kwiatkowska J, Dorniak K. Unusual location of the Libman-Sacks endocarditis in a teenager: a case report. Cardiol Young. (2016) 26(2):365–7. doi: 10.1017/s1047951115000712
29. Al Riyami H, Joshi N, Al Senaidi K, Al ‘Abdul Salam N, Abdwani R. All endocarditis is not infective: Libman-Sacks endocarditis. Cureus. (2022) 14(7):e26526. doi: 10.7759/cureus.26526
Keywords: Libman-Sacks endocarditis, systemic lupus erythematosus, immunosuppressive therapy, children, case report
Citation: Lu J, Bao S, Xu X, Jin Y, Liu C, Zhang Y, Wang Q and Jin Y (2024) Libman-Sacks endocarditis in a child with systemic lupus erythematosus: a case report and literature review. Front. Pediatr. 12:1323943. doi: 10.3389/fped.2024.1323943
Received: 18 October 2023; Accepted: 8 January 2024;
Published: 31 January 2024.
Edited by:
Lovro Lamot, University of Zagreb, CroatiaReviewed by:
Nikola Krmek, Sisters of Charity Hospital, CroatiaKübra Öztürk, Istanbul Medeniyet University Göztepe Prof Dr Süleyman Yalçın City Hospital, Türkiye
© 2024 Lu, Bao, Xu, Jin, Liu, Zhang, Wang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanliang Jin amlueWFubGlhbmdAc2NtYy5jb20uY24=
 Jingyi Lu
Jingyi Lu Shengfang Bao1
Shengfang Bao1