- 1Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 2Department of Pediatrics, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 3Department of Critical Care Medicine, The Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang, China
- 4Department of Critical Care Medicine, The Hongxinglong Hospital of Beidahuang Group, Shuangyashan, Heilongjiang, China
- 5Department of Emergency, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 6Department of Critical Care Medicine, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 7Department of Emergency, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 8Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
Background: Initial choices of antimicrobial therapy for most cases of community-acquired pneumonia (CAP) in children under 5 years of age are typically based on local epidemiology, risk factors assessment, and subsequent clinical parameters and positive cultures, which can lead to the underdiagnosis and underestimation of lung infections caused by uncommon pathogens. Contezolid, an orally administered oxazolidinone antibiotic, gained approval from the National Medical Products Administration (NMPA) of China in June 2021 for managing complicated skin and soft tissue infections (cSSTI) caused by staphylococcus aureus (SA), streptococcus pyogenes, or streptococcus agalactis. Owing to its enhanced safety profile and ongoing clinical progress, the scope of contezolid's clinical application continues to expand, benefiting a growing number of patients with Gram-positive bacterial infections.
Case summary: In this report, we present the first use of contezolid in a toddler with severe CAP caused by SA, aiming to avoid potential adverse drug reactions (ADRs) associated with vancomycin and linezolid.
Conclusion: Although contezolid has not been officially indicated for CAP, it has been shown to be effective and safe in the management of SA-induced severe CAP in this toddler, suggesting its potential as an alternative option in the dilemma, especially for patients who are susceptible or intolerant to ADRs associated with first-line anti-methicillin-resistant staphylococcus aureus (MRSA) antimicrobial agents.
Introduction
Community-acquired pneumonia (CAP) is most prevalent among children under 5 years old (1). Identifying CAP typically involves a thorough assessment of the patient's medical history, clinical symptoms, physical examination, laboratory tests, and imaging results (2). In children under 5 years old, the most commonly identified bacteria causing CAP include streptococcus pneumoniae, streptococcus pyogenes, klebsiella pneumoniae, escherichia coli, staphylococcus aureus (SA), haemophilus influenzae, haemophilus parainfluenzae, and moraxella catarrhalis (3, 4). The presence of significant pleural effusion or extensive multi-lobar infiltrates or consolidations on lung imaging is often indicative of necessity of hospitalization, disease severity, need for intensive care unit (ICU)-level care and organ function support, and a less favorable prognosis (5, 6). Prompt diagnosis, pathogen identification, risk stratification, and subsequent targeted antimicrobial therapy are vital for favorable outcomes in children with CAP (7). In clinical practice, initial choices of antimicrobial therapy for most cases of CAP in children under 5 years of age are typically based on local epidemiology, risk factors assessment, and subsequent clinical parameters and culture results, which can lead to the underdiagnosis and underestimation of lung infections caused by uncommon pathogens (8). By amplifying and sequencing the nucleic acids of isolated pathogens, metagenomic next-generation sequencing (mNGS) can offer superior performance in identifying pathogens from various body parts with higher sensitivity and diagnostic specificity than traditional pathogen detection methods, particularly for rare pathogens (9, 10).
Although not the most common, staphylococcus aureus (SA), including the methicillin-resistant SA (MRSA) variant, does play a role as one of the pathogens responsible for pediatric CAP, which can be partly attributed to its natural colonization in the airways (11, 12). More importantly, SA-induced CAP is associated with unfavorable clinical outcomes compared to the common pneumococcal CAP, involving a higher risk for sepsis and septic shock, need for ICU-level care and organ function support, and significant mortality, regardless of the presence of methicillin-resistance (13, 14). However, the current practice of empirical antimicrobial agents for MRSA-induced lung infections remains suboptimal in consideration of potential adverse drug reactions (ADRs) associated with first-line anti-MRSA antimicrobial agents, leading clinicians have to carefully weigh the pros and cons of antibiotic choices (15, 16). This predicament underscores the pressing need for the development of new antibiotics, leading to the emergence of contezolid. Conteziold, an orally administered oxazolidinone antibiotic, gained approval from the National Medical Products Administration (NMPA) of China in June 2021 for managing complicated skin and soft tissue infections (cSSTI) caused by SA, streptococcus pyogenes, or streptococcus agalactis (17). Owing to its enhanced safety profile and ongoing clinical progress, the scope of contezolid's clinical application continues to expand, benefiting a growing number of patients with Gram-positive bacterial infections (18–21).
Notably, there have been no reports of the clinical application of contezolid in treating severe CAP caused by SA in the toddler. In this report, we present, for the first time, the compassionate use of contezolid in the treatment of severe CAP induced by SA in a toddler, demonstrating its favorable therapeutic efficacy and enhanced safety profile.
Case presentations
Chief complaints
A 28-month-old girl was admitted to the Department of Critical Care Medicine at the Sixth Affiliated Hospital of Harbin Medical University on September 26, 2022, due to a one-day history of fever and recurrent convulsions.
History of present illness
The day before admission, the toddler had intermittent fever, with her body temperature reaching as high as 38.8°C at night, which could be temporarily relieved through physical cooling and oral antipyretic medication. In the early morning of admission, the toddler experienced another episode of fever accompanied by a convulsion, manifested as loss of consciousness, unresponsiveness to calls, cyanosis of the lips, clenching of the teeth, clenched fists, flexion of the upper limbs, stiffness of the lower limbs, and urinary and fecal incontinence, which lasted for a few minutes and resolved spontaneously. A similar convulsion recurred half an hour later and did not improve after receiving chloral hydrate enema and intravenous dexamethasone at the local hospital.
History of past illness
The toddler had no previous history of illness.
Personal and family history
There was no relevant personal or family history.
Physical examination
Upon admission, the physical examination revealed the following parameters: height 0.86 meters, weight 12 kg, body mass index (BMI) 16.22 kg/m2, body temperature 36.0°C, heart rate (HR) 230 beats/min, blood pressure (BP) 96/60 mmHg, respiratory rate (RR) 83 breaths/min, pulse oxygen saturation (SO2) 90%, and children Glasgow Coma Scale (GCS) score of 8 points. Additionally, coma, pharyngeal congestion, mild edema of bilateral bulbar conjunctiva, mild cyanosis of the lips, shortness of breath, three depressions sign, rough breathing sounds, and audible moist rales in both lungs were also observed.
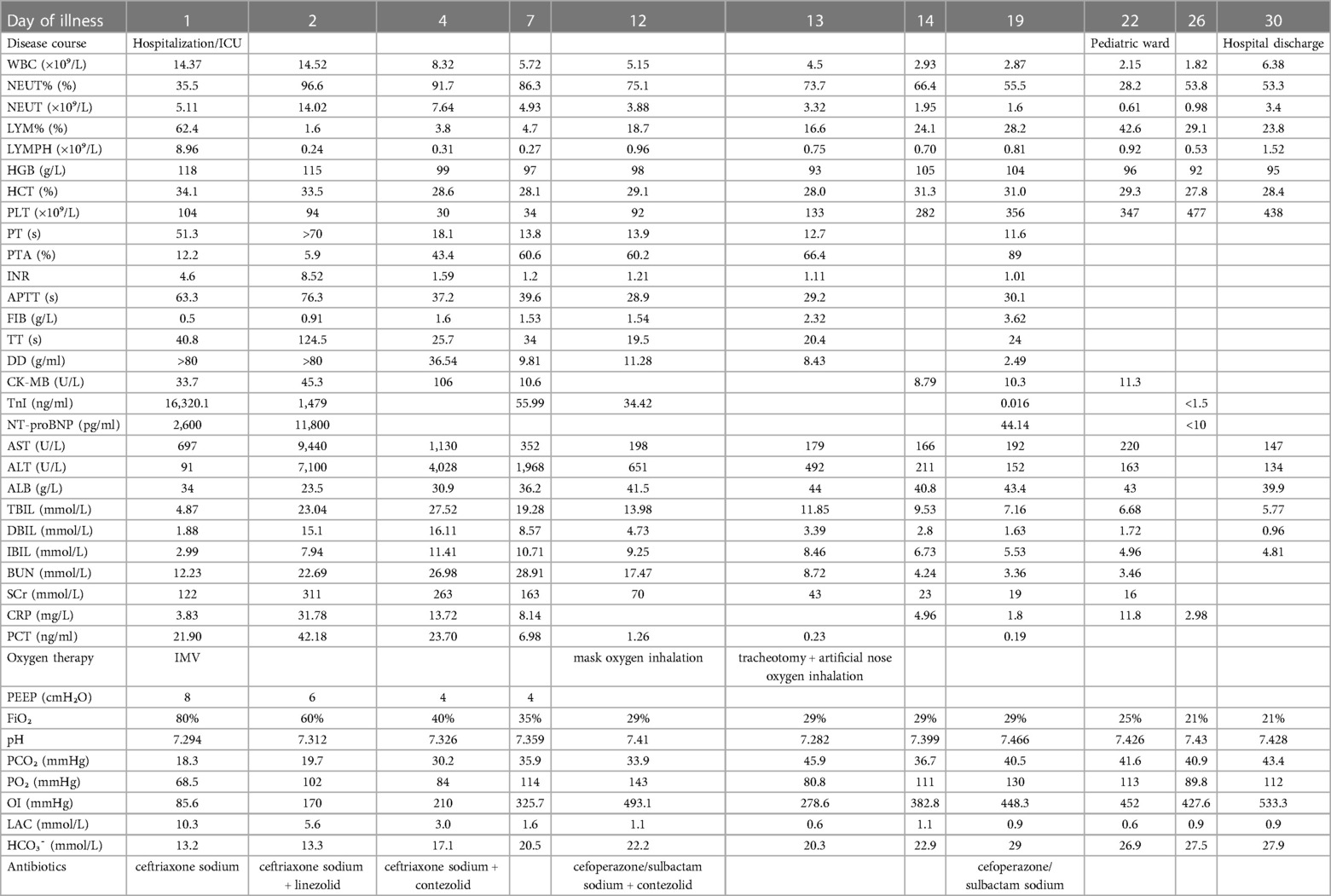
Laboratory parameters
The laboratory parameters upon admission and subsequent re-examinations during hospitalization are presented in Table 1 and Figures 1–3. Arterial blood gas analysis (ABGA) indicated the following abnormal values: pH 7.264, carbon dioxide partial pressure (PCO2) 27.0 mmHg, lactic acid (LAC) 10.3 mmol/L, and bicarbonate (HCO3-) 14.3 mmol/L. Occult blood was detected in gastric juice and stool samples. The Pediatric Critical Illness Score (PCIS) upon admission was 74 points. Nucleic acid tests for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), influenza A and B viruses, respiratory syncytial virus (RSV), as well as serological tests for mycoplasma pneumoniae (MP) and antibodies against various hepatitis viruses, syphilis, and human immunodeficiency virus (HIV) were all negative. During hospitalization, the results of multiple blood cultures were negative.
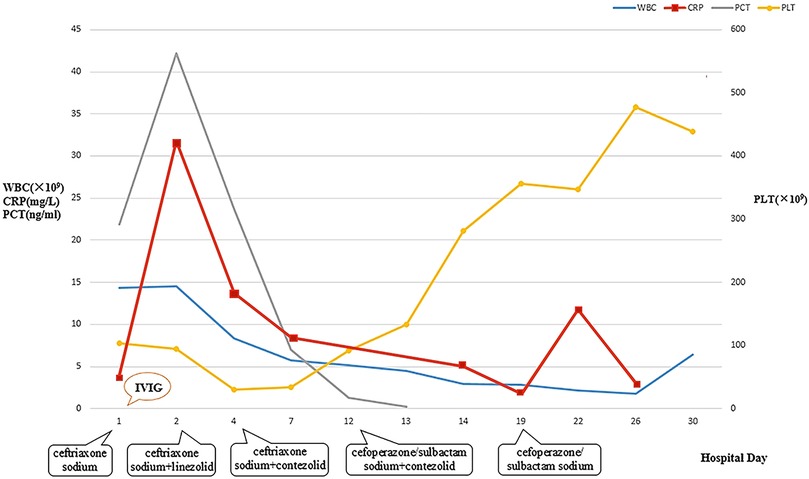
Figure 1. Dynamic changes of WBC, CRP, PCT, and PLT during hospitalization. WBC, white blood cell count; CRP, C-reactive protein; PCT, procalcitonin; PLT, platelet.
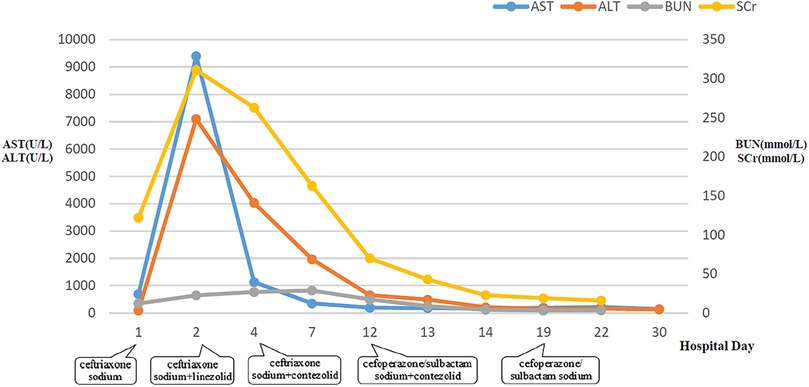
Figure 2. Dynamic changes of AST, ALT, BUN, and SCr during hospitalization. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; SCr, serum creatinine.
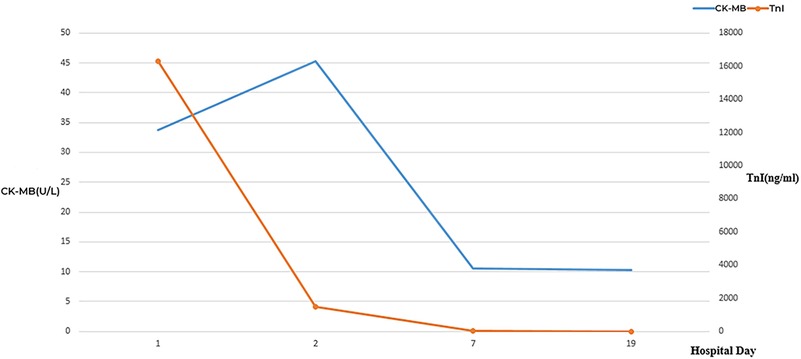
Figure 3. Dynamic changes of CK-MB and TnI during hospitalization. CK-MB, creatine kinase isoenzymes; TnI, troponin I.
Imaging findings
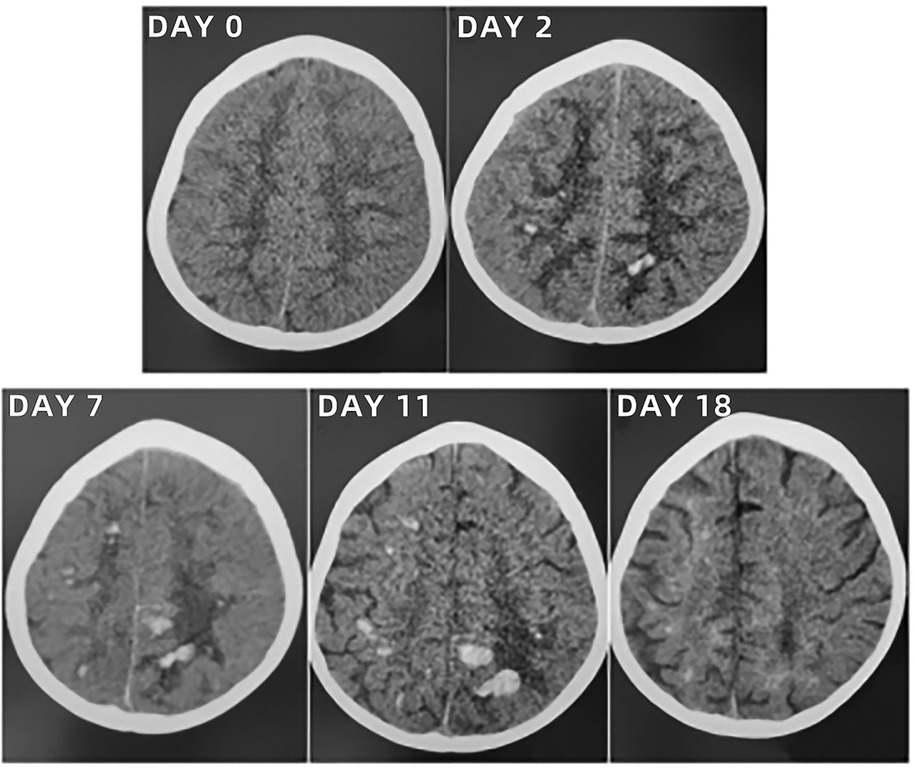
The initial lung computed tomography (CT) scan revealed scattered, patchy shadows in both lungs with indistinct boundaries and visible air-bronchograms. Echocardiography (ECHO) indicated minor regurgitation of the mitral and tricuspid valves, along with a low limit of left ventricular systolic function (LVSF). Abdominal ultrasound (US) displayed upper abdominal bloating, diffuse lesions in both kidneys, and elevated resistance in blood flow (BF) of both renal arteries (RA). Other imagings upon admission, including brain CT scan and US, and chest and lower limbs vascular US, showed no remarkable findings. Imaging findings upon admission and subsequent re-examinations during hospitalization and follow-up were displayed in Figures 4–6.
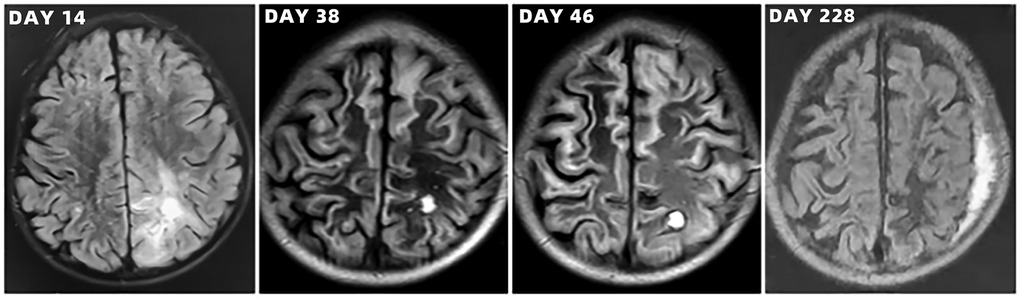
Figure 6. Brain magnetic resonance imaging scans of the toddler during hospitalization and follow-up.
Diagnosis upon admission
The toddler was diagnosed as severe CAP, septic shock, and multiple organ dysfunction syndrome (MODS) based on the history of present illness, clinical presentations, physical examination, laboratory parameters as well as imaging findings. However, the etiology of recurrent convulsions had not been identified at that point.
Treatment
After admission to ICU, comprehensive therapies, including appropriate analgesia and sedation, orotracheal intubation, invasive mechanical ventilation (IMV) with lung-protective ventilation strategies, prone position, goal-directed fluid resuscitation, infusion of vasoactive and positive inotropic agents, empirical selection of ceftriaxone sodium as the initial antibiotic, application of corticosteroids to mitigate inflammation-induced multiple organs damage, immunoglobulin (Ig) pulse (400 mg/kg) to enhance immunity, levetiracetam for anti-epileptic therapy, dehydration for brain protection, protection of multiple organs function, and administration of H2 receptor antagonists to prevent stress ulcers, were implemented. Flexible fiberoptic bronchoscopy (FFB) revealed a moderate amount of yellow mucus sputum in the airway with congestion and swelling of the airway mucosa. Subsequently, bronchoalveolar lavage (BAL) was performed to collect samples for mNGS. On the second day after admission, mNGS of bronchoalveolar lavage fluid (BALF) identified the pathogen as SA with the highest sequence number of 10,954 and natural resistance to aztreonam, polymyxin B/colistin, and nalidixic acid. Due to a sharp increase in serum creatinine (SCr) to 311.00 ummol/L and a rapid decrease in platelet (PLT) to 94 × 109/L, in the dilemma of vancomycin and linezolid, we chose the latter combined with ceftriaxone sodium for anti-infective therapy. A repeat ECHO showed that cardiac function had returned to normal. However, it was important to note that the re-examination of brain CT scan revealed multiple intracerebral hemorrhage (ICH) and ischemic changes in the white matter behind the bilateral lateral ventricles, the etiology of which needed to be further clarified after laboratory parameters approached normal levels. On the fourth day after admission, the re-examination of lung CT scan indicated no significant change in the extent and severity of bilateral pneumonia compared to the initial assessment. However, PLT had decreased to 30 × 109/L, and coagulation and kidney function had recovered somewhat but remained noticeably abnormal, which seriously undermined our confidence in continuing to use linezolid or switching to vancomycin. The continued decline in PLT leading to an increase in ICH or the deterioration of kidney function requiring continuous renal replacement therapy (CRRT) was too much for this toddler. After careful consideration and obtaining informed consent from the legal guardian of the toddler, given that enteral nutrition (EN) had been reestablished, compassionate use of contezolid (200 mg twice daily) was chosen in place of linezolid to avoid potential ADRs associated with vancomycin and linezolid. On the 12th day after admission, the toddler was successfully weaned from IMV and transitioned to mask oxygen inhalation following cuff leak test (CLT) and 30-minute spontaneous breathing trial (SBT). With PLT and coagulation function nearly returning to normal, lumbar puncture was performed for laboratory examinations of cerebrospinal fluid (CSF), including mNGS, microbial cultivation, routine analysis, biochemical detection, Ig determination, and acid-fast bacillus testing, all of which came back negative except for elevated IgA and α2-macroglobulin. After ruling out the possibility of central nervous system (CNS) infection, ceftriaxone sodium was replaced with cefoperazone/sulbactam sodium. On the 13th day after admission, the toddler underwent a tracheotomy procedure to maintain airway patency and prevent the accumulation of airway secretions and recurrence of lung infection, as she remained in a comatose state with occasional convulsions and limited self-cleaning ability of the airway. On the 14th day after admission, brain magnetic resonance imaging (MRI) scan revealed multiple ICH in bilateral cerebral parenchyma, primarily located in the left parieto-occipital lobe and in the early stage of sub-acute phase, with no abnormalities detected in intracranial vessels (Figure 3). On the 19th day after admission, contezolid was discontinued. On the 22nd day after admission, the toddler was stable and transferred to the pediatric ward, but remained unconscious with children GCS score of 11 points and occasional convulsions, manifested as limb stiffness.
Outcome and follow-up
On the 30th day after admission, the toddler was discharged and transferred to the rehabilitation hospital for further neurological rehabilitation, with children GCS score of 13 points. Follow-up CT and MRI scans of the toddler are shown in Figures 4–6. The last follow-up lung CT scan on the 126th day after discharge showed bronchitis with localized inflammation. The last follow-up brain MRI scan on the 199th day after discharge revealed left parietal subdural hematoma, cerebral atrophy, and multiple encephalomalacia foci. At the last follow-up on the 280th day after discharge, it was noted that the toddler had regained consciousness, presented as completing simple actions on command, pronouncing a single sound and standing up with support from a chair, except for poor hand-eye coordination and inability to roll over independently.
Discussion
CAP, usually considered as a major global health issue, represents a leading cause of hospitalization, large consumption of antibiotics, need for ICU-level care and organ function support, and substantial mortality in pediatric population younger than 5 years, thus posing a significant threat to childhood health, placing a huge burden on healthcare systems, and presenting a grand challenge to clinical management (7, 22, 23). As a common acute lower respiratory tract infection, pediatric CAP is characterized by a predominance of respiratory viruses over other pathogens, with RSV being the most frequently isolate, especially in pediatric population under 2 years of age (24, 25). However, the etiologies and prevalences of CAP vary substantially depending on the age breakdowns, individual susceptibilities, clinical settings, geographical regions, seasons and epidemiological characteristics (26, 27). For instance, in Saudi Arabia, SA, a Gram-positive bacterium, has been identified as one of the major contributors to pediatric CAP (28). Additionally, SA is a frequent pathogen of bloodstream infection (BSI), which can worsen the condition in children with CAP (29, 30). Finally, local or systemic complications, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), multiple organ involvement or even dysfunction often serve as crucial clinical indicators of disease deterioration and poor prognosis in children with CAP, and SA is one of the common culprits (31).
SA can be categorized into methicillin-susceptible SA (MSSA) and MRSA based on the presence or absence of methicillin-resistance, with panton-valentine leukocidin (PVL) being one of the most important virulence factors to cause leukocyte lysis and tissue necrosis in clinical settings (12, 32). Consequently, SA is an extreme-virulent and high-pathogenic Gram-positive bacterium and capable of rapidly developing antibiotic resistance (33). Furthermore, the prevalences of SA-induced a series of diseases have shown a steadily increasing trend over the years due to the introduction of pneumococcal conjugate vaccines (PCVs) (34, 35). Prompt and accurate pathogen identification provides objective scientific evidences for optimal amtimicrobial stewardship strategies to reduce excessive use of antibiotics, secondary resistance of strains, potential ADRs and unnecessary costs. However, challenges such as insufficient awareness of pathogen detection, limited access to pathogen acquisition, low positive yield and relative hysteresis of traditional detection methods, and uncertainty of detection results significantly impede the timely and precise management of CAP in clinical practice (36). Rapid, highly accurate, readily available, and cost-effective microbiological diagnosis for the etiologies of CAP may be a breakthrough for this predicament. With its extensive clinical application, mNGS has gradually demonstrated greater potential than traditional detection methods for early pathogen identification to facilitate rational use of antibiotics, especially in BALF samples from patients undergoing IMV (37–39).
In this toddler, mNGS of BALF sample collected immediately after IMV revealed the culprit was SA with natural resistance to aztreonam, polymyxin B/colistin and nalidixic acid. Despite the lack of detection of methicillin-resistance, first-line anti-MRSA antimicrobial agents were chosen for salvage therapy in view of disease severity, progress in lung imagings, and multi-organ involvement of the toddler upon admission. Even if the responsible pathogens are identified, antibiotic regimens must take into account some specific clinical characteristics such as pharmacokinetics (PK), pharmacodynamics (PD) and potential ADRs associated with the selected antibiotics, disease severity, immune status, organ function and economic affordability of patients, and history of antibiotic use (40). More importantly, failure in initial empirical antimicrobial therapy can be associated with unfavorable outocmes (41). The decision to off-label use of contezolid was driven by concern about potential ADRs associated with vancomycin and linezolid, which could further make the already serious condition worse (18). Although contezolid is officially approved for managing cSSTI induced by SA, streptococcus pyogenes or streptococcus agalactis, but in fact, it has demonstrated broad-spectrum and potent activity against Gram-positive bacteria from various parts of the body, including multidrug-resistant (MDR) strains, in both in-vivo and in-vitro studies with an improved safety and tolerability profile through minimizing effects associated with myelosuppression and monoamine oxidase (MAO) inhibition (18, 19, 42–45). As the only completely synthetic class of antibiotic, contezolid exerts a unique mechanism of action by inhibiting targeted protein synthesis and exhibits excellent dissemination to lung tissues (46). After continuous oral administration of contezolid (200 mg twice daily) for 15 days on ordinary fed status, the toddler achieved the expected therapeutic effect and favorable outcome without any ADRs. In addition, high CSF permeability of unbound contezolid has been demonstrated in a patient with tuberculous meningoencephalitis, suggesting the therapeutic potential of contezolid for CNS infections (47).
Another noteworthy issue in this case was the occurrence of secondary multiple ICH and ischemic changes in the white matter behind the bilateral lateral ventricles following severe CAP, which was really a rare phenomenon in clinical practice. These near-complete negative CSF tests dispelled our initial suspicions of a CNS infection. During the subsequent multidisciplinary team (MDT) discussion, the majority consensus was that these neurological abnormalities were primarily linked to severe CAP-induced MODS, especially coagulation dysfunction. Insufficient evidence existed to suggest the involvement of secondary cytokine storm in the brain. An open question for consideration was whether 12-day systemic antibiotic therapy in conjunction with 5-day Ig pulse could eliminate the pathogens responsible for these neurological abnormalities, even though we were not inclined to favor this possibility. The last follow-up lung CT scan on the 126th day after discharge indicated bronchitis with localized inflammation, suggesting that it might take longer to repair SA-caused severe lung damage in the toddler. The results of the last brain MRI scan and neurological follow-up revealed the persistent severity of brain injury and sequelae in the toddler with SA-induced severe CAP, which could have a substantial impact on the growth and development of children.
A limitiation of this study is the lack of objective conditions for monitoring PK and PD of contezolid, which is particularly important when using newly developed drugs in pediatric patients, especially for off-label use. In addition, the successful application of contezolid in the management of SA-induced severe CAP in this toddler is only a case report, which is not universal, and can not be directly extended to similar patient populations or indications. Lastly, although this toddler was followed up to 280 days after discharge, it was still insufficient.
Conclusion
Although contezolid has not been officially indicated for CAP, it has been shown to be effective and safe in the management of SA-induced severe CAP in this toddler, suggesting its potential as an alternative option in the dilemma, especially for patients who are susceptible or intolerant to ADRs associated with first-line anti-MRSA antimicrobial agents. Future efforts should concentrate on confirming our findings through more similar cases and well-designed clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Sixth Affiliated Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
H-YL: Investigation, Writing – original draft, Writing – review & editing. X-FB: Methodology, Writing – original draft, Writing – review & editing. Y-JW: Resources, Writing – original draft, Writing – review & editing. F-JX: Methodology, Writing – review & editing. HZ: Investigation, Writing – review & editing. Y-CZ: Investigation, Writing – review & editing. YZ: Methodology, Writing – review & editing. Z-HW: Resources, Writing – review & editing. DW: Methodology, Writing – review & editing. HM: Resources, Writing – original draft. Y-LL: Investigation, Writing – original draft. L-QL: Methodology, Writing – original draft. S-XQ: Data curation, Investigation, Writing – original draft. YAG: Resources, Supervision, Writing – original draft, Writing – review & editing. KK: Supervision, Writing – original draft, Writing – review & editing. YNG: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study received financial support from the following sources: the National Natural Science Foundation of China (No.82372172), the Key Research and Development Plan Project of Heilongjiang Province (No.GA23C007), the Heilongjiang Province Postdoctoral Start-up Fund (No.LBH-Q20037), the Special Fund for Clinical Research of Wu Jie-Ping Medical Foundation (No.320.6750.2022-02-16), the Research Project of Heilongjiang Provincial Health Commission (No.20231717010461), and the Scientific Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (No.2021M08 and 2018L006).
Acknowledgments
The authors extend their gratitude to all those who provided valuable assistance, guidance, and support for this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1321447/full#supplementary-material
References
1. Cardinale F, Cappiello AR, Mastrototaro MF, Pignatelli M, Esposito S. Community-acquired pneumonia in children. Early Hum Dev. (2013) 89(Suppl 3):S49–52. doi: 10.1016/j.earlhumdev.2013.07.023
2. Womack J, Kropa J. Community-acquired pneumonia in adults: rapid evidence review. Am Fam Physician. (2022) 105(6):625–30. 35704808.35704808
3. Leung AKC, Wong AHC, Hon KL. Community-acquired pneumonia in children. Recent Pat Inflamm Allergy Drug Discov. (2018) 12:136–44. doi: 10.2174/1872213X12666180621163821
4. Ning G, Wang X, Wu D, Yin Z, Li Y, Wang H, et al. The etiology of community-acquired pneumonia among children under 5 years of age in Mainland China, 2001–2015: a systematic review. Hum Vaccin Immunother. (2017) 13:2742–50. doi: 10.1080/21645515.2017.1371381
5. Nascimento-Carvalho CM. Community-acquired pneumonia among children: the latest evidence for an updated management. J Pediatr (Rio J. (2020) 96(Suppl 1):29–38. doi: 10.1016/j.jped.2019.08.003
6. Yun KW, Wallihan R, Desai A, Alter S, Ambroggio L, Cohen DM, et al. Clinical characteristics and etiology of community-acquired pneumonia in US children, 2015–2018. Pediatr Infect Dis J. (2022) 41:381–7. doi: 10.1097/INF.0000000000003475
7. Yun KW, Wallihan R, Juergensen A, Mejias A, Ramilo O. Community-acquired pneumonia in children: myths and facts. Am J Perinatol. (2019) 36:S54–7. doi: 10.1055/s-0039-1691801
8. Torres A, Chalmers JD, Dela Cruz CS, Dominedò C, Kollef M, Martin-Loeches I, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. (2019) 45:159–71. doi: 10.1007/s00134-019-05519-y
9. Grumaz S, Grumaz C, Vainshtein Y, Stevens P, Glanz K, Decker SO, et al. Enhanced performance of next-generation sequencing diagnostics compared with standard of care microbiological diagnostics in patients suffering from septic shock. Crit Care Med. (2019) 47:e394–402. doi: 10.1097/CCM.0000000000003658
10. Chen M, Lu W, Wu S, Wang S, Lu T, Peng C. Metagenomic next-generation sequencing in the diagnosis of leptospirosis presenting as severe diffuse alveolar hemorrhage: a case report and literature review. BMC Infect Dis. (2021) 21:1230. doi: 10.1186/s12879-021-06923-w
11. Aguilera-Alonso D, Kirchschläger Nieto S, Ara Montojo MF, Sanz Santaeufemia FJ, Saavedra-Lozano J, Soto B, et al. Staphylococcus aureus community-acquired pneumonia in children after 13-valent pneumococcal vaccination (2008–2018): epidemiology, clinical characteristics and outcomes. Pediatr Infect Dis J. (2022) 41:e235–42. doi: 10.1097/INF.0000000000003503
12. He H, Wunderink RG. Staphylococcus aureus pneumonia in the community. Semin Respir Crit Care Med. (2020) 41:470–9. doi: 10.1055/s-0040-1709992
13. Lee LN, Chou WR, Wang JY, Kuo YL, Chang CY, Lee YC, et al. Characteristics and local risk factors of community-acquired and health-care-associated Staphylococcus aureus pneumonia. Sci Rep. (2022) 12:18670. doi: 10.1038/s41598-022-23246-1
14. Cilloniz C, Dominedò C, Gabarrús A, Garcia-Vidal C, Becerril J, Tovar D, et al. Methicillin-susceptible staphylococcus aureus in community-acquired pneumonia: risk factors and outcomes. J Infect. (2021) 82:76–83. doi: 10.1016/j.jinf.2020.10.032
15. Hu B, Ye L, Li T, Feng Z, Huang L, Guo C, et al. Drug-induced kidney injury in Chinese critically ill pediatric patients. Front Pharmacol. (2022) 13:993923. doi: 10.3389/fphar.2022.993923
16. Shi Y, Wu HL, Wu YH, Li S, Zhang LY, Xu SS, et al. Safety and clinical efficacy of linezolid in children: a systematic review and meta-analysis. World J Pediatr. (2023) 19:129–38. doi: 10.1007/s12519-022-00650-1
18. Li B, Liu Y, Luo J, Cai Y, Chen M, Wang T. Contezolid, a novel oxazolidinone antibiotic, may improve drug-related thrombocytopenia in clinical antibacterial treatment. Front Pharmacol. (2023) 14:1157437. doi: 10.3389/fphar.2023.1157437
19. Zhao S, Zhang W, Zhang L, Zhang J, Li J, Si L, et al. Use of contezolid for the treatment of refractory infective endocarditis in a patient with chronic renal failure: case report. Infect Drug Resist. (2023) 16:3761–5. doi: 10.2147/IDR.S413452
20. Chen P, An L, Zhang Z. Sequential therapy of linezolid and contezolid to treat vancomycin-resistant Enterococcus faecium pneumonia in a centenarian patient: case report. Infect Drug Resist. (2023) 16:1573–8. doi: 10.2147/IDR.S401533
21. Wang K, Hu Y, Duan Z, Fu H, Hu X, Zhao Y, et al. Severe community-acquired pneumonia caused by methicillin-sensitive Staphylococcus aureus: successfully treated with contezolid - A case report and literature review. Infect Drug Resist. (2023) 16:3233–42. doi: 10.2147/IDR.S406799
22. Chee E, Huang K, Haggie S, Britton PN. Systematic review of clinical practice guidelines on the management of community acquired pneumonia in children. Paediatr Respir Rev. (2022) 42:59–68. doi: 10.1016/j.prrv.2022.01.006
23. Nascimento-Carvalho AC, Nascimento-Carvalho CM. Clinical management of community-acquired pneumonia in young children. Expert Opin Pharmacother. (2019) 20:435–42. doi: 10.1080/14656566.2018.1552257
24. Pratt MTG, Abdalla T, Richmond PC, Moore HC, Snelling TL, Blyth CC, et al. Prevalence of respiratory viruses in community-acquired pneumonia in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2022) 6:555–70. doi: 10.1016/S2352-4642(22)00092-X
25. Rueda ZV, Aguilar Y, Maya MA, López L, Restrepo A, Garcés C, et al. Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. (2022) 22:169. doi: 10.1186/s12887-022-03235-z
26. Cheng W, Li M, Yu S, Peng X, Zhang L, Zhou C, et al. Epidemiological characteristics of community-acquired pneumonia and effects from the COVID-19 pandemic in Shenzhen of China. J Trop Pediatr. (2022) 69:fmac111. doi: 10.1093/tropej/fmac111
27. Roh EJ, Lee MH, Lee JY, Kim HB, Ahn YM, Kim JK, et al. Analysis of national surveillance of respiratory pathogens for community-acquired pneumonia in children and adolescents. BMC Infect Dis. (2022) 22:330. doi: 10.1186/s12879-022-07263-z
28. Alshammari MK, Alotaibi MA, AlOtaibi AS, Alosaime HT, Aljuaid MA, Alshehri BM, et al. Prevalence and etiology of community- and hospital-acquired pneumonia in Saudi Arabia and their antimicrobial susceptibility patterns: a systematic review. Medicina (Kaunas). (2023) 59:760. doi: 10.3390/medicina59040760
29. Hu F, Yuan L, Yang Y, Xu Y, Huang Y, Hu Y, et al. A multicenter investigation of 2,773 cases of bloodstream infections based on China antimicrobial surveillance network (CHINET). Front Cell Infect Microbiol. (2022) 12:1075185. doi: 10.3389/fcimb.2022.1075185
30. Wattal C, Goel N. Pediatric blood cultures and antibiotic resistance: an overview. Indian J Pediatr. (2020) 87:125–31. doi: 10.1007/s12098-019-03123-y
31. de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. (2020) 396:786–98. doi: 10.1016/S0140-6736(20)31550-6
32. Darboe S, Dobreniecki S, Jarju S, Jallow M, Mohammed NI, Wathuo M, et al. Prevalence of panton-valentine leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an urban gambian hospital: a 11-year period retrospective pilot study. Front Cell Infect Microbiol. (2019) 9:170. doi: 10.3389/fcimb.2019.00170
33. Esposito S, Blasi F, Curtis N, Kaplan S, Lazzarotto T, Meschiari M, et al. New antibiotics for staphylococcus aureus infection: an update from the world association of infectious diseases and immunological disorders (WAidid) and the Italian society of anti-infective therapy (SITA). Antibiotics (Basel). (2023) 12:742. doi: 10.3390/antibiotics12040742
34. Berger Y, Adler A, Ariel T, Rokney A, Averbuch D, Grisaru-Soen G. Paediatric community-acquired bacteraemia, pneumococcal invasive disease and antibiotic resistance fell after the pneumococcal conjugate vaccine was introduced. Acta Paediatr. (2019) 108:1321–8. doi: 10.1111/apa.14670
35. Darboe S, Okomo U, Muhammad AK, Ceesay B, Jallow M, Usuf E, et al. Community-acquired invasive bacterial disease in Urban Gambia, 2005–2015: a hospital-based surveillance. Clin Infect Dis. (2019) 69:S105–13. doi: 10.1093/cid/ciz463
36. Fritz CQ, Edwards KM, Self WH, Grijalva CG, Zhu Y, Arnold SR, et al. Prevalence, risk factors, and outcomes of bacteremic pneumonia in children. Pediatrics. (2019) 144:e20183090. doi: 10.1542/peds.2018-3090
37. Chen S, Kang Y, Li D, Li Z. Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: systematic review and meta-analysis. Int J Infect Dis. (2022) 122:867–73. doi: 10.1016/j.ijid.2022.07.054
38. Yang A, Chen C, Hu Y, Zheng G, Chen P, Xie Z, et al. Application of metagenomic next-generation sequencing (mNGS) using bronchoalveolar lavage fluid (BALF) in diagnosing pneumonia of children. Microbiol Spectr. (2022) 10:e0148822. doi: 10.1128/spectrum.01488-22
39. Yang SL, Gao Y, Han ZY, Du X, Liu W, Jin SG, et al. Successful treatment of near-fatal pulmonary embolism and cardiac arrest in an adult patient with fulminant psittacosis-induced severe acute respiratory distress syndrome after veno-venous extracorporeal membrane oxygenation rescue: a case report and follow-up. Heliyon. (2023) 9:e20562. doi: 10.1016/j.heliyon.2023.e20562
40. Gao Y, Wang HL, Zhang ZJ, Pan CK, Wang Y, Zhu YC, et al. A standardized step-by-step approach for the diagnosis and treatment of sepsis. J Intensive Care Med. (2022) 37:1281–7. doi: 10.1177/08850666221085181
41. Kollef MH, Betthauser KD. New antibiotics for community-acquired pneumonia. Curr Opin Infect Dis. (2019) 32:169–75. doi: 10.1097/QCO.0000000000000526
42. Kaul G, Dasgupta A, Chopra S. Contezolid in complicated skin and soft tissue infection. Drugs Today (Barc). (2022) 58:315–26. doi: 10.1358/2022.58.7.3389002
43. Wang W, Voss KM, Liu J, Gordeev MF. Nonclinical evaluation of antibacterial oxazolidinones contezolid and contezolid acefosamil with low serotonergic neurotoxicity. Chem Res Toxicol. (2021) 34:1348–54. doi: 10.1021/acs.chemrestox.0c00524
44. Zhao X, Huang H, Yuan H, Yuan Z, Zhang Y. A phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections. J Antimicrob Chemother. (2022) 77:1762–9. doi: 10.1093/jac/dkac073
45. Carvalhaes CG, Duncan LR, Wang W, Sader HS. In vitro activity and potency of the novel oxazolidinone contezolid (MRX-I) tested against gram-positive clinical isolates from the United States and Europe. Antimicrob Agents Chemother. (2020) 64:e01195–20. doi: 10.1128/AAC.01195-20
46. El-Kimary EI, Allam AN, Khafagy ES, Hegazy WAH. Analytical methodologies for the estimation of oxazolidinone antibiotics as key members of anti-MRSA arsenal: a decade in review. Crit Rev Anal Chem. (2023):1–30. doi: 10.1080/10408347.2023.2228902
Keywords: Contezolid, Compassionate use, Toddler, Community-acquired pneumonia, Staphylococcus aureus, Gram-positive bacterial infection, Adverse drug reactions
Citation: Liu H-Y, Bi X-F, Wang Y-J, Xie F-J, Zhang H, Zhu Y-C, Zhang Y, Wang Z-H, Wu D, Meng H, Lin Y-L, Liu L-Q, Qiu S-X, Gao Y, Kang K and Gao Y (2024) Compassionate use of contezolid in a toddler with severe community-acquired pneumonia induced by staphylococcus aureus: a case report and follow-up. Front. Pediatr. 12:1321447. doi: 10.3389/fped.2024.1321447
Received: 16 October 2023; Accepted: 22 January 2024;
Published: 7 February 2024.
Edited by:
Jun Zheng, Tianjin Central Hospital of Obstetrics and Gynecology, ChinaReviewed by:
Fangrui Ding, Tianjin Central Hospital of Obstetrics and Gynecology, ChinaRu Xifang, Peking University, China
© 2024 Liu, Bi, Wang, Xie, Zhang, Zhu, Zhang, Wang, Wu, Meng, Lin, Liu, Qiu, Gao, Kang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Gao aWN1Z2FveWFuQDE2My5jb20= Kai Kang amFuZWtrNzlAMTI2LmNvbQ== Yang Gao Z2FveWFuZzAzMTJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Abbreviations ADRs, adverse drug reactions; ALB, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BALF, bronchoalveolar lavage fluid; BUN, blood urea nitrogen; CAP, community-acquired pneumonia; CK-MB, creatine kinase isoenzymes; CNS, central nervous system; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CSF, cerebrospinal fluid; cSSTI, complicated skin and soft tissue infections; CT, computed tomography; DBIL, direct bilirubin; DD, D-dimer; ECHO, echocardiography; FIB, fibrinogen; FiO2, fraction of inspiration oxygen; GCS, Glasgow coma scale; GGT, gamma-glutamyl transferase; HCT, hematocrit; HGB, haemoglobin; IBIL, indirect bilirubin; ICH, intracerebral hemorrhage; ICU, intensive care unit; IMV, invasive mechanical ventilation; INR, international normalized ratio; Ig, immunoglobulin; LYM%, lymphocyte proportion; LYMPH, lymphocyte count; mNGS, metagenomic next-gengeration sequencing; MODS, multiple organ dysfunction syndrome; MRI, magnetic resonance imaging; MRSA, methicillin resistant staphylococcus aureus; NEUT, neutrophil count; NEUT%, neutrophil proportion; NT-proBNP, N-terminal pro-brain natriuretic peptide; OI, oxygenation index; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; PTA, prothrombin activity; RSV, respiratory syncytial virus; SA, staphylococcus aureus; SCr, serum creatinine; TBIL, total bilirubin; TnI, troponin I; TP, total protein; TT, thrombin time; US, ultrasound; WBC, white blood cell count.
 Hui-Ying Liu
Hui-Ying Liu Xiao-Fei Bi1,†
Xiao-Fei Bi1,† Kai Kang
Kai Kang Yang Gao
Yang Gao