- 1Department of Pediatrics, Hebei Medical University, Shijiazhuang, China
- 2Department of Pediatric Cardiology, Children’s Hospital of Hebei Province, Shijiazhuang, China
- 3Hebei Provincial Key Laboratory of Pediatric Cardiovascular Disease, Shijiazhuang, China
- 4Department of Neurosurgery, Hebei General Hospital, Shijiazhuang, China
- 5Department of Pediatric Neurology, Children’s Hospital of Hebei Province, Shijiazhuang, China
Background and purpose: Vasovagal syncope (VVS) and psychogenic pseudosyncope (PPS) can be difficult to distinguish, given their similar clinical presentations. This study was conducted to explore the clinical value of catecholamine levels in the differential diagnosis of VVS and PPS in children.
Methods: This retrospective case-control study was conducted with data from children with VVS and PPS who underwent head-up tilt tests (HUTTs) at the Children's Hospital of Hebei Province between March 2021 and March 2023. The data collected were baseline clinical characteristics, HUTT results, serum catecholamine levels in the supine and upright positions, and 24 h urinary catecholamine concentrations. These variables were compared between the VVS and PPS groups.
Results: From 328 potentially eligible cases, 54 (16.46%) cases of VVS and 24 (7.32%) cases of PPS were included in the analysis. No significant difference in age, sex, body mass index, or syncope frequency was observed between the VVS and PPS groups. The main predisposing factors for syncope were body position changes in the VSS group (83.33%) and emotional changes in the PPS group (41.67%). The episode duration was significantly shorter in the VSS group than in the PPS group (4.01 ± 1.20 vs. 24.06 ± 5.56 min, p < 0.05). The recovery time was also shorter in the VVS group than in the PPS group (1.91 ± 0.85 vs. 8.62 ± 2.55 min, p < 0.05). Relative to patients with PPS, those with VVS had significantly higher serum epinephrine (EP) levels in the upright position [199.35 (102.88, 575.00) vs. 147.40 (103.55, 227.25), p < 0.05] and lower serum epinephrine levels in the supine position [72.70 (42.92, 122.85) vs. 114.50 (66.57, 227.50), p < 0.05].
Conclusions: Serum EP levels have potential value in the differential diagnosis of VVS and PPS.
Introduction
Transient loss of consciousness (TLOC) is a common clinical symptom that accounts for approximately 3% of all emergency department visits (1, 2). It can be caused by mechanisms ranging from reflex syncope to arrhythmia and heart block (3). Syncope, the most common cause of TLOC, is characterized by the inability to maintain an autonomous body position due to a reduction in oxygen delivery to the central nervous system induced by cerebral hypoperfusion. It is characterized by sudden TLOC followed by rapid and complete recovery (4). Vasovagal syncope (VVS) accounts for approximately 60%–70% of syncope cases and is especially common among children and adolescents (5). It is an abnormal response mediated by the autonomic nervous system and can be divided into vascular inhibitory, cardiac inhibitory, and mixed types (6). Psychogenic pseudosyncope (PPS) is another clinical syndrome that occurs without defective cerebral perfusion or function (7). As PPS and VVS share clinical manifestations such as falling and recurrent TLOC episodes, their timely and accurate diagnosis in symptomatic children is difficult (8, 9). It is essential, however, as the treatment and prognosis of these two conditions are quite different.
The head-up tilt test (HUTT) is a routine clinical test used in the differential diagnosis of VVS and PPS, but it alone is not sufficient due to its low sensitivity (10). In addition, the HUTT is time consuming and inconvenient and may induce shock, limiting its broad clinical application. Thus, the development of simpler, more specific and reliable methods to distinguish VVS from PPS in clinical scenarios is needed. Several groups are currently trying to find innovative methods to aid the differentiation of VVS and PPS in children (10, 11).
Catecholamines, including epinephrine (EP), norepinephrine (NE), and dopamine (DP), are important neurotransmitters secreted from the adrenal medulla. Extensive neurohumoral changes are related to VVS onset (12). As early as 1965, Chosy and Graham (13) reported that the urine EP level was higher in patients with than in those without VVS. Subsequently, changes in catecholamine levels associated with the pathogenesis of VVS have been foci of research. In the upright position, the NE and EP levels rise to a greater extent in patients with than in those without syncope. In proximity to syncope, however, the EP level continues to rise, peaking at the end of episode, while the NE level returns to normal (14–16). Based on HUTT results, EP has been identified as a possible contributor to VVS susceptibility (17). However, the potential syncope-related diagnostic value and mechanisms of action of catecholamines remain unclear. This study was conducted to evaluate whether catecholamine levels can be used as auxiliary indicators for the differential diagnosis of VVS and PPS in clinical practice.
Method
Participants
In this retrospective case-control study, children with unexplained syncope who presented to the Children's Hospital of Hebei Province between March 2021 and March 2023 were considered for inclusion in this study. The inclusion criteria were: (1) diagnosis of VVS or PPS, (2) age <18 years, (3) HUTT performance, (4) measurement of supine and upright plasma catecholamine levels, and (5) measurement of 24-hour urine catecholamine concentrations. Patients (1) diagnosed with PPS and VVS and those with (2) syncope caused by cardiogenic, neurogenic, and other diseases and (3) insufficient clinical information were excluded. The hospital's ethics committee approved the study (Medical Ethics no. 24), the patients’ [legal guardian/next of kin] provided written informed consent to participate in this study. The clinical data were collected from the electronic medical records system of our hospital.
Diagnoses
VVS was diagnosed in accordance with the 2018 guidelines for the diagnosis and treatment of syncope in children and adolescents in China (18). The criteria were: (1) a clear history of syncope with spontaneous recovery; (2) attacks usually induced by prolonged uprightness, mental stress, and environment factors, such as sultry; (3) HUTT positivity; (4) sudden hypotension and/or inappropriate bradycardia during onset; and (5) the exclusion of other disorders, such as cerebrovascular, cardiogenic, and metabolic diseases.
PPS was diagnosed according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (19). The criteria were: (1) a clear history of recurrent syncope with spontaneous recovery; (2) eye closure and muscle tone loss; (3) normal heart rate (HR) and blood pressure (BP) before, during, and after the clinical event; and (4) the exclusion of other disorders, such as neurogenic, cardiogenic, and metabolic diseases (18).
HUTT performance and catecholamine detection
The HUTT was performed according to the 2018 guidelines for the diagnosis and treatment of syncope in children and adolescents in China (18). The subjects were asked to fast for at least 4 h before the test and to stop any vasoactive medication for at least five half-lives. The test was conducted in a temperature-controlled, quiet, dimly lit room. After 10 min rest, the subjects’ HR, BP, and heart function were recorded continuously with an ambulatory BP meter, an echocardiographic monitor, and a tilting bed (MedStandard, Suzhou, China) in the supine position and then with a head-up (60°) tilt for 45 min or until a positive response occurred. Blood samples in the supine position were collected firstly. And after 10 min in the upright positions, the next sample for upright positions were collected. In addition, 24 h urine samples were collected. The levels of catecholamines in these samples were detected by high-performance liquid chromatography/mass spectrometry (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions.
Data collection from medical records
Participants’ basic clinical and demographic data, including sex, age, and body mass index (BMI), were retrieved from their medical records. Data on their syncope-related past medical histories, such as the LOC duration, attack frequency, predisposing factors, and family history, were characterized via self-administered questionnaire. A dedicated staff member recorded the medical information, and another investigator independently checked it.
Statistical analysis
The statistical analyses were performed using SPSS (version 24.0; IBM Corporation, Armonk, NY, USA). The normality of data distribution was assessed using the Shapiro–Wilk test. Normally distributed measurement data are expressed as means ± standard deviations and were compared between groups using the unpaired t test. Non-normally distributed variables are expressed as medians with interquartile ranges and were compared between groups using the nonparametric Mann–Whitney U test. Enumeration data are expressed as rates or percentages and were analyzed using the chi-squared test. Differences were considered to be significant with p < 0.05.
Results
Baseline characteristics
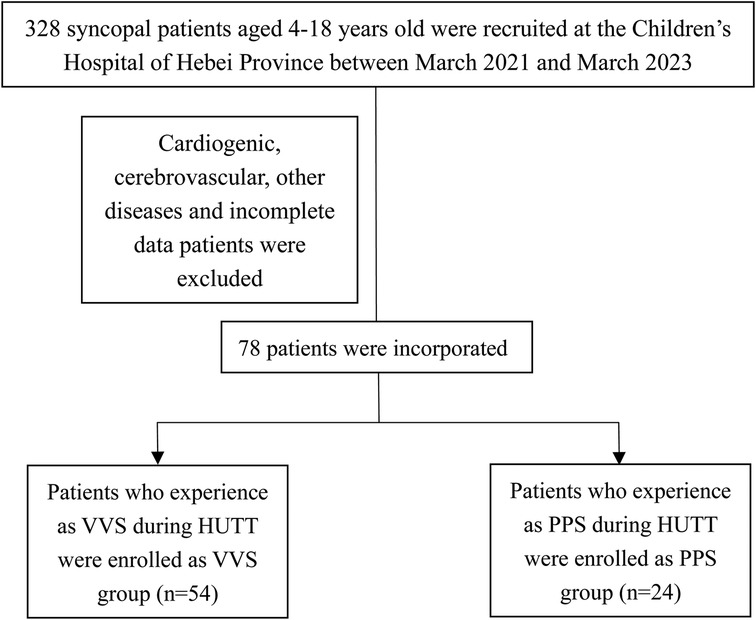
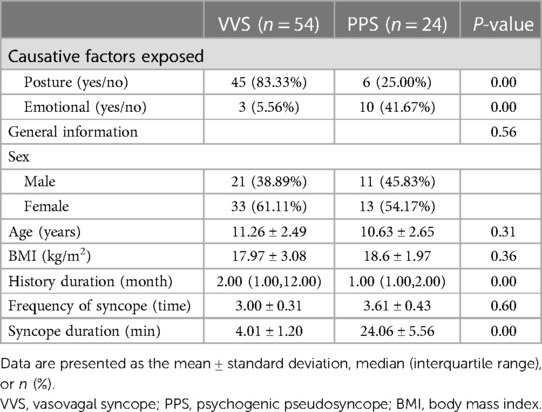
Of 328 patients with syncope assessed at the hospital during the study period, including HUTT performance 182 (55.48%) and 24 (7.33%) patients were diagnosed with VVS and PPS, respectively. After the exclusion of patients for whom catecholamine measurements were lacking, 54 patients with VVS (21 males, 33 females) and 24 patients with PPS (11 males, 13 females) were enrolled in the study (Figure 1). The patients’ baseline and demographic characteristics are provided in Table 1. No significant difference in age, sex, or BMI was observed between groups.
Clinical features
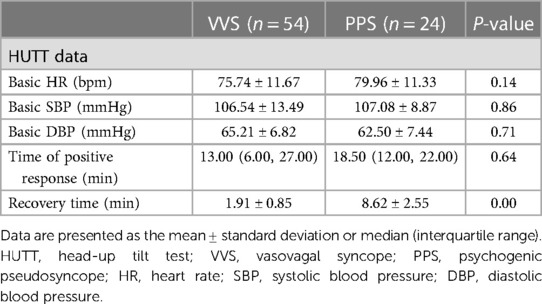
The main predisposing factors for syncope were body position changes in the VSS group (83.33%) and emotional changes in the PPS group (41.67%; p < 0.05; Table 1). Syncope history durations were longer in the VSS group than in the PPS group [2.00 (1.00, 12.00) vs. 1.00 (1.00, 2.00) months, p < 0.05; Table 1]. The frequency of syncope did not differ significantly between the VVS and PPS groups (3.00 ± 0.31 and 3.61 ± 0.43 events, p = 0.6; Table 1). The syncopal episode duration was significantly shorter in the VVS group than in the PPS group (4.01 ± 1.20 vs. 24.06 ± 5.56 min, p < 0.05; Table 1). HUTT results indicated that the recovery time was shorter in the VVS group than in the PPS group (1.91 ± 0.85 vs. 8.62 ± 2.55 min, p < 0.05; Table 2). No significant difference in the baseline HR, systolic or diastolic BP, or positive response time was observed between groups (p > 0.05).
Catecholamine levels
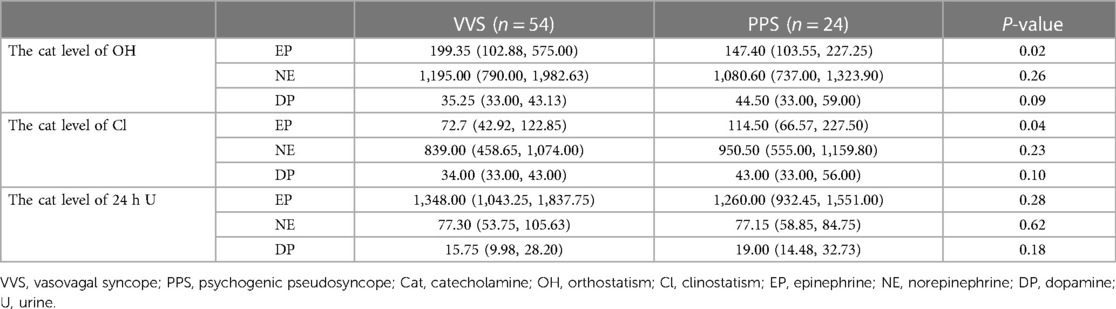
We then performed catecholamine levels analysis among VVS and PPS patients. As presented in Table 3, the serum NE [upright: 1,195.00 (790.00, 1,982.63) pmol/L vs. 1,080.60 (737.00, 1,323.90) pmol/L, p > 0.05; supine: 72.7 839.00, (458.65, 1,074.00) pmol/L vs. 950.50 (555.00, 1,159.80) pmol/L, p > 0.05] and NP [upright: 35.25 (33.00, 43.13) pmol/L vs. 44.50 (33.00, 59.00) pmol/L, p > 0.05; supine: 34.00 (33.00, 43.00) pmol/L vs. 43.00 (33.00, 56.00) pmol/L, p > 0.05] levels did not differ between groups, regardless of the body position. Notably, relative to patients with VVS, those with PPS had significantly lower serum EP levels [199.35 (102.88, 575.00) vs. 147.40 (103.55, 227.25) pmol/L, p < 0.05] in the upright position and higher serum EP levels [72.7 (42.92, 122.85) pmol/L vs. 114.5 (66.57, 227.50) pmol/L, p < 0.05] in the supine position. As for 24-h urine sample, no significant difference was observed in EP [1,348.00 (1,043.25, 1,837.75) nmol/24 h vs. 1,260.00 (932.45, 1,551.00) nmol/24 h, p > 0.05], NE [77.30 (53.75, 105.63) nmol/24 h vs. 77.15 (58.85, 84.75) nmol/24 h, p > 0.05] and NP [15.75 (9.98, 28.20) nmol/24 h vs. 19.00 (14.48, 32.73) nmol/24 h, p > 0.05] between groups.
Discussion
In the present study, we analyzed the clinical features, HUTT results, and catecholamine levels of children with VVS and PPS. Notably, we found that the serum EP level in upright posture and EP level in supine posture were statistical significance, suggesting that it can aid the differential diagnosis between VVS and PPS.
VVS is among the most common causes of syncope in children and adolescents and is triggered mainly by postural change and/or emotional stress (8, 20). It is characterized by sympathetic withdrawal and increased vagal tone (20). Most patients with VVS exhibit hypotension and bradycardia during attacks, which last for a few minutes and self-terminate (21). PPS is a TLOC entity, the prevalence of which may be underestimated in children (22, 23). It is believed to be a conversion (i.e., psychiatric) disorder. Generally, PPS attacks in children are induced by emotional stress, such as that caused by abuse, abandonment, or school phobia/transfer (9). This type of syncope usually occurs at rest, rather than during exertion. Presyncope symptoms may include dizziness, dyspnea with hyperventilation, and tingling (24). During a PPS episode, TLOC may last for several minutes or up to 50 min (22, 24). We initially assessed the demographic characteristics and clinical characteristics in VVS and PPS patients. Consistent with previous reports, no difference was observed in gender, age and BMI. Typical VVS was usually triggered by posture changes, while PPS was induced by emotional factors. Furthermore, syncope duration was significant longer in PPS patients when compared to VVS patients. Remarkably, patients with VVS regained complete consciousness within 1–2 min after syncope onset, whereas for PPS, the recovery time was approximately 8 min. A similar phenomenon was observed in another study (2, 11).
In clinical practice, physicians usually make initial differential diagnoses between these disorders based on clinical symptoms and HUTT results. In contrast to VVS, the typical feature of PPS is eye closure during an episode; another important difference is that the syncope duration is longer in PPS than in VVS. However, direct observation of entire episode courses rarely occurs in clinical settings. In this study, we also found that the syncope duration was longer in the PPS group than in the VVS group. Thus, a detailed information of disease during episode is helpful for disease diagnosis and management. However, it is not always possible to obtain such complete record in clinical settings. Indeed, evidence suggests that the number of PPS cases is grossly underestimated (11, 25). Consequently, finding a simple and reliable indicator for differential diagnosis between VVS and PPS is urgently needed.
Catecholamines, including EP, NE, and DP, are hormones play critical roles in regulating metabolism, immune function, BP, stress responses, and other essential biological processes (15, 26). However, whether the EP level can be used to distinguish VVS from PPS in children remains unclear. Herein, we found that the upright serum EP level was higher in patients with VVS than in those with PPS, whereas supine serum EP level was exactly the opposite. It suggests that the serum EP level in either upright or supine posture can serve as an auxiliary indicator for the differential diagnosis of PPS and VVS.
This study has some limitations. Due to the small sample, we could not determine the optimal cut-off value for the upright serum EP level for diagnosis. Thus, multi-center studies with large samples should be conducted in the future. Meanwhile, the practical application in clinic deserves further exploration.
Conclusion
Our results suggest that the serum EP level can be used as an auxiliary indicator for the differential diagnosis of VVS and PPS, in combination with clinical symptoms and HUTT results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Children’s Hospital of Hebei Province (Medical Ethics no. 24). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HW: Conceptualization, Methodology, Writing – review & editing. WM: Conceptualization, Methodology, Writing – original draft. MJ: Formal Analysis, Writing – review & editing. BL: Writing – review & editing. SS: Conceptualization, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Hebei Medical Science research project (no. 20200654), China.
Acknowledgments
We thank Medjaden, Inc. for the scientific editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tannemaat M, van Niekerk J, Reijntjes R, Thijs R, Sutton R, van Dijk J. The semiology of tilt-induced psychogenic pseudosyncope. Neurology. (2013) 81(8):752–8. doi: 10.1212/WNL.0b013e3182a1aa88
2. Villafane J, Miller J, Glickstein J, Johnson J, Wagner J, Snyder C, et al. Loss of consciousness in the young child. Pediatr Cardiol. (2021) 42(2):234–54. doi: 10.1007/s00246-020-02498-6
3. van Dijk N, Boer K, Colman N, Bakker A, Stam J, van Grieken J, et al. High diagnostic yield and accuracy of history, physical examination, and ECG in patients with transient loss of consciousness in FAST: the fainting assessment study. J Cardiovasc Electrophysiol. (2008) 19(1):48–55. doi: 10.1111/j.1540-8167.2007.00984.x
4. Kenny R, Brignole M, Dan G, Deharo J, van Dijk J, Doherty C, et al. Syncope unit: rationale and requirement–the European heart rhythm association position statement endorsed by the heart rhythm society. Europace. (2015) 17(9):1325–40. doi: 10.1093/europace/euv115
5. Anderson J, Willis M, Lancaster H, Leonard K, Thomas C. The evaluation and management of pediatric syncope. Pediatr Neurol. (2016) 55:6–13. doi: 10.1016/j.pediatrneurol.2015.10.018
6. Zou R, Wang S, Wen W, Cai H, Wang Y, Liu P, et al. Risk factors and prognostic follow-up of vasovagal syncope children with seizure-like activities during head-up tilt test induced-syncope. Front Cardiovasc Med. (2022) 9:916542. doi: 10.3389/fcvm.2022.916542
7. Walsh K, Baneck T, Page R, Brignole M, Hamdan M. Psychogenic pseudosyncope: not always a diagnosis of exclusion. Pacing Clin Electrophysiol. (2018) 41(5):480–86. doi: 10.1111/pace.13316
8. Ali M, Pachon Maetos J, Kichloo A, Masudi S, Grubb B, Kanjwal K. Management strategies for vasovagal syncope. Pacing Clin Electrophysiol. (2021) 44(12):2100–08. doi: 10.1111/pace.14402
9. Liao Y, Du J, Benditt D, Jin H. Vasovagal syncope or psychogenic pseudosyncope: a major issue in the differential diagnosis of apparent transient loss of consciousness in children. Sci Bull. (2022) 67(16):1618–20. doi: 10.1016/j.scib.2022.07.024
10. Li C, Zhang Y, Liao Y, Han L, Zhang Q, Fu J, et al. Differential diagnosis between psychogenic pseudosyncope and vasovagal syncope in children: a quantitative scoring model based on clinical manifestations. Front Cardiovasc Med. (2022) 9:839183. doi: 10.3389/fcvm.2022.839183
11. Zhang Z, Jiang X, Han L, Chen S, Tao L, Tao C, et al. Differential diagnostic models between vasovagal syncope and psychogenic pseudosyncope in children. Front Neurol. (2019) 10:1392. doi: 10.3389/fneur.2019.01392
12. Benditt D, van Dijk J, Krishnappa D, Adkisson W, Sakaguchi S. Neurohormones in the pathophysiology of vasovagal syncope in adults. Front Cardiovasc Med. (2020) 7:76. doi: 10.3389/fcvm.2020.00076
13. Chosy J, Graham D. Catecholamines in vasovagal fainting. J Psychosom Res. (1965) 9(2):189–94. doi: 10.1016/0022-3999(65)90032-2
14. Jardine D, Melton I, Crozier I, Bennett S, Donald R, Ikram H. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am J Cardiol. (1997) 79(9):1302–6. doi: 10.1016/s0002-9149(9x)00084-9
15. Eisenhofer G, Kopin I, Goldstein D. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. (2004) 56(3):331–49. doi: 10.1124/pr.56.3.1
16. Ermis C, Samniah N, Lurie K, Sakaguchi S, Benditt D. Adrenal/renal contribution to circulating norepinephrine in posturally induced neurally mediated reflex syncope. Am J Cardiol. (2003) 91(6):746–50. doi: 10.1016/s0002-9149(02)03422-7
17. Kohno R, Detloff B, Chen L, Norby F, Benditt D. Greater early epinephrine rise with head-up posture: a marker of increased syncope susceptibility in vasovagal fainters. J Cardiovasc Electrophysiol. (2019) 30(3):289–96. doi: 10.1111/jce.13792
18. Wang C, Li Y, Liao Y, Tian H, Huang M, Dong X, et al. 2018 Chinese pediatric cardiology society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull (Beijing). (2018) 63(23):1558–64. doi: 10.1016/j.scib.2018.09.019
19. First M. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. (2013) 201(9):727–9. doi: 10.1097/NMD.0b013e3182a2168a
20. Moloney D, Romero-Ortuno R, Kenny R. Vasovagal syncope. JAMA Intern Med. (2021) 181(6):880. doi: 10.1001/jamainternmed.2020.9151
21. Li H, Gao L, Yuan Y. Advance in the understanding of vasovagal syncope in children and adolescents. World J Pedia. (2021) 17(1):58–62. doi: 10.1007/s12519-020-00367-z
22. Raj V, Rowe A, Fleisch S, Paranjape S, Arain A, Nicolson S. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci. (2014) 184:66–72. doi: 10.1016/j.autneu.2014.05.003
23. Alciati A, Shiffer D, Dipaola F, Barbic F, Furlan R. Psychogenic pseudosyncope: clinical features, diagnosis and management. J Atr Fibrillation. (2020) 13(1):2399. doi: 10.4022/jafib.2399
24. Tannemaat M, Thijs R, van Dijk J. Managing psychogenic pseudosyncope: facts and experiences. Cardiol J. (2014) 21(6):658–64. doi: 10.5603/CJ.a2014.0070
25. Benbadis S, Chichkova R. Psychogenic pseudosyncope: an underestimated and provable diagnosis. Epilepsy Behav. (2006) 9(1):106–10. doi: 10.1016/j.yebeh.2006.02.011
Keywords: vasovagal syncope, psychogenic pseudosyncope, catecholamine, differential diagnosis, head-up tilt tests
Citation: Wang H, Ma W, Jin M, Li B and Sun S (2024) Value of catecholamine levels in the differential diagnosis of vasovagal syncope and psychogenic pseudosyncope in children. Front. Pediatr. 12:1281196. doi: 10.3389/fped.2024.1281196
Received: 2 October 2023; Accepted: 20 May 2024;
Published: 31 May 2024.
Edited by:
Marco Carotenuto, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Shuo Wang, Central South University, ChinaEwelina Kolarczyk, Medical University of Silesia, Poland
© 2024 Wang, Ma, Jin, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzhen Sun, c3V6aGVuc3VuMTk4OEAxNjMuY29t
 Hua Wang
Hua Wang Wandong Ma4
Wandong Ma4 Mei Jin
Mei Jin