- 1Department of Pediatric Hematology and Oncology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
Aeromonas caviae infection of the bloodstream and intestine is a rare and severe opportunistic infection in immunocompromised people. In Southwest China, we first reported a case of bloodstream and intestinal infection with multidrug-resistant (MDR) Aeromonas caviae in a 4-year-old child with T-cell acute lymphoblastic leukemia. Blood and stool cultures were used to identify the infection. The selection of antibiotics was based on clinical expertise and medication sensitivity tests. We used linezolid, levofloxacin, and polymyxin B to treat the patient aggressively. Aeromonas caviae infection is uncommon in juvenile acute lymphoblastic leukemia. Doctors should be aware of the likelihood of opportunistic infection during the post-chemotherapy bone marrow suppression period. We further conducted a review of the literature and performed a detailed analysis of Aeromonas infection in pediatric leukemia. It is becoming increasingly apparent that antibiotic is abused domestically and abroad, resulting in the sharp increase of MDR bacteria. In general, most of the Aeromonas isolates are susceptible to third- or fourth-generation cephalosporins, aminoglycosides, quinolones, and carbapenem, but drug-resistant strains are being reported increasingly. We summarized the drug resistance rate of Aeromonas caviae and Aeromonas hydrophila in China in the last 10 years. Early recognition and effective treatment will improve prognosis and reduce mortality.
Introduction
The Aeromonas genus is found ubiquitously in freshwater and soil environments. Aeromonas infection mainly occurs in patients with an underlying disease or immune deficiency and can cause serious multiple organ dysfunction (1). The incidence of Aeromonas septicemia was approximately 0.07 per 1,000 admissions, and in some regions, Aeromonas hydrophila and A. caviae are common pathogens (2, 3). The mortality rate, which can reach up to 24%–63%, not only increases the clinical work pressure but also threatens public health (4). Definitive diagnosis and appropriate antibiotic treatment are necessary to control disease progression.
Underlying hematological diseases, especially leukemia, apparently increase the likelihood and severity of illness caused by Aeromonas. Bloodstream infection is common among hematological diseases (5, 6). Over 90% of Aeromonas bloodstream infections occur during the neutropenic stage (7). Cytotoxic drugs and immunosuppressants can cause the reduction of neutrophils and a decrease in defense and immunity capacity. During this time, Aeromonas can invade the body through multiple pathways, especially damaged skin and mucosal.
In the past, Aeromonas strains were sensitive to third- or fourth-generation cephalosporins. Aminoglycosides, fluoroquinolones, and carbapenems are used empirically to treat multidrug-resistant (MDR) Aeromonas species. However, with the wide use of antibiotics in agriculture, fish farming, and clinical settings, increasing resistance has been noted (8, 9). The widespread application of broad-spectrum antibiotics has promoted the appearance of antimicrobial resistance genes (10, 11). An immunosuppressed individual is susceptible to opportunistic pathogen infection because of disease features. A retrospective study found that over 90% of Aeromonas bloodstream infections occur during the neutropenic stage. Leukemia patients with prolonged neutropenia are the high-risk population for Aeromonas infection (7, 12). The manifestations are not specific, but the infection often progresses rapidly. Hence, this case report aims to describe the clinical manifestation and summarize the treatment experience of MDR A. caviae infection in acute lymphoblastic leukemia.
Case report
A 4-year-old girl admitted to West China Second University Hospital presented with epistaxis, ecchymosis, and paleness, followed by fever with a decreased neutrophil count (absolute neutrophil counts less than 1.5 × 109/L). She was diagnosed with T-cell acute lymphoblastic leukemia with the TLX1::TCRA fusion gene after the morphology, immunology, cytogenetics, and molecular biology (MICM) classification. The brain, chest, and abdomen radiological examination showed a negative result, and no chromosomal abnormality or primary immune deficiency was detected. Before chemotherapy, cefoperazone/sulbactam (50 mg/kg q8 h) was given as an anti-infective regimen. She was stratified into the intermediate-risk group and successfully treated with induction therapy using the Chinese Children Cancer Group 2020 (CCCG-ALL-2020) protocol. The bone marrow response on day 19 (calculated from the start of chemotherapy) was evaluated using bone marrow aspiration and minimal residual disease (MRD). The bone marrow smear suggested complete remission, and the MRD was negative (less than 0.01%).
She developed significant neutropenia on day 4 of her first course of induction chemotherapy (measured from the commencement of chemotherapy). She also complained of mouth pain and face edema. Meropenem (40 mg/kg every 8 h) and vancomycin (15 mg/kg every 6 h) were administered as empiric antibiotics, and voriconazole (6 mg/kg every 12 h) was added as prophylactic for antifungal therapy because neutropenia persisted (for more than 14 days). Vancomycin and voriconazole blood concentrations were within normal limits. Her temperatures were consistently normal, and she had no additional signs of infection. On day 28, however, she developed a fever, diarrhea, and vomiting. Peritoneal discomfort and perianal skin erosion were discovered during a physical examination. Her neutrophil count was 0 × 109/L, and her C-reactive protein (CRP) level was 110.1 mg/L. The ALL induction chemotherapy was not administered. Considering the risk of MDR Gram-positive coccus infection, vancomycin was changed to linezolid (10 mg/kg q8 h) and meropenem, and voriconazole was continuously administered at the same dose. However, just a day later (on day 29), she had septic shock, which manifested as wilt, hypoxemia, hypotension, and extended capillary refilling time. She was promptly transported to the Pediatric Intensive Care Unit after her blood volume was replenished. Noradrenaline and venoclysis hydrocortisone were administered, and she still had worsening dyspnea and continuous hypoxia.
The CRP and procalcitonin (PCT) increased to 299.8 mg/L and 23.67 ng/ml, respectively, with the disturbance of the internal environment, including hypoproteinemia, hyponatremia, hypokalemia, hypocalcemia, and hyperlactatemia (ALB 22.1 g/L, Na+ 128 mmol/L, K+ 2.82 mmol/L, Ca2+ 1.62 mmol/L, Lac 3.7 mmol/L). Aminotransferase, creatinine, and urea were normal. The stool culture identified MDR A. caviae (susceptible to amikacin, levofloxacin, and tigecycline) (Table 1). The microbial was cultured by Salmonella-Shigella agar medium and MacConkey agar medium. The microbial identification used matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) with 99% sensitivity. The micro-broth dilution method for antimicrobial susceptibility testing was used to determine the minimal inhibitive concentration (MIC) of antibiotics against the pathogen. The chest X-ray and abdomen computed tomography showed ground glass opacification in the post-basal segment of the left inferior pulmonary lobe and peritoneal thickening of the left paracolic sulcus (Figures 1A,B). Invasive mechanical ventilation was adopted, and antibiotics were adjusted to linezolid (10 mg/kg q8 h), amikacin (15 mg/kg qd), and tigecycline (1.2 mg/kg q12 h) simultaneously. Despite the shock subsiding through energetic treatment, she still had a high spiking fever, and the inflammatory cytokines did not descend to the normal level (CRP 183.7 mg/L, PCT 3.78 ng/ml). The 1,3-β-D glucan test and the serum galactomannan antigen (GM) test were negative. The cerebrospinal fluid examination was normal. However, the blood culture identified MDR A. caviae (susceptible to amikacin, levofloxacin, and tigecycline) and carbapenem-resistant Acinetobacter baumannii (susceptible to polymyxin B, tigecycline, aminoglycosides, and quinolones) (Table 1). The microbial was cultured using a Columbia blood agar base medium. Due to obstinate fever and persistent abnormal inflammatory cytokines, the antibiotics were adjusted to polymyxin B (1.5 million units/kg q12 h), levofloxacin (8 mg/kg q12 h), and linezolid (10 mg/kg q8 h) according to drug sensitivity results. On day 39, the temperature was back to normal, and the CRP and PCT decreased to 5.8 mg/L and 0.3 ng/ml, respectively. However, a repeat chest CT revealed a new exudative lesion in the post-basal segment of the left inferior pulmonary lobe (Figure 1C).
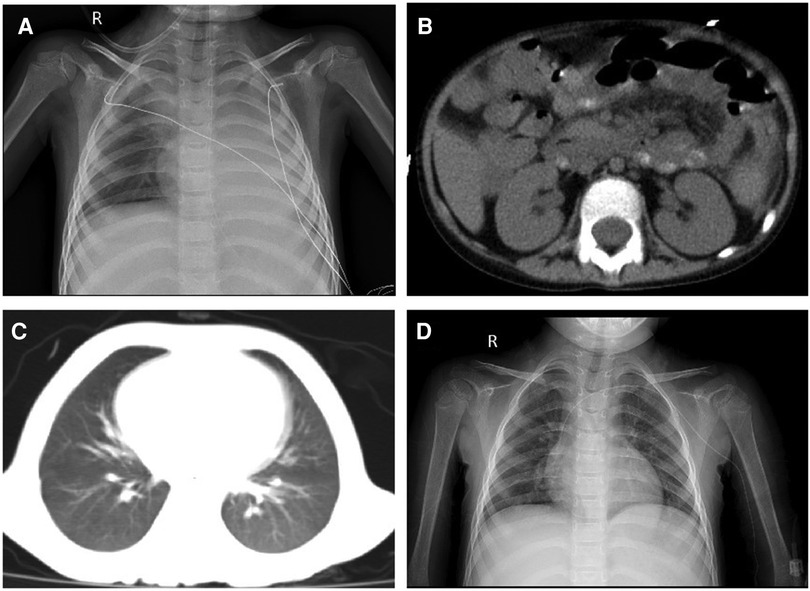
Figure 1. (A) Chest X-ray showing a significantly decreased transmittance in the left lung; (B) peritoneal thickening of the left paracolic sulcus; (C) chest CT showing a new exudative lesion in the post-basal segment of the left inferior pulmonary lobe; and (D) chest X-ray showing that the transmittance of the lung was improved.
On day 57, her neutrophils reverted to normal levels, and her pulmonary lesions in the chest X-ray showed no advancement (Figure 1D). She began the second round of induction therapy, which included cyclophosphamide, cytarabine, and mercaptopurine. The A. caviae and A. baumannii infections did not return after the treatment. Figure 2 depicts a chronology of the temperature and the C-reaction protein.
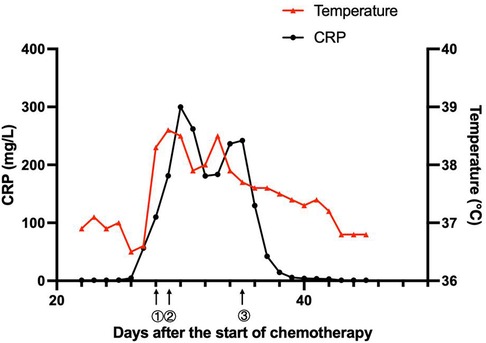
Figure 2. Clinical course of the patient. ① On day 28, the patient had a fever, diarrhea, and vomiting. The antibiotic therapy was switched to linezolid (10 mg/kg q8 h) combined with meropenem (40 mg/kg q8 h). ② On day 29, the patient suffered from septic shock. The antibiotics were adjusted to linezolid (10 mg/kg q8 h), amikacin (15 mg/kg qd), and tigecycline (1.2 mg/kg q12 h). ③ On day 35, with the child having an obstinate fever, persistent abnormal inflammatory cytokines, and drug susceptibility results, the antibiotics were adjusted to polymyxin B (1.5 million units/kg q12 h), levofloxacin (8 mg/kg q12 h) and linezolid (10 mg/kg q8 h).
Discussion
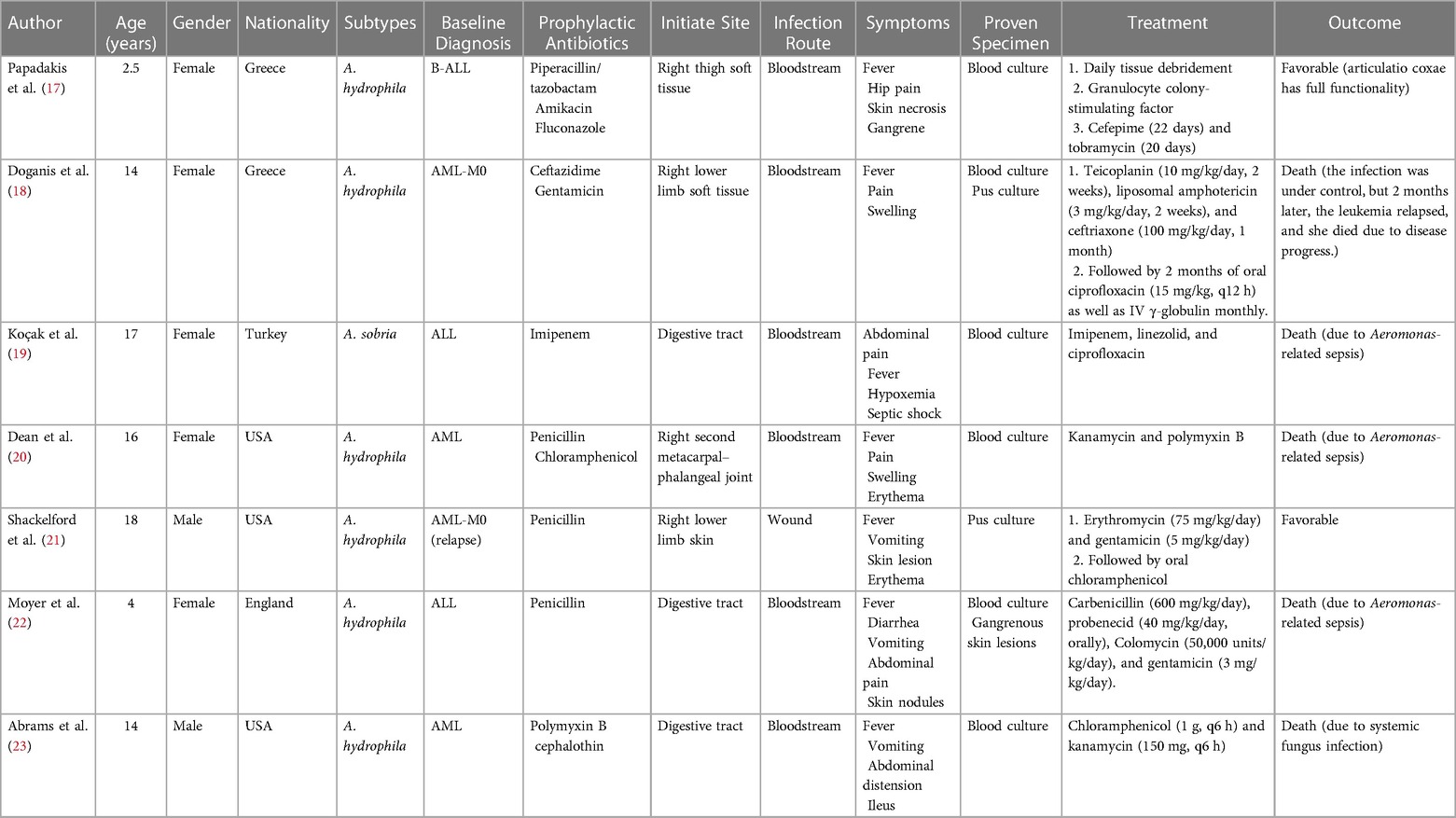
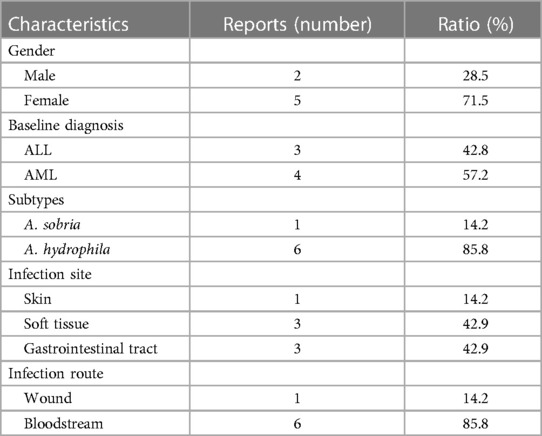
The Aeromonas genus is a bacterial group of cosmopolitan distribution and isolated from a broad range of sources like fresh water, soil, fruits, and vegetables (13). The first documented isolation of Aeromonas as a causative agent dates from 1954 in a Jamaican woman presenting with myositis (10). The bacteria are characterized facultatively as anaerobic, oxidase, and catalase-positive, Gram-negative bacillus and are considered opportunistic pathogens that mainly occur in patients with underlying diseases (14). The incidence of Aeromonas infection varies by geographical location and can be related to bad hygiene habits in underdeveloped regions (15). In pediatric patients, the Aeromonas genus is a common causal agent of intestinal infections, with A. hydrophila and A. caviae being the species mostly identified as causative agents (16). For the cases reviewed, we performed a detailed analysis of Aeromonas infection in pediatric leukemia (17–23) (Table 2). The seven cases are all younger than 18 years old, with a male-to-female ratio of 5:2, including acute lymphoblastic leukemia and acute myeloid leukemia. Only two subtypes have been found in the clinical samples, and the most predominant clinical species is A. hydrophila. The characteristics of the reported cases are summarized in Table 3. The common infection sites were the skin, soft tissue, and gastrointestinal tract. The clinical appearances were non-specific and included fever, pain, swelling, diarrhea, and vomiting. Before identifying the pathogen, penicillin and cephalosporin were used empirically. Once the diagnosis was clear, the antibiotics were adjusted to third- and fourth-generation cephalosporins, carbapenems, polymyxin, and amphotericin B. Three of the seven cases died due to Aeromonas-related sepsis (19, 20, 22).
Although Aeromonas infection is usually reported in immunocompromised hosts, MDR A. caviae infection among children with leukemia is rare. Here, we first reported a case of bloodstream and intestinal infection by MDR A. caviae in pediatric acute lymphoblastic leukemia. Considering that A. caviae is an opportunistic pathogen, the risk factors of infection are indeed a matter of great concern to us. Through a thorough search for risk factors, it would be reasonable to suppose that the infection occurrence is related to the immunocompromised status induced by post-chemotherapeutic bone marrow suppression. In addition, the patients came from regions with a relatively backward economy and poor sanitation and nutritional status, which may increase the risk of severe infection. Improving the living environment and hygiene practices and strengthening nutritional support are beneficial for preventing the infection.
Aeromonas infection causes a wide range of clinical symptoms, from acute self-limiting diarrhea to systemic inflammatory response syndrome (SIRS) (24). Acute gastroenteritis is one of the most common manifestations among healthy people, and the major cause is the consumption of contaminated food (25). Aeromonas-related gastroenteritis most usually manifests as self-limiting watery diarrhea, but it can also manifest more severely as dysentery- and cholera-like disease (26). Primary or spontaneous Aeromonas-related peritonitis is a severe consequence in the elderly or immunocompromised population due to impaired intestinal motility and inadequate host defenses (27). Aeromonas can also cause a variety of skin and soft tissue infections, particularly in people who have been injured or have been exposed to a contaminated aquatic environment (28). The common Aeromonas skin infections are impetigo, erysipelas, cellulitis, folliculitis, abscesses, and necrotizing fasciitis, and the reported common subtype is A. hydrophila (24, 25). A. caviae-associated skin and soft tissue infection can be seen in some sporadic cases. One case involved a 22-year-old healthy Korean female, who underwent cosmetic liposuction and presented to the hospital with necrotizing fasciitis in both calves. She subsequently developed multiorgan dysfunction and skin necrosis with consequent massive skin loss. Culture reports on wound swabs and debrided tissue specimens proved an A. caviae infection. Through broad-spectrum antibiotics and repeated aggressive wound exploration and debridement, she eventually recovered from this severe invasive infection (26). Aeromonas septicemia has a fulminant course, with its mortality rate ranging from 25 to 30%. It is usually reported in immunocompromised hosts. The signs or symptoms do not distinguish from systemic infections caused by other bacteria; therefore, diagnosis needs to rely on pathogenic evidence (10, 27). An increasing number of laboratories and medical institutions have started to adopt next-generation sequencing (NGS) to search for rare pathogens. NGS is a rapid and non-invasive diagnostic method. Using NGS is recommended when an infection with a rare pathogen is suspected, especially immunocompromised individuals who need emergency treatment. Aeromonas meningitis is an uncommon clinical entity, and few cases have been described in pediatrics. The high risks are associated with hematological disease, surgical craniotomy, and traumatic skull fracture (28, 29). We recommend that intracranial examinations, including head computed tomography and cerebrospinal fluid examination, should be implemented as soon as possible, even if the patient does not have neurological signs and symptoms.
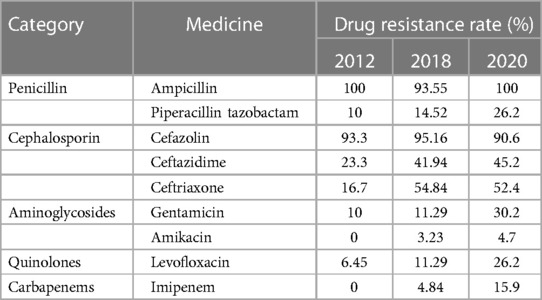
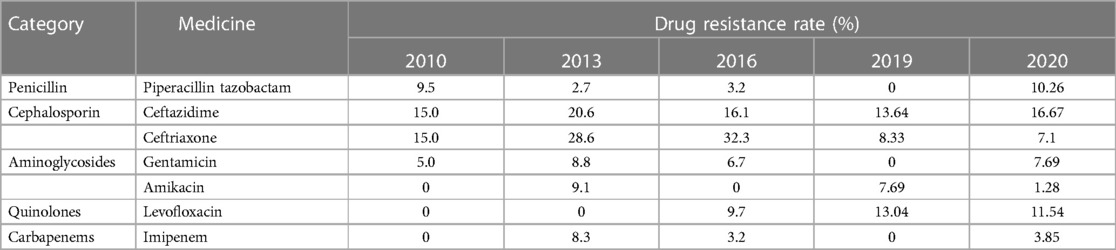
The patient we reported was in the period of post-chemotherapeutic bone marrow suppression. During the therapy, she developed persistent fever, diarrhea, vomiting, and perianal skin erosion. The clinical manifestations resembled infections with other pathogens, but she rapidly developed septic shock. Clinical manifestation and stool culture results highly indicated an enterogenic infection. The patient was hospitalized for chemotherapy, but the diet was prepared by her parents outside the hospital based on their wishes and demands. The intake of contaminated food or water was the possible infection route. Through blood and stool culture, A. caviae was eventually identified. The early symptoms of A. caviae infection are not typical, but the clinical course proceeded rapidly. The diagnosis relies on etiology, that is, once the Aeromonas are confirmed by sterile body fluid or secretion culture (blood, cerebrospinal fluid, sputum, urine, stool, and wound secretions), potent antibiotics are then immediately adopted. The use of antibiotics in clinical practice is undoubtedly a revolutionary milestone in medicine, and antibiotics are on the front line in the fight against Aeromonas infection. Aeromonas are resistant to penicillin and first-generation cephalosporins due to beta-lactamases, but they are susceptible to third- and fourth-generation cephalosporins, aminoglycosides, and fluoroquinolones. Our patient was also infected with carbapenem-resistant A. baumannii, which was equally worrying because the coexistence of two bacteria may exacerbate the patient's condition. We eventually chose levofloxacin and polymyxin B based on the drug sensitivity test of two bacteria. Her temperatures and levels of inflammatory cytokines were back to normal through regular potent antibiotic treatment. We further summarized herein the drug resistance rate of A. caviae and A. hydrophila in China for the last 10 years (Tables 4, 5). The Aeromonas strains extracted from the literature all corresponded to clinical cases. They were mainly isolated from the bile, followed by wound secretion and blood (30–35). The uncontrolled and indiscriminate usage of antibiotics has prompted the generation of drug-resistant bacteria. The spread of MDR bacteria in various situations has resulted in an inescapable public health issue: the number of effective antibiotics accessible for treatment is decreasing. Some hospital strains are resistant to numerous medicines, including carbapenems, colistin, and tigecycline, which are utilized as a last resort in situations involving multiply-resistant bacteria (34, 35). We summarized the MDR bacteria diagnosis and treatment flow chart in Figure 3, along with three clinical treatment experiences: first, early detection of MDR bacteria is critical; second, understanding the evolution of pathogen resistance profiles is critical for providing effective treatments; through the national or regional antimicrobial surveillance network, the types and proportions of pathogens can dynamically be investigated; the communication between laboratorians and clinicians should be further improved to strengthen hospital infection control and stewardship of antimicrobial agents; and third, therapeutic administration should consider the risk factors, clinical manifestations, and drug susceptibility and should not be limited to specific drugs.
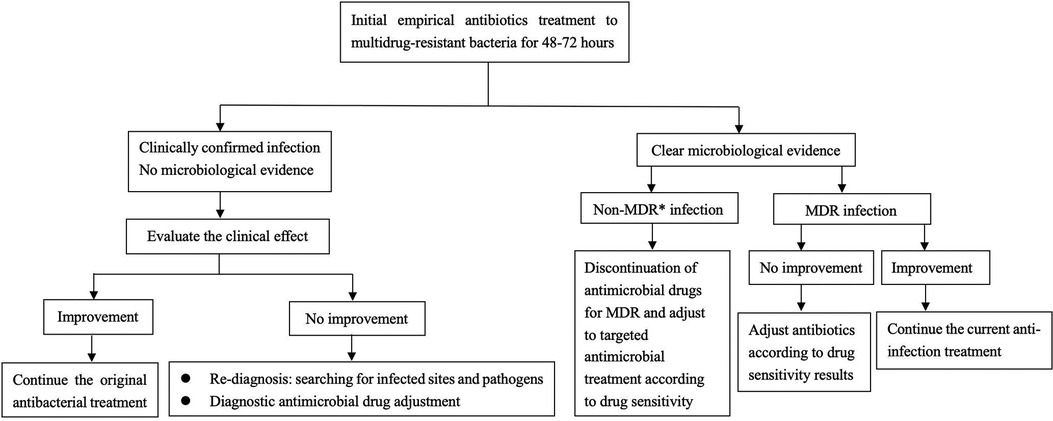
Figure 3. Diagnosis and treatment flow chart of multidrug-resistant bacteria. *MDR multidrug-resistant bacteria.
Conclusion
Aeromonas are common aquatic bacteria that are widely spread in the environment and can infect humans and animals. The presence of an underlying disease, particularly neoplastic disease, appears to enhance the risk of Aeromonas infection and the disease severity. Ever since the widespread use of antibiotics, drug-resistant microorganisms, particularly carbapenem-resistant bacteria, have proliferated. Under the management of antibiotic selection, early identification and reasonable antibiotic selection are crucial for improving prognosis and reducing mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving human participants was conducted in accordance with the local legislation and institutional requirements. Ethical review or approval was not required for the study on human volunteers. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
MJ and YD designed the experiments. JG performed the experiments. YD and JG collected and analyzed the data. YD and MJ drafted the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the patient and her parents for providing blood samples, coordinating with the treatment, and agreeing to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang X, Pan J, Chen L, Li R, Han Y, Di Z, et al. Prevalence, virulence-related genes and antimicrobial resistance of Aeromonas spp. from loach Misgurnus anguillicaudatus with skin ulcer and healthy controls in Southern China. Aquaculture. (2022) 552:738040. doi: 10.1016/j.aquaculture.2022.738040
2. Kimura M, Araoka H, Yoneyama A. Aeromonas caviae is the most frequent pathogen amongst cases of Aeromonas bacteremia in Japan. Scand J Infect Dis. (2013) 45(4):304–9. doi: 10.3109/00365548.2012.737474
3. Morinaga Y, Yanagihara K, Araki N, Harada Y, Yamada K, Akamatsu N, et al. Clinical characteristics of seven patients with Aeromonas septicemia in a Japanese hospital. Tohoku J Exp Med. (2011) 225(2):81–4. doi: 10.1620/tjem.225.81
4. Chen PL, Wu CJ, Tsai PJ, Tang HJ, Chuang YC, Lee NY, et al. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS One. (2014) 9(11):e111213. doi: 10.1371/journal.pone.0111213
5. Nolla-Salas J, Codina-Calero J, Valles-Angulo S, Sitges-Serra A, Zapatero-Ferrandiz A, Climent MC, et al. Clinical significance and outcome of Aeromonas spp. infections among 204 adult patients. Eur J Clin Microbiol Infect Dis. (2017) 36(8):1393–403. doi: 10.1007/s10096-017-2945-4
6. Lau SM, Peng MY, Chang FY. Outcomes of Aeromonas bacteremia in patients with different types of underlying disease. J Microbiol Immunol Infect. (2000) 33(4):241–7.11269369
7. Xu C, Lin Q, Zhao Y, Zhu G, Jiang E, Li S, et al. Clinical characteristics and risk factors of Aeromonas bloodstream infections in patients with hematological diseases. BMC Infect Dis. (2022) 22(1):303. doi: 10.1186/s12879-022-07277-7
8. Anandan S, Gopi R, Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Gunasekaran P, Walia K, et al. First report of bla(OXA-181)-mediated carbapenem resistance in Aeromonas caviae in association with pKP3-A: threat for rapid dissemination. J Glob Antimicrob Resist. (2017) 10:310–4. doi: 10.1016/j.jgar.2017.07.006
9. Wickramanayake M, Dahanayake PS, Hossain S, Heo GJ. Antimicrobial resistance of pathogenic Aeromonas spp. isolated from marketed pacific abalone (Haliotis discus hannai) in Korea. J Appl Microbiol. (2020) 128(2):606–17. doi: 10.1111/jam.14485
10. Fernandez-Bravo A, Figueras MJ. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms. (2020) 8(1):129. doi: 10.3390/microorganisms8010129
11. Hilt EE, Fitzwater SP, Ward K, Maurice A, Chandrasekaran S, Garner OB, et al. Carbapenem resistant Aeromonas hydrophila carrying bla(cphA7) isolated from two solid organ transplant patients. Front Cell Infect Microbiol. (2020) 10:563482. doi: 10.3389/fcimb.2020.563482
12. Maschmeyer G, De Greef J, Mellinghoff SC, Nosari A, Thiebaut-Bertrand A, Bergeron A, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia. (2019) 33(4):844–62. doi: 10.1038/s41375-019-0388-x
13. Goncalves Pessoa RB, de Oliveira WF, Marques DSC, Dos Santos Correia MT, de Carvalho E, Coelho L. The genus Aeromonas: a general approach. Microb Pathog. (2019) 130:81–94. doi: 10.1016/j.micpath.2019.02.036
14. Bravo L, Morier L, Castañeda N, Ramírez M, Silva M, Castro-Escarpulli G. Aeromonas: an emerging pathogen associated with extraintestinal infection in Cuba. Rev Cubana Med Trop. (2003) 55(3):208–9.15849928
15. Ghenghesh KS, Ahmed SF, El-Khalek RA, Al-Gendy A, Klena J. Aeromonas-associated infections in developing countries. J Infect Dev Ctries. (2008) 2(2):81–98. doi: 10.3855/T2.2.81
16. Bello-Lopez JM, Sanchez-Garibay C, Ibanez-Cervantes G, Leon-Garcia G, Gonzalez-Avila LU, Hernandez-Cortez C, et al. Genetic and phenotypic determinants of resistance to antibiotics in Aeromonas spp., strains isolated from pediatric patients. J Infect Dev Ctries. (2020) 14(10):1146–54. doi: 10.3855/jidc.12966
17. Papadakis V, Poniros N, Katsibardi K, Charissiadou AE, Anastasopoulos J, Polychronopoulou S. Fulminant Aeromonas hydrophila infection during acute lymphoblastic leukemia treatment. J Microbiol Immunol Infect. (2012) 45(2):154–7. doi: 10.1016/j.jmii.2011.09.008
18. Doganis D, Baka M, Tsolia M, Pourtsidis A, Lebessi E, Varvoutsi M, et al. Multifocal Aeromonas osteomyelitis in a child with leukemia. Case Rep Infect Dis. (2016) 2016:8159048. doi: 10.1155/2016/8159048
19. Kocak Toprak S, Ilhan G, Erdogan E, Karakus S. Aeromonas sobria bacteremia in an acute lymphoblastic leukemia case in remission. Turk J Haematol. (2011) 28(1):79–80. Remisyondaki akut lenfoblastik losemi olgusunda Aeromonas sobria bakteriyemisi. doi: 10.5152/tjh.2011.13
20. Dean HM, Post RM. Fatal infection with Aeromonas hydrophila in a patient with acute myelogenous leukemia. Ann Intern Med. (1967) 66(6):1177–9. doi: 10.7326/0003-4819-66-6-1177
21. Shackelford PG, Ratzan SA, Shearer WT. Ecthyma gangrenosum produced by Aeromonas hydrophilia. J Pediatr. (1973) 83(1):100–1. doi: 10.1016/s0022-3476(73)80326-9
22. Moyer CD, Sykes PA, Rayner JM. Aeromonas hydrophila septicaemia producing ecthyma gangrenosum in a child with leukaemia. Scand J Infect Dis. (1977) 9(2):151–3. doi: 10.3109/inf.1977.9.issue-2.20
23. Abrams E, Zierdt CH, Brown JA. Observations on Aeromonas hydrophila septicaemia in a patient with leukaemia. J Clin Pathol. (1971) 24(6):491–2. doi: 10.1136/jcp.24.6.491
24. Ugarte-Torres A, Perry S, Franko A, Church DL. Multidrug-resistant Aeromonas hydrophila causing fatal bilateral necrotizing fasciitis in an immunocompromised patient: a case report. J Med Case Rep. (2018) 12(1):326. doi: 10.1186/s13256-018-1854-1
25. Clebak KT, Malone MA. Skin infections. Prim Care. (2018) 45(3):433–54. doi: 10.1016/j.pop.2018.05.004
26. Park SY, Jeong WK, Kim MJ, Lee KM, Lee WS, Lee DH. Necrotising fasciitis in both calves caused by Aeromonas caviae following aesthetic liposuction. J Plast Reconstr Aesthet Surg. (2010) 63(9):e695–8. doi: 10.1016/j.bjps.2010.04.006
27. Parker JL, Shaw JG. Aeromonas spp. clinical microbiology and disease. J Infect. (2011) 62(2):109–18. doi: 10.1016/j.jinf.2010.12.003
28. Vanzo C, Gareis S, Gomila A, Caliva S, Paredes M, Garnero A. Meningitis due to carbapenemase-producing Aeromonas hydrophila: a case report. Arch Argent Pediatr. (2023) 121(1):e202102448. Meningitis por Aeromonas complejo hydrophila productora de carbapenemasa: a proposito de un caso. doi: 10.5546/aap.2021-02448.eng
29. Kali A, Kalaivani R, Charles P, Seetha KS. Aeromonas hydrophila meningitis and fulminant sepsis in preterm newborn: a case report and review of literature. Indian J Med Microbiol. (2016) 34(4):544–7. doi: 10.4103/0255-0857.195383
30. Chao CM, Lai CC, Tang HJ, Ko WC, Hsueh PR. Biliary tract infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis. (2013) 32(2):245–51. doi: 10.1007/s10096-012-1736-1
31. Jiang HHX, Liu P, Wu A, Liu Y, Wu Y. Clinical characteristics and drug resistance analysis of 62 cases of Aeromonas caviae infection. Chin J Nosocomio. (2018) 28(24):3730–3. doi: 10.11816/cn.ni.2018-183070
32. Meng GYQ, Ma Q, Ma B, Li Y. Analysis of clinical distribution characteristics and drug sensitivity of 41 patients with Aeromonas caviae infection. J Henan Med Coll. (2020) 32(3):307–9. doi: 10.3969/j.issn.1008-9276.2020.03.023
33. He T, Yang SS, Deng LJ, Yan L, Zhang LP. Antimicrobial resistance profile of the Aeromonas strains isolated from extraintestinal specimens in a hospital in Chongqing. Chin J Infect Chemother. (2017) 17(6):653–7.
34. Zhu YDH, Xu Y, Pan F, Tian W. Clinical characteristics and prognostic factors analysis of extraintestinal Aeromonas hydrophila and treatment of the disease. Chin J Health Lab Tec. (2019) 29(9):1077–9.
Keywords: Aeromonas caviae, bloodstream infection, pediatric acute lymphoblastic leukemia, drug sensitivity test, treatment
Citation: Dai Y, Gao J and Jiang M (2024) Case Report: A rare infection of multidrug-resistant Aeromonas caviae in a pediatric case with acute lymphoblastic leukemia and review of the literature. Front. Pediatr. 12:1233600. doi: 10.3389/fped.2024.1233600
Received: 14 June 2023; Accepted: 30 April 2024;
Published: 13 May 2024.
Edited by:
Oral Alpan, Amerimmune, United StatesReviewed by:
Lekshmi Narendrakumar, Translational Health Science and Technology Institute, IndiaJose Ramos-Vivas, Universidad Europea del Atlántico, Spain
© 2024 Dai, Gao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyan Jiang, amlhbmdteTA5MDRAMTYzLmNvbQ==
 Yiling Dai
Yiling Dai Ju Gao1,2
Ju Gao1,2 Mingyan Jiang
Mingyan Jiang