- 1Research Centre for Medical Genetics, Moscow, Russia
- 2Veltischev Research and Clinical Institute for Pediatrics of the Pirogov Russian National Research Medical University, Moscow, Russia
- 3Genomed Ltd., Moscow, Russia
- 4Republican Research and Clinical Center of Neurology and Neurosurgery, Minsk, Belarus
Congenital myasthenic syndrome with episodic apnea is associated with pathogenic variants in the CHAT gene. While respiratory disorders and oculomotor findings are commonly reported in affected individuals, a subset of patients only present with muscle weakness and/or ptosis but not apneic crises. In this case series, we describe five individuals with exercise intolerance caused by single nucleotide variants in the CHAT gene. The age of onset ranged from 1 to 2.5 years, and all patients exhibited a fluctuating course of congenital myasthenic syndrome without disease progression over several years. Notably, these patients maintained a normal neurological status, except for the presence of abnormal fatigability in their leg muscles following prolonged physical activity. We conducted a modified protocol of repetitive nerve stimulation on the peroneal nerve, revealing an increased decrement in amplitude and area of compound muscle action potentials of the tibialis anterior muscle after 15–20 min of exercise. Treatment with 3,4-diaminopyridine showed clear improvement in two children, while one patient experienced severe adverse effects and is currently receiving a combination of Salbutamol Syrup and pyridostigmine with slight positive effects. Based on our findings and previous cases of early childhood onset with muscle fatigability as the sole manifestation, we propose the existence of a mild phenotype characterized by the absence of apneic episodes.
1 Introduction
Congenital myasthenic syndromes (CMSs) comprise a diverse group of hereditary neuromuscular junction disorders characterized by exercise intolerance and muscle weakness that worsens with fatigue. Some CMSs manifest with ocular and bulbar symptoms, such as ptosis, ophthalmoplegia, and respiratory disturbances. Currently, 35 genes have been associated with CMS (1). Congenital myasthenic syndromes (CMSs) can be classified based on various features, encompassing the type of inheritance (autosomal dominant, autosomal recessive), the location of the mutant protein (presynaptic, synaptic, postsynaptic, abnormal glycosylation of acetylcholine receptor subunits), the primary clinical presentation (limb-girdle, ocular), the response to acetylcholine inhibitors, and the long-term course (progressive, fluctuating, regressive) (2, 3).
Pathogenic variants in the CHAT gene are associated with CMS with episodic apnea (CMS-EA), also known as CMS type 6. The first case of CMS-EA was published 20 years ago (4), and since then, approximately 50 patients with CMS-EA have been reported (3, 5). The CHAT gene encodes choline acetyltransferase, an enzyme involved in the resynthesis of acetylcholine in the nerve terminal (6, 7). CMS-EA is an autosomal-recessive presynaptic disorder, with most known variants in the CHAT gene being missense variants (8).
The majority of reported patients with CMS-EA exhibit respiratory disorders and/or oculomotor findings (9). Typically, CMS-EA presents in infancy, characterized by apnea or respiratory insufficiency. However, there have been only a few reported cases without apneic crises, and only one case without both respiratory and oculomotor disturbances (10–14).
In this study, we present five patients from four families with compound heterozygous variants in the CHAT gene. Remarkably, all patients exhibit a mild phenotype of CMS without respiratory signs or oculomotor weakness.
2 Subjects and methods
Between 2019 and 2022, genetic testing confirmed a diagnosis of CHAT-associated CMS in five children (four boys and one girl) from four families. All parents provided informed consent for their children's participation in the study.
The patients were diagnosed at the age of 7–15 years, while the manifestation of the disease occurred at 1–2.5 years of age. Two siblings reside in Belarus, while the remaining three unrelated patients are Russian, residing in different regions of Russia.
Neurological examinations and electrophysiological studies were conducted on all patients (Dantec Keypoint G2, Denmark and Neuro-MEP-micro, Neurosoft, Ivanovo, Russia). The electrophysiological studies included nerve conduction studies and repetitive nerve stimulation (RNS). Standard electrodiagnostic techniques were employed, involving single or repetitive supramaximal nerve stimulation. RNS was performed using a 3 Hz stimulation of the facial nerve (with registration from the orbicularis oculi muscle) and axillary nerve (deltoid muscle) with a 20 s muscle activation period. In addition, a modified 3 Hz RNS technique was applied to the peroneal nerve (tibialis anterior muscle). This procedure was performed immediately following a 15 min session of voluntary physical exercises, with a subsequent RNS applied at the 2–4 min of the rest period. The physical exercises consisted of activities such as walking, running, and stair climbing.
All patients underwent next-generation sequencing (NGS), including either whole-exome sequencing (WES) or whole-genome sequencing (WGS). The WES analysis was carried out using paired-end sequencing (2 × 150 bp) on an IlluminaNextSeq 500 sequencer (Illumina, San Diego, California, U.S.). The library was constructed using the KAPA Hyper Prep Kit (F. Hoffmann-La Roche Ltd, Switzerland). Target enrichment was performed with an IDT xGen® Exome Research Panel v.1 solution capture array (IDT inc., USA), including the coding regions of 19,396 known genes. The detected variants were annotated according to the HGVS (Human Genome Variation Society) nomenclature: https://varnomen.hgvs.org/recommendations/DNA/ (version 20.05) (HGVS, RRID:SCR_012989). The sequencing data were analyzed using the NGS-data-Genome program developed at the Department of Bioinformatics of FSBSI RCMG (registration number 2021662113). Mean coverage was ×76.1, with 0.68% of fragments with less than ×10 coverage. The following predictive algorithms to analyze the pathogenicity of the variants were used: SIFT (Sort Intolerant from Tolerant Human Protein) (SIFT, RRID:SCR_012813) (15), DEOGEN2 (16), MVP (17), and MutationAssesor (18). The detected variants were classified according to the ACMG/AMP guidelines (19). Whole exome sequencing was performed using the equipment of the Genome Shared Use Center (FSBSI RCMG, Moscow, Russia).
WGS was performed using a DNBSEQ-G400 instrument in a pair-ended mode (2 × 150 b.p.) with an average on-target coverage of 30× with MGIEasy FS PCR-Free DNA Library Prep Set (BGI, Beijing, China) for library preparation (Genomed Ltd., Moscow, Russia). Bioinformatic analysis was performed using an in-house software pipeline as described earlier (20) with modifications. In brief, it included quality control of raw reads (FastQC tool v. 0.11.5) followed by read mapping to the hg19 human genome assembly (minimap2 v.2.24-r1122), sorting of the alignments, and marking duplicates (Picard Toolkit v. 2.18.14). Base recalibration and variant calling were performed with GATK3.8. Variant annotation was done using Annovar tool (v.2018Apr16). CNV and SV analysis was performed using Manta tool (v. 1.6.0). Further filtering was performed by functional consequences and population frequencies according to the ACMG recommendations as well as clinical relevance determined by Human Phenotype Ontology database (21).
Segregation analysis and validation of all detected variants were performed by Sanger sequencing carried out using ABIPrism 3,500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Primer sequences were designed and variants were named according to the NM_020549.5 transcript variant.
3 Case descriptions
Five patients from four unrelated families were diagnosed with CHAT-associated myasthenic syndrome. A total of six different single nucleotide variants (SNVs) were identified through NGS studies, with two of them being previously reported. Among these variants, one is a nonsense variant, while the others are missense variants. Three SNVs are classified as pathogenic or likely pathogenic, while the remaining three SNVs are categorized as variants of uncertain significance (Supplementary Table S1).
The first case is a 7-year-old boy (1.1) who presented with episodic weakness in his leg muscles. The proband is the first child of healthy, non-consanguineous Russian parents. The pregnancy and delivery proceeded without complications, and early motor development was unremarkable. The initial episode of severe muscle weakness occurred at the age of 2.5 years while the patient was swimming in a lake with cool water and was unable to exit independently due to muscle weakness. Subsequently, episodes of moderate to severe weakness in the leg muscles would typically occur after walking a distance of around 500 meters. In some instances, the weakness would worsen up to an ambulatory loss. However, these episodes lasted no longer than a few minutes, and muscle strength fully recovered after resting. No other complaints or symptoms were reported. Neurological examination revealed no signs of muscle weakness or fatigability after 2–3 min of exercise or after 10–15 squats. No other neurological abnormalities were detected. Initially, the patient was referred for gene panel sequencing associated with periodic paralysis, but no SNVs were identified. Subsequently, whole-genome sequencing (WGS) was conducted, which revealed two SNVs in the CHAT gene (NM_020549.5). One of the SNVs was a previously reported nonsense variant c.451C>T, p.(Arg151Ter) (22, 23), and the other was a novel missense variant c.404C>G, p.(Pro135Arg). Validation and segregation analysis confirmed the compound heterozygous state of the detected SNVs. The treatment with pyridostigmine did not yield a positive response. Further outcome information is unavailable due to loss of communication with proband's parents.
The second patient (proband 2.1) diagnosed with CHAT-associated CMS is an 8-year-old boy who experiences similar complaints of severe leg muscle fatigability after walking for 15 min. The proband was born at 39 weeks of gestation from a normal first pregnancy, with a normal height and weight. The parents first noticed symptoms when the boy was 2 years old, as he frequently complained of fatigue while walking. The disease has not progressed, and for several years, it has manifested only with this one symptom. A routine clinical examination revealed a normal neurological status, with no signs of facial or limb muscle weakness during rest or after 5 min of exercises. WGS identified two heterozygous SNVs in the CHAT gene: c.404C>G, p.(Pro135Arg) and c.1156T>C, p.(Cys386Arg). Both variants are novel. Segregation analysis confirmed that each parent is an asymptomatic carrier of one of these variants. Notably, a different substitution at the amino acid position c.403C>T, p.(Pro135Ser) has previously been submitted in ClinVar database as a variant of uncertain significance in patient with gait disturbances and muscle weakness. The patient was given 3,4-diaminopyridine at a daily dose of 15 mg, resulting in severe adverse effects, including blurred vision and generalized tonic-clonic seizures. AChE inhibitor therapy (pyridostigmine 180 mg/day) demonstrated insufficient effectiveness. Consequently, Salbutamol Syrup (2 mg/day) was added to the treatment, exhibiting a slight positive effect by increasing the patient's walking distance.
Two male siblings (3.1 and 3.2) exhibited different disease onset and clinical presentations. The older brother experienced a waddling gait and frequent falls starting from the age of 1 year when he began walking independently. These symptoms persisted until around 3–4 years of age. Subsequently, the gait disturbance occurred episodically following physical activity, and the disease stabilized for several years. At the age of 12 years, the patient noticed improvement due to adapting to physical limitations and adopting an appropriate daily activity regimen. Currently, the patient experiences milder symptoms, primarily in the form of transient fatigue. The most recent neurological examination was conducted at the age of 15 years, revealing pathological muscle fatigue only after 35 squats, resulting in a decrease in proximal leg muscle strength to 4/5 (MRC scale). The second boy (3.2) demonstrated increased fatigability during normal physical activities compared to children of the same age, starting at 2 years old. The parents also observed changes in gait following prolonged walking. Both siblings experienced worsening of symptoms during the cold winter season. The younger boy underwent examination at the age of 10 years. After performing repetitive leg lifts and head lifts in the prone position (20 times and 25 times, respectively), a decrease in leg and neck muscle strength to 3/5 was observed. WES was conducted for the younger brother, revealing two SNVs in the CHAT gene: one previously reported variant c.1061C>T, p.(Thr354Met) (24–26) and one novel variant c.1841C>G, p.(Ala614Gly). Both SNVs were also identified in the older brother. Each parent is an asymptomatic carrier of one of these variants. AChE inhibitor therapy showed no positive response in both children. Subsequently, they received a dosage of 40 mg/day of 3,4-diaminopyridine and demonstrated clear improvement, as evidenced by an increase in the distance walked without experiencing fatigue.
The fifth proband is an 11-year-old girl who experiences episodic weakness in her leg muscles associated with physical exercises or walking. Fatigability typically occurs after walking approximately 1 km, and muscle strength is restored within several minutes of rest. The disease initially manifested at the age of 1.5 years and is characterized by a gait change while walking. Since then, no new symptoms have occurred, and the disease has shown no signs of progression. Neurological examination revealed decreased strength in the thigh and lower leg muscles only after 15 min of walking and climbing stairs. Muscle weakness following exercise resulted in a waddling gait and Gowers sign when standing up from the floor. The upper limb muscles remain unaffected, and no other neurological abnormalities were detected. Through WES, two single nucleotide variants (SNVs) in a heterozygous state were identified in the CHAT gene: c.1061C>T, p.(Thr354Met), and c.971T>G, p.(Leu324Arg). Sanger sequencing was performed for the proband and her parents and confirmed the compound heterozygous state of these SNVs. Pyridostigmine treatment was initiated, but no apparent positive effects were observed within the first 2 weeks.
All five probands underwent nerve conduction studies and RNS of the facial, ulnar, and peroneal nerves. The compound muscle action potentials (CMAP) in all patients exhibited normal amplitude and unchanged form. Standard 3 Hz RNS with 20 s of muscle exercise revealed no abnormalities in all patients. However, considering the development of weakness in the legs after 15–20 min of walking, running, or climbing stairs, the RNS protocol was modified. The RNS study was performed on the peroneal nerve with CMAP registration from the tibialis anterior muscle after 15 min of voluntary muscle activation (Figure 1). In all patients, an increased decrement in amplitude and area of negative CMAP, ranging from 22% to 37%, was detected. This decrement normalized after a rest period of 3–5 min.
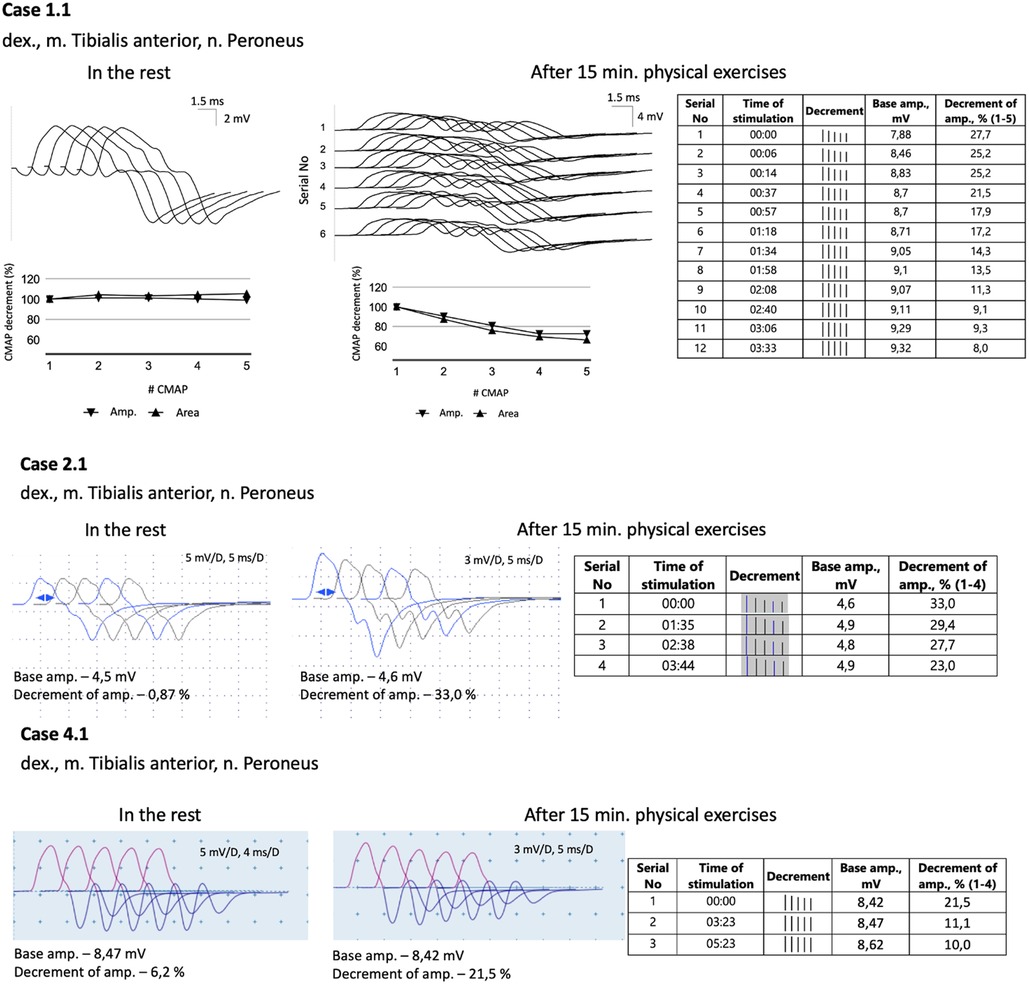
Figure 1. A modified 3 Hz RNS technique applied to the peroneal nerve with CMAPs registration from tibialis anterior muscle. This procedure was carried out after 15 min of physical exercises, which included walking, running, and stair climbing. Subsequently, a 3 Hz RNS was performed after a rest period of 2–5 min.
4 Discussion
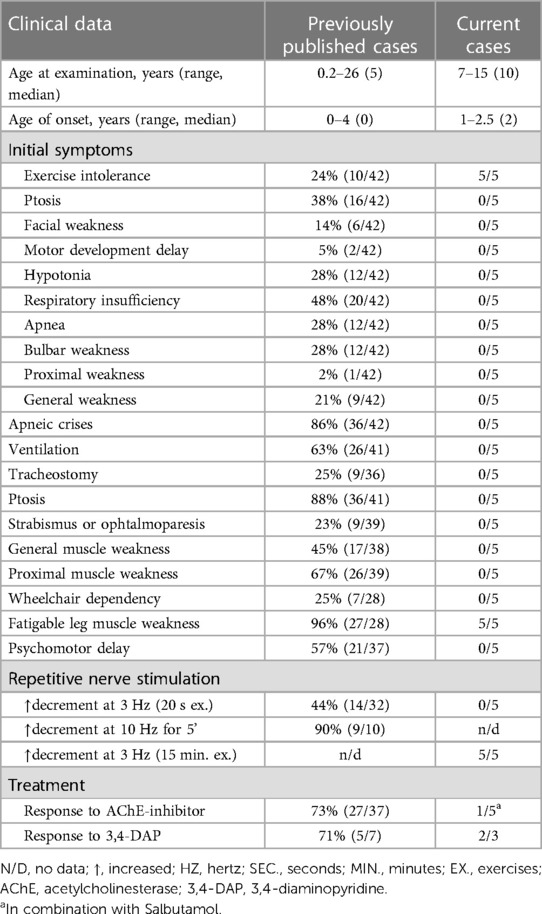
We present a case series of five individuals with CHAT-associated CMS who exhibited solely exercise intolerance in their leg muscles. Our analysis included a comprehensive review of the literature, identifying a total of 42 cases with clinical descriptions (Table 1). Among these cases, one patient presented with isolated muscle fatigability (10), while an additional five patients demonstrated muscle weakness and ptosis but did not experience apneic crises (11–14). Notably, previously published cases have reported a more severe disease course, characterized by episodic or permanent respiratory disturbances (4, 9–13, 24, 25–35). In nine patients, tracheostomy was necessary to provide respiratory support. The majority of the reported patients (90%, n = 38) had disease onset in infancy, with the latest reported onset occurring at the age of four years in a single case report (10). The initial symptoms displayed considerable variation, with four patients presenting isolated exercise intolerance, while most cases exhibited a combination of apnea, hypotonia, and general weakness.
In our cohort of five patients, exercise intolerance was the initial and sole symptom observed (Table 1). The age of onset ranged between 1 and 2.5 years. Importantly, all patients consistently demonstrated a normal neurological status, with the sole exception being the presence of abnormal muscle fatigability subsequent to prolonged physical activity. None of our patients presented with ptosis or ophthalmoparesis, which is in contrast to earlier reported cases where 88% of patients demonstrated ocular symptoms (Table 1).
All five of our patients exhibited a fluctuating course of CMS without progression over the course of several years. Long-term follow-up studies focusing on patients with CHAT-associated CMS have been conducted (11, 27). Schara et al. proposed the existence of two distinct phenotypes based on age of onset and clinical progression: neonatal onset and manifestation in early infancy (11). The first group of patients experiences an extremely severe clinical course, with some individuals presenting seizures and intellectual disability. Fatal outcomes during infancy due to CHAT-associated CMS have also been documented (11, 28). However, patients with early infantile manifestation often show symptom improvement and a reduction in the number of respiratory crises (11, 27). Considering our patients and additional cases previously described with onset in early childhood and solely presenting muscle fatigability as the single sign of the disease, we propose the existence of a third mild phenotype characterized by the absence of apneic episodes.
Three out of the five patients in our cohort experienced symptom worsening in exposure to cold. This phenomenon has been previously described in five patients with CHAT-associated CMS (12, 13, 28). The presence of this symptom is unique to CHAT-associated CMS when compared to other forms of CMS and can serve as a valuable clinical clue for diagnosis (28). It is possible that symptom worsening in the cold is more common in CHAT-associated CMS, but patients or their parents may not pay adequate attention to it, or it may be rarely detected due to the onset of the disease in infancy in the majority of patients. Studies in Drosophila have demonstrated a significant reduction in ChAT activity and impairment of synaptic transmission, which could explain the exacerbation of symptoms in the cold in patients with CHAT-associated CMS (36).
Diverse results of RNS studies in CHAT-associated CMS have been reported in the literature. Only a small percentage of patients show a decrement of more than 10% during standard 3 Hz nerve stimulation (11, 12, 27–29). As a result, alternative RNS methods have been proposed and utilized for patients with CMS. One commonly employed method is prolonged subtetanic repetitive nerve stimulation at 10 Hz for 5 min, which has yielded positive results in 9 out of 10 patients (Table 1). However, this method and single-fiber electromyography can be rather painful, especially for children. In light of these considerations, we decided to modify the 3 Hz RNS protocol by conducting it after 15 min of physical exercises. Since all the children in our study exhibited weakness in their leg muscles, we chose to register CMAP with tibialis anterior muscle in response to peroneal nerve stimulation. Immediately following the exercises, we observed an increased decrement of up to 22%–37%, which improved significantly within 3–4 min of rest. Thus, we found this modification of RNS to be informative and applicable even for young children.
The management of CHAT-associated CMS presents challenges with regard to treatment. Acetylcholinesterase inhibitors (AChE inhibitors) are typically the first-line therapy, resulting in positive outcomes for approximately 73% of patients (Table 1). However, in cases where there is a limited or absent response, the combination of 3,4-diaminopyridine with AChE inhibitors is considered (11). Within our cohort, only one patient exhibited a mild positive response to treatment with AChE inhibitors in combination with Salbutamol Syrup. Other our patients demonstrated no apparent positive effects from AChE inhibitors therapy, potentially attributed to the delayed initiation of treatment in all cases (ranging from 5 to 14 years from age of onset). Two patients demonstrated significant symptom improvement when treated with 3,4-diaminopyridine. All our patients exhibited a mild disease course since childhood without obvious signs of pronounced disease progression. This leads us to cautiously predict a favorable prognosis for patients. However, the scarcity of cases depicting a mild phenotype makes it challenging to definitively ascertain the long-term prognosis for such patients.
Four novel missense SNVs were identified in our study. Three of these SNVs are currently classified as variants of unknown significance, while one SNV [c.404C>G, p.(Pro135Arg)] is considered likely pathogenic due to a different missense change detected at the same position. In two of our families, a previously reported missense variant [c.1061C>T, p.(Thr354Met)] was found which has been associated with a severe course of CMS characterized by onset in infancy and episodic apneic crises in several patients (24–26). Interestingly, we also observed a nonsense variant that has not been previously reported in patients with CMS but has been documented in individuals with conotruncal defect (transposition of the great arteries) in a heterozygous state with de novo origin (22, 23). No second variant in the CHAT gene was identified in the two patients with the cardiac phenotype. However, no cardiac defects were observed in our patient with this variant or in their relatives.
Establishing precise phenotype-genotype correlations in autosomal recessive diseases presents challenges due to the genotypic heterogeneity within patient cohorts. Numerous cohort studies have been conducted to explore genotype-phenotype correlations in CHAT-associated CMS (11, 12, 30). CHAT is an enzyme composed of binding and catalytic domains, with an interfacial active-site tunnel (37, 38). Previous studies have attempted to establish a correlation between the severity of clinical manifestations and the proximity of the variant to the substrate binding and catalytic sites, revealing phenotype-genotype correlations for specific residues (12, 30). Notably, variants in our cohort are uniformly distributed across the entire gene (Figure 2). Contrary to expectations, our analysis revealed that patients sharing the same genotype (e.g., homozygous for I336T) exhibit a broad spectrum of clinical severity (Supplementary Table S2). Furthermore, our observations suggest that loss-of-function variants in the CHAT gene may not be exclusively associated with a severe disease course. This inference is supported by both our cohort and previously published cases, where individuals with these variants manifest both mild and severe phenotypes of the disease. To gain a more comprehensive understanding, further functional analysis of novel variants in the CHAT gene is imperative, particularly focusing on cases exhibiting a milder disease course that has been identified.
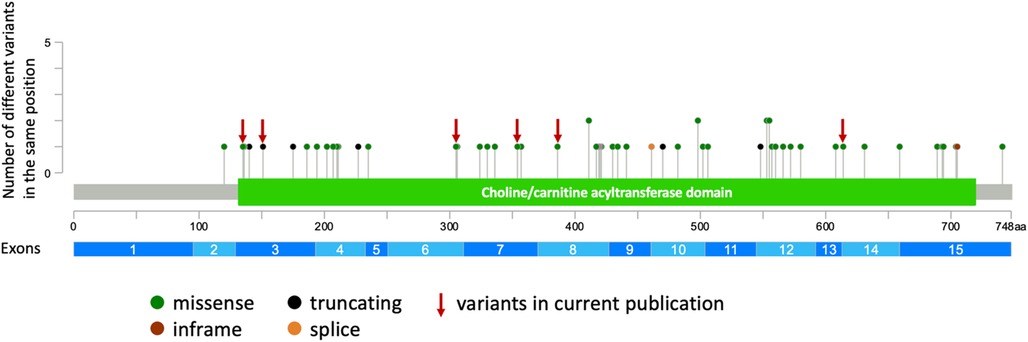
Figure 2. The representation of all described and novel variants in the CHAT gene (generated using cBioPortal https://www.cbioportal.org/mutation_mapper).
In conclusion, our findings add to the existing literature, which includes six previously published cases without respiratory disorders, and highlight the existence of a milder variant of CHAT-associated CMS. This subgroup of patients predominantly presents with abnormal muscle fatigue as the sole or predominant sign, distinguishing them from the more severe presentations reported earlier. Additionally, we introduce a modified RNS protocol that is specifically tailored for pediatric patients with CMS. This modified protocol offers an informative and reproducible approach for assessing neuromuscular function in this patient population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Research Centre for Medical Genetics, Moscow, Russia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing – original draft. AB: Conceptualization, Investigation, Resources, Writing – original draft. AMa: Methodology, Writing – review & editing. AS: Investigation, Resources, Writing – review & editing. IS: Investigation, Resources, Writing – review & editing. AMi: Resources, Writing – review & editing. SvK: Resources, Writing – review & editing. RG: Visualization, Writing – original draft. VZ: Methodology, Writing – review & editing. OR: Methodology, Writing – review & editing. SN: Investigation, Resources, Writing – review & editing. ED: Supervision, Writing – review & editing. SeK: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was carried out within the state assignment of the Ministry of Science and Higher Education of the Russian Federation for Research Centre for Medical Genetics.
Conflict of interest
AS is employed by Genomed Ltd., Russia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1280394/full#supplementary-material
References
1. Ohno K, Ohkawara B, Shen XM, Selcen D, Engel AG. Clinical and pathologic features of congenital myasthenic syndromes caused by 35 genes-a comprehensive review. Int J Mol Sci. (2023) 24(4):3730. doi: 10.3390/ijms24043730
2. Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. (2015) 14:420–34. doi: 10.1016/S1474-4422(14)70201-7
3. Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis. (2019) 14:57. doi: 10.1186/s13023-019-1025-5
4. Ohno K, Tsujino A, Brengman JM, Harper CM, Bajzer Z, Udd B, et al. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci U S A. (2001) 98:2017–22. doi: 10.1073/pnas.98.4.2017
5. Zhang Y, Cheng X, Luo C, Lei M, Mao F, Shi Z, et al. Congenital myasthenic syndrome caused by a novel hemizygous CHAT mutation. Front Pediatr. (2020) 8:185. doi: 10.3389/fped.2020.00185
6. Rossier J. Choline acetyltransferase: a review with special reference to its cellular and subcellular localization. Int Rev Neurobiol. (1977) 20:283–337. doi: 10.1016/s0074-7742(08)60656-x
7. Mora M, Lambert EH, Engel AG. Synaptic vesicle abnormality in familial infantile myasthenia. Neurology. (1987) 37:206–14. doi: 10.1212/wnl.37.2.206
8. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. (2014) 133:1–9. doi: 10.1007/s00439-013-1358-4
9. Arican P, Gencpinar P, Cavusoglu D, Olgac Dundar N. Clinical and genetic features of congenital myasthenic syndromes due to CHAT mutations: case report and literature review. Neuropediatrics. (2018) 49:283–8. doi: 10.1055/s-0038-1654706
10. Natera-de Benito D, Töpf A, Vilchez JJ, González-Quereda L, Domínguez-Carral J, Díaz-Manera J, et al. Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul Disord. (2017) 27:1087–98. doi: 10.1016/j.nmd.2017.08.003
11. Schara U, Christen HJ, Durmus H, Hietala M, Krabetz K, Rodolico C, et al. Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Paediatr Neurol. (2010) 14:326–33. doi: 10.1016/j.ejpn.2009.09.009
12. Arredondo J, Lara M, Gospe SM Jr, Mazia CG, Vaccarezza M, Garcia-Erro M, et al. Choline acetyltransferase mutations causing congenital myasthenic syndrome: molecular findings and genotype-phenotype correlations. Hum Mutat. (2015) 36:881–93. doi: 10.1002/humu.22823
13. Tan JS, Ambang T, Ahmad-Annuar A, Rajahram GS, Wong KT, Goh KJ. Congenital myasthenic syndrome due to novel CHAT mutations in an ethnic kadazandusun family. Muscle Nerve. (2016) 53:822–6. doi: 10.1002/mus.25037
14. Öztürk S, Güleç A, Erdoğan M, Demir M, Canpolat M, Gümüş H, et al. Congenital myasthenic syndromes in Turkey: clinical and molecular characterization of 16 cases with three novel mutations. Pediatr Neurol. (2022) 136:43–9. doi: 10.1016/j.pediatrneurol.2022.08.001
15. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. (2003) 31:3812–4. doi: 10.1093/nar/gkg509
16. Raimondi D, Tanyalcin I, Ferté J, Gazzo A, Orlando G, Lenaerts T, et al. DEOGEN2: prediction and interactive visualization of single amino acid variant deleteriousness in human proteins. Nucleic Acids Res. (2017) 45:W201–6. doi: 10.1093/nar/gkx390
17. Qi H, Zhang H, Zhao Y, Chen C, Long JJ, Chung WK, et al. MVP predicts the pathogenicity of missense variants by deep learning. Nat Commun. (2021) 12:510. doi: 10.1038/s41467-020-20847-0
18. Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. (2011) 39:e118. doi: 10.1093/nar/gkr407
19. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
20. Marakhonov AV, Konovalov FA, Makaov AK, Vasilyeva TA, Kadyshev VV, Galkina VA, et al. Primary microcephaly case from the Karachay-Cherkess Republic poses an additional support for microcephaly and seckel syndrome spectrum disorders. BMC Med Genomics. (2018) 11:8. doi: 10.1186/s12920-018-0326-1
21. Köhler S, Gargano M, Matentzoglu N, Carmody LC, Lewis-Smith D, Vasilevsky NA, et al. The human phenotype ontology in 2021. Nucleic Acids Res. (2021) 49:D1207–17. doi: 10.1093/nar/gkaa1043
22. Edwards JJ, Rouillard AD, Fernandez NF, Wang Z, Lachmann A, Shankaran SS, et al. Systems analysis implicates WAVE2 complex in the pathogenesis of developmental left-sided obstructive heart defects. JACC Basic Transl Sci. (2020) 5:376–86. doi: 10.1016/j.jacbts.2020.01.012
23. Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. (2017) 49:1593–601. doi: 10.1038/ng.3970
24. Barisic N, Müller JS, Paucic-Kirincic E, Gazdik M, Lah-Tomulic K, Pertl A, et al. Clinical variability of CMS-EA (congenital myasthenic syndrome with episodic apnea) due to identical CHAT mutations in two infants. Eur J Paediatr Neurol. (2005) 9:7–12. doi: 10.1016/j.ejpn.2004.10.008
25. Tofilo M, Voskoboeva E, Timonina V, Staufner C, Hoffmann GF, Poschl J, et al. A new family with congenital myasthenic syndrome caused by compound heterozygous CHAT mutations. Biomed J Sci Tech Res. (2022) 44:35972–83. doi: 10.26717/BJSTR.2022.44.007122
26. Mallory LA, Shaw JG, Burgess SL, Estrella E, Nurko S, Burpee TM, et al. Congenital myasthenic syndrome with episodic apnea. Pediatr Neurol. (2009) 41:42–5. doi: 10.1016/j.pediatrneurol.2009.02.017
27. McMacken G, Whittaker RG, Evangelista T, Abicht A, Dusl M, Lochmüller H. Congenital myasthenic syndrome with episodic apnoea: clinical, neurophysiological and genetic features in the long-term follow-up of 19 patients. J Neurol. (2018) 265:194–203. doi: 10.1007/s00415-017-8689-3
28. Maselli RA, Chen D, Mo D, Bowe C, Fenton G, Wollmann RL. Choline acetyltransferase mutations in myasthenic syndrome due to deficient acetylcholine resynthesis. Muscle Nerve. (2003) 27:180–7. doi: 10.1002/mus.10300
29. Schmidt C, Abicht A, Krampfl K, Voss W, Stucka R, Mildner G, et al. Congenital myasthenic syndrome due to a novel missense mutation in the gene encoding choline acetyltransferase. Neuromuscul Disord. (2003) 13:245–51. doi: 10.1016/s0960-8966(02)00273-0
30. Shen XM, Crawford TO, Brengman J, Acsadi G, Iannaconne S, Karaca E, et al. Functional consequences and structural interpretation of mutations of human choline acetyltransferase. Hum Mutat. (2011) 32:1259–67. doi: 10.1002/humu.21560
31. Liu Z, Zhang L, Shen D, Ding C, Yang X, Zhang W, et al. Compound heterozygous CHAT gene mutations of a large deletion and a missense variant in a Chinese patient with severe congenital myasthenic syndrome with episodic apnea. Front Pharmacol. (2019) 10:259. doi: 10.3389/fphar.2019.00259
32. Kraner S, Laufenberg I, Strassburg HM, Sieb JP, Steinlein OK. Congenital myasthenic syndrome with episodic apnea in patients homozygous for a CHAT missense mutation. Arch Neurol. (2003) 60:761–3. doi: 10.1001/archneur.60.5.761
33. Yeung WL, Lam CW, Fung LW, Hon KL, Ng PC. Severe congenital myasthenia gravis of the presynaptic type with choline acetyltransferase mutation in a Chinese infant with respiratory failure. Neonatology. (2009) 95:183–6. doi: 10.1159/000155612
34. Yiş U, Becker K, Kurul SH, Uyanik G, Bayram E, Haliloğlu G, et al. Genetic landscape of congenital myasthenic syndromes from Turkey: novel mutations and clinical insights. J Child Neurol. (2017) 32:759–65. doi: 10.1177/0883073817705252
35. Dilena R, Abicht A, Sergi P, Comi GP, Di Fonzo A, Chidini G, et al. Congenital myasthenic syndrome due to choline acetyltransferase mutations in infants: clinical suspicion and comprehensive electrophysiological assessment are important for early diagnosis. J Child Neurol. (2014) 29:389–93. doi: 10.1177/0883073812470000
36. Wang W, Kitamoto T, Salvaterra PM. Drosophila choline acetyltransferase temperature-sensitive mutants. Neurochem Res. (1999) 24:1081–7. doi: 10.1023/a:1021021213625
37. Kim AR, Rylett RJ, Shilton BH. Substrate binding and catalytic mechanism of human choline acetyltransferase. Biochemistry. (2006) 45:14621–31. doi: 10.1021/bi061536l
Keywords: CHAT, congenital myasthenic syndrome, CMS, mild phenotype, case report
Citation: Murtazina A, Borovikov A, Marakhonov A, Sharkov A, Sharkova I, Mirzoyan A, Kulikova S, Ganieva R, Zabnenkova V, Ryzhkova O, Nikitin S, Dadali E and Kutsev S (2024) Mild phenotype of CHAT-associated congenital myasthenic syndrome: case series. Front. Pediatr. 12:1280394. doi: 10.3389/fped.2024.1280394
Received: 20 August 2023; Accepted: 2 January 2024;
Published: 18 January 2024.
Edited by:
Simona Sestito, University "Magna Graecia", Catanzaro, ItalyReviewed by:
Edoardo Malfatti, Hôpitaux Universitaires Henri Mondor, FranceEmanuele Micaglio, IRCCS San Donato Polyclinic, Italy
© 2024 Murtazina, Borovikov, Marakhonov, Sharkov, Sharkova, Mirzoyan, Kulikova, Ganieva, Zabnenkova, Ryzhkova, Nikitin, Dadali and Kutsev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aysylu Murtazina YXlzeWx1bXVydGF6aW5hQGdtYWlsLmNvbQ==
 Aysylu Murtazina
Aysylu Murtazina Artem Borovikov
Artem Borovikov Andrey Marakhonov
Andrey Marakhonov Artem Sharkov
Artem Sharkov Inna Sharkova1
Inna Sharkova1 Alena Mirzoyan
Alena Mirzoyan