- 1Department of Medical Ultrasound, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Ultrasound, The First People’s Hospital of Huaihua, Huaihua, China
- 3Department Allgemeine Innere Medizin, Kliniken Hirslanden Bern, Beau Site, Salem und Permanence, Bern, Switzerland
- 4Department of Ultrasound, The Third Hospital of Wuhan, Wuhan, China
Purpose: We mainly aimed to perform a narrative review of clinical applications of the three intracavitary contrast-enhanced ultrasonography (CEUS) including contrast-enhanced voiding urosonography (ceVUS), contrast-enhanced retrograde urethrosonography (ceRUG), and contrast-enhanced genitosonography (ceGS) in pediatric lower genitourinary anomalies.
Method: A literature search in the PubMed and Web of Science databases was conducted up to 1 July 2022 on all studies published in English using the search terms “contrast-enhanced voiding urosonography”, “contrast-enhanced retrograde urethrosonography”, and “contrast-enhanced genitosonography”. Trials were limited to pediatric subjects (ages ≤18 years) with no time restrictions. The inclusion criteria were studies on ceVUS, ceRUG, and ceGS to evaluate pediatric lower genitourinary anomalies. Two independent authors summarized the included articles.
Results: Finally, a total of 48 original articles and 6 case reports or case series were included, of which 50 (93%) were only relevant to ceVUS, 3 (5%) articles involved ceGS, while only one (2%) article involved ceRUG, and 87% of the applications of ceVUS were focused on vesicoureteral reflux (VUR). We also searched 24 related reviews, of which 20 involved ceVUS in diagnosing VUR and 4 involved ceRUG and ceGS for other lower genitourinary anomalies.
Conclusion: Intracavitary CEUS including ceVUS, ceRUG, and ceGS in pediatrics has many advantages over other radiological examinations in diagnosing lower genitourinary anomalies. Although ceVUS is widely used in detecting VUR, ceRUG and ceGS have also become promising techniques for evaluating the urethral pathologies and urogenital sinus.
Introduction
There is a broad disease spectrum of lower genitourinary tract anomalies in children, among which vesicoureteral reflux (VUR) is the most common urological disorder (1). Due to end-stage renal failure and infertility caused by abnormalities of the lower urinary tract and genital tract anomalies, early diagnosis and treatment are important for the overall outcomes and prognoses of the patients (2). Historically, radiographic voiding cystourethrography (VCUG) and direct or indirect radionuclide cystography (RNC) were mainly used in diagnosing pediatric VUR (3), while VCUG and retrograde urethrography were applied for imaging the urethra (4), and the genitography was established to diagnose the urogenital sinus (5). However, due to the prolonged radiation exposure of the children's gonads with VCUG and genitography, and poor anatomical resolution of RNC, a safe and non-radiologic imaging modality, the intracavitary contrast-enhanced ultrasonography (CEUS), was selected alternatively as a further diagnostic option for the lower urinary and genital tract anomalies (6). Based on the administration of ultrasound contrast agents (UCAs) into the bladder, urethra, or perineal orifice through a catheter, intracavitary CEUS including contrast-enhanced voiding urosonography (ceVUS), contrast-enhanced retrograde urethrosonography (ceRUG), and contrast-enhanced genitosonography (ceGS) has become a rapidly developing imaging examination mainly for detecting and grading VUR as well as for diagnosing varieties of urethral diseases and urogenital sinus malformation (7–9). Another intracavitary CEUS application, hysterosalpingo-contrast sonography, with its utility not yet developed in the pediatric population, is not covered in this article.
In this review, we mainly aim to present a comprehensive review of intracavitary CEUS applications (ceVUS, ceRUG, and ceGS) in pediatric genitourinary anomalies including lower urinary tract anomalies and urogenital sinus malformation, which could be helpful for pediatricians and radiologists to choose the right ultrasonographic imaging method when confronted with these diseases. This review article will be presented in three sections. The first section will introduce the evolution history of the three intracavitary CEUS techniques (ceVUS, ceRUG, and ceGS) in pediatrics. In the second section, we will present the appropriate dosages and safety of ceVUS, ceRUG, and ceGS in pediatrics. Lastly, and most importantly, related pediatric clinical applications of ceVUS, ceRUG, and ceGS in lower genitourinary diseases (e.g., VUR, megaureter, ectopic ureter, ureterocele, bladder diverticulum, posterior urethral valves (PUVs), anterior urethral valves (AUVs), diverticula of the prostatic utricle, stricture of the bulbar urethra, spinning top urethra, and urogenital sinus) that available documents refer to will be described.
Materials and methods
We searched the PubMed and Web of Science databases for all research published in English up to 1 July 2022, using the search terms “contrast-enhanced voiding urosonography”, “contrast-enhanced retrograde urethrosonography”, and “contrast-enhanced genitosonography”. Trials were limited to pediatric subjects (ages ≤18 years) with no time restrictions. We also included some narrative and systematic reviews to provide our readers with adequate details within the allowed number of references. In addition to the database searching, a hand search was performed, consisting of the reference lists of related articles and reviews and Google scholar search engines.
The articles were reviewed to determine the relevance based on the following criteria: studies that involved pediatric subjects using ceVUS, ceRUG, and ceGS to evaluate pediatric lower genitourinary anomalies including VUR, megaureter, ectopic ureter, ureterocele, bladder diverticulum, PUV, AUV, diverticula of the prostatic utricle, stricture of the bulbar urethra, spinning top urethra, and urogenital sinus. The articles that were beyond this coverage or referred to mixed pediatric and adult subjects or adult subjects were excluded.
All the titles and abstracts for all eligible articles were reviewed by two authors independently. If the abstracts were not relevant, then they were discarded, and the full-text articles were accessed. Further reviewing the full-text papers may lead to deserting some irrelevant documents and finally retaining articles that met the inclusion criteria in this review. When there was any discrepancy in the ultimately included articles, a consensus negotiation was reached to form the final inclusion result between the two authors.
Results
Through a comprehensive search in the PubMed and Web of Science databases, a total of 195 records were retrieved for our review up to 1 July 2022, including 62 papers from the PubMed and 133 articles from the Web of Science. After eliminating duplicate literatures preliminarily, two investigators independently reviewed the titles and abstracts of the remaining 128 studies, excluding editorials, meeting abstracts, non-human literature, adult subjects, and not related articles. The 96 full-text papers obtained were further reviewed and eventually included 48 original articles, 6 case reports or case series, and 24 reviews eligible for this review. The detailed PRISMA flowchart is summarized in Figure 1.
Among the 54 included clinical trials, 50 studies (93%) were only related to ceVUS, 3 (5%) articles involved ceGS, while only one (2%) article involved ceRUG. The proportion of published studies involving the use of ceVUS was significantly higher than ceRUG and ceGS, and 87% of the applications of ceVUS were focused on VUR. When referring to the types of UCAs used in these studies, 56% of the studies were implemented with SonoVue®/Lumason®, and 41% of the studies used the first-generation UCAs (Levovist, Echovist, and Albunex) (Table 1).
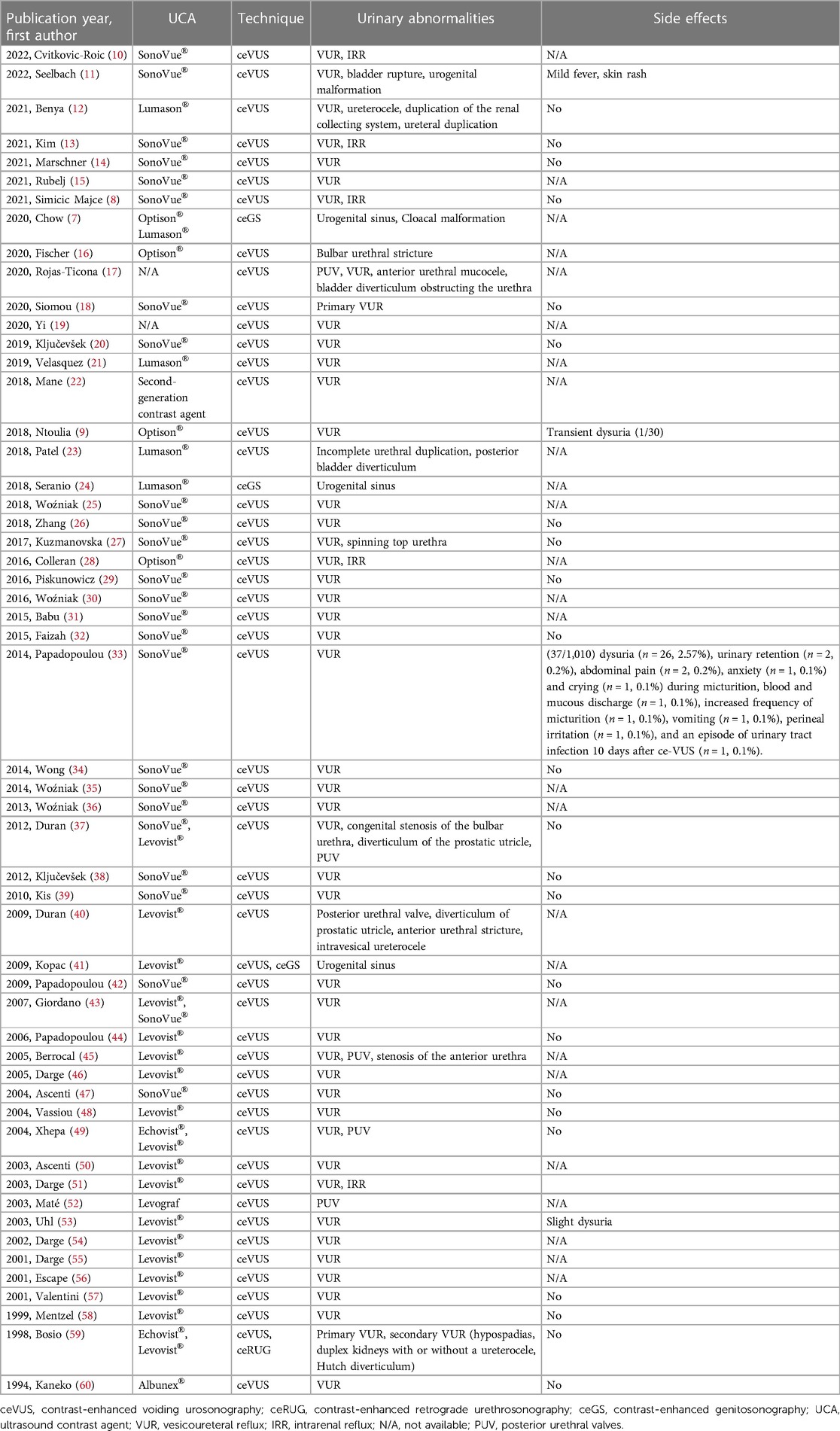
Table 1. Pediatric-only included studies that report the lower urinary anomalies using intracavitary contrast-enhanced ultrasonography including ceVUS, ceRUG, and ceGS performed with UCAs published in English.
We also searched 24 related narrative and systematic reviews, of which 20 (%) articles involved ceVUS in diagnosing VUR and 4 involved ceRUG and ceGS for other lower genitourinary anomalies (Table 2). When reviewing these clinical trials and reviews, we could get some information on the evolution history, dosages, and safety of ceVUS, ceRUG, and ceGS in pediatrics simultaneously. Hence, we also described the related content referring to the evolution history, dosages, and safety of ceVUS, ceRUG, and ceGS in this review.
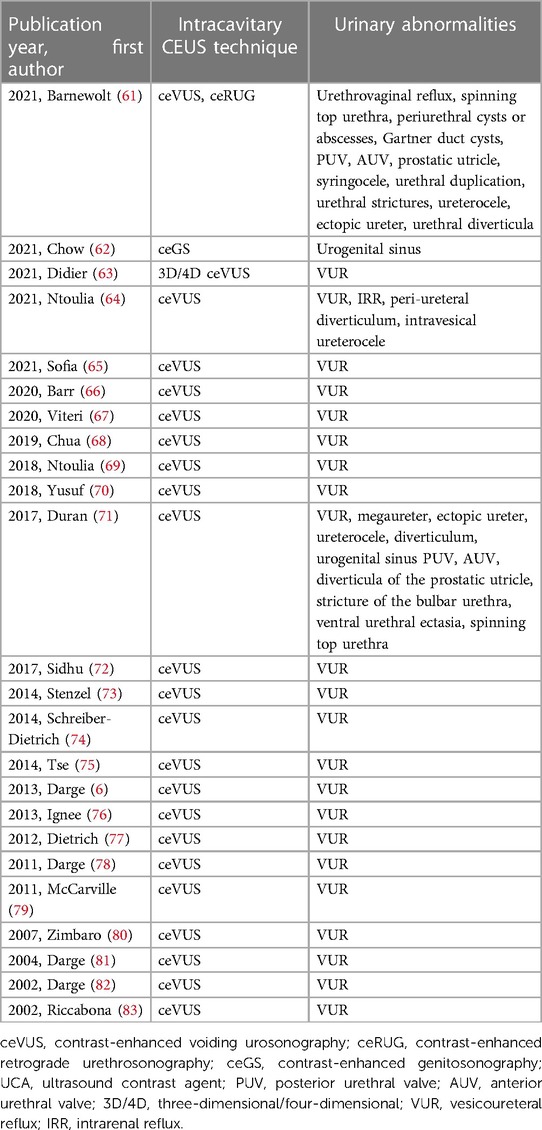
Table 2. Included reviews of intracavitary contrast-enhanced ultrasonography including ceVUS, ceRUG, and ceGS in pediatric lower genitourinary anomalies performed with UCAs published in English.
The evolution of ceVUS, ceRUG, and ceGS in pediatrics
In 1976, VUR was first diagnosed with ultrasound using M-mode imaging by detecting the changes in renal pelvis echoes in three adults (84). Since then, varieties of attempts including using indirect or direct ultrasound methods in the diagnosis of VUR have been implemented. Due to low sensitivity and specificity to reliably predict VUR, indirect methods without instilling any kind of material into the bladder using B-mode or duplex and color Doppler ultrasound were abandoned (85, 86). Direct ultrasound methods with the administration of various substances (e.g., saline, iodine, air bubbles made by shaking saline or iodine, or adding carbon dioxide, or pure air) into the bladder appeared in the 1980s (87–90). However, the direct ultrasound methods mentioned above have many limitations. One is a false-positive with saline caused by transient dilatation of the renal pelvis due to bladder filling or pre-existing ureteral and/or pelvicalyceal dilatation. The other is a false-negative due to an acoustic shadow caused by air preventing or obscuring reflux of air into the terminal ureters and possible pelvicalyceal dilatation. Another is that the air bubbles lack homogeneity and quantity, and dissolve fast (20–30 s), limiting the time available to detect reflux. Later in 1994, it was first reported that a novel UCA consisting of sonicated air-filled human albumin microspheres called Albunex® was applied to diagnose VUR in a child (60). In 1995, von Rohden et al. used Echovist® (an air-filled microbubble with a galactose shell) to examine VUR (91). However, both Albunex® and Echovist® did not last a long time (approximately 5 min), which is impractical for sufficient reflux evaluation.
The breakthrough in the ultrasound diagnosis of pediatric VUR came in the mid-1990s as the first-generation UCA, Levovist® (air-filled microbubbles with galactose and palmitic acid), became available for clinical practice in Europe (92). It was stable and the only approved UCA for intravesical use in children in 13 European countries and in Australia, but is no longer marketed currently. In the early 2000s, the second-generation UCAs, Lumason®/SonoVue® (sulfur hexafluoride lipid-type A microspheres; Lumason® in the United States and SonoVue® outside the United States) and Optison® (perflutren protein-type A microspheres), came into use. As the second-generation UCAs are even more stable, have fewer adverse events, and need less dose to achieve comparable contrast enhancement compared with Levovist®, it is recommended to use the second-generation UCAs in performing ceVUS.
The technique with the introduction of UCAs into the bladder was widely known as ceVUS since 2000. Before that, different names about this technique were used, such as cystosonography, contrast sonography, micturition sonography, ultrasound cystography, echo-enhanced cystosonography, and retrograde echocystography (59, 89, 93–96). But the use of ceVUS in pediatrics was not approved by the official organization until 22 December 2016; the US Food and Drug Administration (FDA) approved intravesical administration of Lumason® for pediatric suspected or known VUR under the collaboration of both the Society for Pediatric Radiology (SPR) CEUS task force and the International Contrast Ultrasound Society (ICUS). Encouragingly as a milestone event, the FDA's approval of ceVUS in pediatrics stimulated more scholars to get into this sort of research for children, thus promoting the development of intracavitary CEUS applications in VUR and other genitourinary system anomalies. In June 2017, the European Medicines Agency approved the use of SonoVue® for ceVUS in pediatrics. In China, SonoVue® was also approved by the Chinese National Medical Products Administration for the intravesical use in VUR. It is worth mentioning that ceVUS is the only officially approved intracavitary CEUS indication in children.
In addition to the most common application in VUR, ceVUS can also be used to identify urethral abnormalities both in girls and boys. It can be dated back early to the 1980s when several authors used non-voiding ultrasound to diagnose PUV as the dilated posterior urethra could be easily detected by gray-scale ultrasonography (97, 98). Although non-distended urethra can be visualized on B-mode ultrasound, distending the urethra during physiological voiding or with retrograde administration of saline into the bladder or urethra is preferable (45, 99). Recently, with the introduction of UCAs, the urethral sonographic images have been improved significantly. As the length of the female urethra is short, the voiding phase as a natural extension of ceVUS is enough to detect urethral structural and functional abnormalities associated with voiding disorders. While in males, detection of the urethra involves anterior and posterior urethra mainly associated with obstructing pathologies, including PUV, AUV and diverticula, and urethral strictures. To achieve high-resolution ultrasound images of the anterior urethra, ceRUG through retrograde instillation of contrast agents is used primarily for evaluating urethral strictures.
Another application of intracavitary CEUS in children is ceGS. It is a novel technique of instilling UCAs into the perineal orifice to reveal the connections between the urethra and müllerian duct remnants (vagina and prostatic utricle) mainly for diagnosing the urogenital sinus or cloacal malformation. The ceGS technique could be performed as a standalone imaging technique or as an extension of ceVUS. Kopac et al. first reported in 2009 that ceGS combined with ceVUS using Levovist® as a contrast agent was applied to identify urogenital sinus in a female newborn baby admitted due to the the absence of a normal vaginal opening (41). Since then, only a small quantity of specialized pediatric centers performed ceGS in a few cases (7, 24). Although normal saline can also be instilled into the perineal orifice to delineate the urogenital anatomic structure, the administration of UCAs makes revealing the anatomical connections more conspicuous.
Appropriate dosages and safety of UCAs in pediatric ceVUS, ceRUG, and ceGS
There are two ways of performing ceVUS with the introduction of UCAs into the bladder. The first one is injecting UCAs directly into the bladder. Through this technique, SonoVue®/Lumason® was reported to be administered at a dose of 0.5 ml–2.5 ml, among which most authors preferred a dose of 0.5 ml or 1 ml (18, 31, 32, 100). The other method is infusing UCA/normal saline solution into the bladder. When using the second method, the concentration of the bladder filling volume ranges from 0.2% to 1% with SonoVue®/Lumason® and 0.06%–0.5% with Optison® (9, 28). However, no matter which method is selected, the current recommended dose of SonoVue®/Lumason® is 1 ml. Moreover, with recent great improvements of ultrasound scanner technologies, the ability of contrast-specific imaging modality to clearly depict the microbubbles increased, and it might be too high for a 1 ml dose.
For scanning the urethra through either the voiding or the retrograde method, the dose of UCAs is similar to that used for detecting VUR (i.e., 0.1%–0.2% of UCA/normal saline solution). However, when the retrograde technique is performed, it might require a higher dose of UCAs due to the use of higher-frequency linear probes and the superficial site of the examined region.
The dosage and concentration of UCA/normal saline dilution that is used for ceVUS can also be used equally for ceGS but is variable due to the different types of UCAs and the sensitivity of the ultrasonic device and software. In the limited number of case reports or several case series about ceGS studies, a 5% of bladder filling volume with Levovist®, a 0.1% solution of Lumason®, or a 0.06% Optison®/normal saline solution was used (7, 24, 41).
In general, the ceVUS technique is quite safe. A review of 31 studies encompassing 12,362 children who underwent ceVUS examinations using the second-generation UCAs SonoVue®/Lumason® or Optison® concluded that there were no serious adverse events (101). Among the 31 studies, only two reported 38 non-serious adverse events (0.31%) including dysuria (n = 27), transient abdominal pain or discomfort (n = 2), urinary retention (n = 2), anxiety during voiding (n = 1), crying during voiding (n = 1), frequency of urination (n = 1), blood and mucous discharge (n = 1), vomiting (n = 1), perineal irritation and discomfort (n = 1), and urinary tract infection (UTI) (n = 1). All these non-serious adverse events present subacute onset and are self-limited, with uneventful outcomes. They are more likely attributed to the catheterization into the bladder leading to discomfort or its psychological impact on the children than the UCA itself. A relatively small number of studies performed with ceGS reported no adverse events (24).
Clinical applications of intracavitary CEUS in lower genitourinary anomalies
Vesicoureteral reflux
VUR is the most common urinary tract anomaly in children with a high prevalence of 31.1% in children with UTI (102). It is defined as the presence of UCA microbubbles in the ureter and/or pelvicalyceal system (Figure 2). VUR can be solitary or complicated with other congenital anomalies (e.g., PUV, duplex pelvis, crossed fused renal ectopia, ureteropelvic junction stenosis, prune belly syndrome, and neurogenic bladder) (Figure 3). Solitary VUR is also called primary VUR, which is present at birth due to the deficiency in the ureterovesical junction impairing the anti-reflux mechanism. When VUR is secondary to neurogenic bladder, video urodynamic studies should be recommended as the gold standard to evaluate the function of the lower urinary tract early and to regularly follow up and monitor the deterioration of bladder function for the first few years (103).
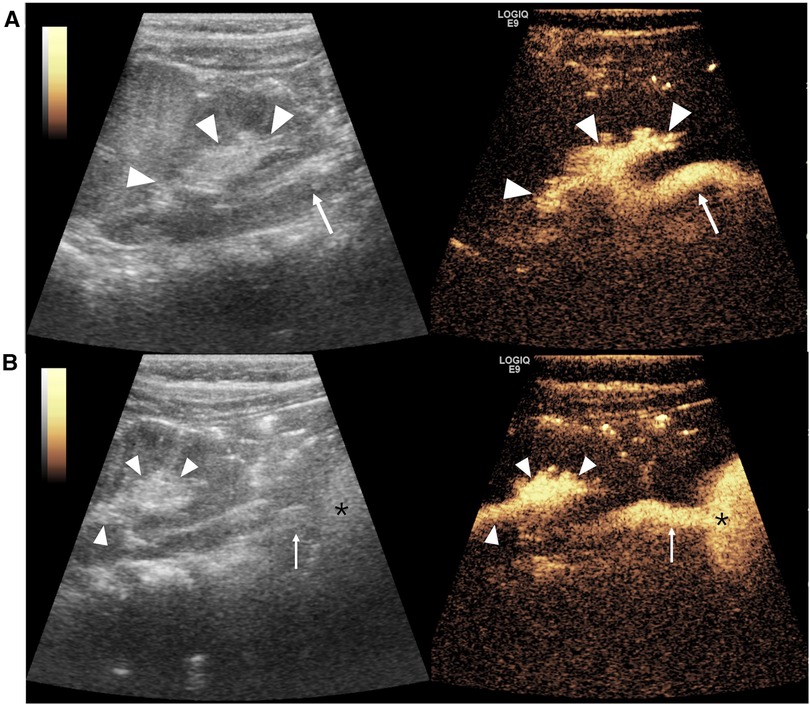
Figure 2. Vesicoureteral reflux imaging on contrast-enhanced voiding urosonography of the dual-screen mode of the left kidney in a 1-year-old boy. (A) The sagittal plane of the left kidney displays contrast agent refluxing into the ureter (arrow) and pelvicalyceal system (arrowheads), indicating grade III reflux. (B) Panoramic coronal view image: simultaneous visualization of the left kidney (arrowheads), ureter (arrow), and bladder (asterisk) from the left flank full of echogenic microbubbles in a single view.
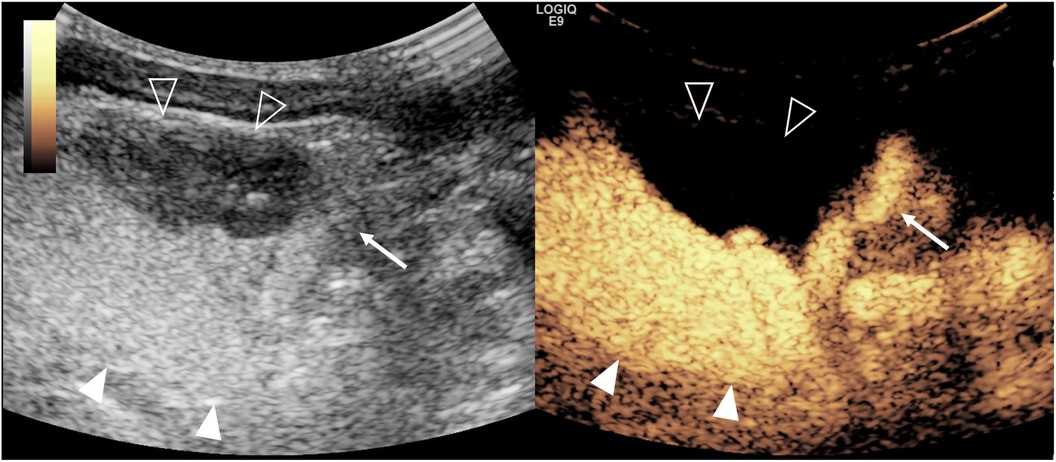
Figure 3. Duplex pelvis complicated with vesicoureteral reflux on contrast-enhanced voiding urosonography (ceVUS) of the dual-screen mode of the left kidney in a 14-day-old girl. Sagittal gray-scale ultrasound (left) and ceVUS (right) images show hydronephrosis of the upper moiety (solid arrowhead) of the duplex kidney and microbubbles refluxing and filling the upper moiety pelvicalyceal (solid arrowheads) with marked dilation and papillary impressions invisible and tortuous dilated ureter (arrow), indicating grade V reflux. There is no dilation and reflux of the lower moiety pelvicalyceal system (open arrowheads).
Intrarenal reflux occurs in 3%–10% of VUR patients, indicating an increased risk of renal scar formation especially due to high-pressure and high-grade reflux and acting as a risk factor for reflux nephropathy (Figure 4). A VUR-associated reflux nephropathy can lead to chronic kidney insufficiency and end-stage renal failure. So early diagnosis of VUR accurately, especially high-grade VUR, is crucial for patient outcomes. Traditionally, VCUG is recommended as the gold standard for diagnosing VUR in children. However, ceVUS with no radiation is gradually becoming an alternative imaging technique to VCUG as it is not only comparable or even superior compared with VCUG in the sensitivity of detecting VUR but also can detect higher grades of VUR (9). A meta-analysis published in 2019 revealed that the pooled AUC, sensitivity, and specificity of the second-generation UCA with harmonic imaging in diagnosing VUR compared with VCUG for pediatrics were 0.965, 90.43%, and 92.82%, respectively (104). In addition, no matter whether the first-generation or second-generation UCAs are used, the diagnostic value of ceVUS in diagnosing VUR is very high (105).
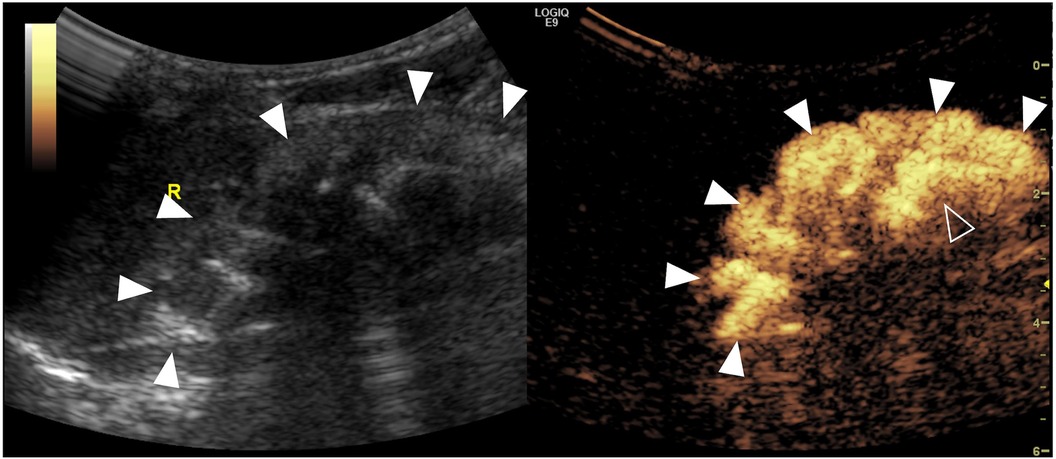
Figure 4. Severe intrarenal reflux complicated with vesicoureteral reflux on contrast-enhanced voiding urosonography of the dual-screen mode of the right kidney in a 14-day-old girl. Sagittal images of the right kidney show microbubbles refluxing into the pelvicalyceal system (open arrowheads) and diffusing into the entire renal parenchyma (solid arrowheads).
However, there are still 3% of false-negative rates, which is a limitation (105). False-negatives occur when ceVUS is performed after other diagnostic procedures, such as intravenous injection of iodine or gadolinium contrast media, or intravesical injection of iodine because of higher specific gravity of the residual contrast material depositing at the bottom of the bladder preventing the reflux of the UCA into the ureters. But this is a rare situation that can be avoided by performing the ceVUS examination on different days. Another reason is that the residual urine within the bladder does not optimally mix with the UCAs due to incomplete emptying of the bladder after bladder catheterization. To avoid this situation, ceVUS examinations should always be started with an empty or urine-free bladder.
Megaureter
A megaureter is defined as a markedly dilated ureter (larger than 7 mm in diameter) regardless of the specific pathological conditions (106). In 1976, the International Conference on Pediatric Urology classified megaureter into three categories: obstructed, refluxing, and non-refluxing non-obstructed, each of which could be subdivided into primary and secondary megaureter according to its pathogenesis. Later in 1980, King noted the presence of distal obstruction in refluxing megaureter and added the category of refluxing megaureter with obstruction (Table 3) (107). Especially when megaureter is combined with VUR, contrast material refluxing into the distal dilated ureter, even the pyelocalyceal system, could be detected by ceVUS.
Ectopic ureter
An ectopic ureter is described as the ureter inserting into the abnormal location except for the trigone. Complete duplex kidney is the most common phenotype of duplex collecting systems, which has two separate ureters called as the upper and lower moiety ureters. An ectopic ureter is usually associated with the upper moiety ureter based on Weigert–Meyer rule. The ectopic ureter opens into the proximal urethra, seminal vesicles, vas deferens, or ejaculatory track in boys; the distal urethra, vagina, or uterus in girls; and the bladder neck both in boys and girls. VUR can happen both in the upper and lower moieties with the lower moiety the frequent location (108), which can be detected by ceVUS. It is also possible for ceVUS to detect the openings of the ectopic ureters.
Ureterocele
Ureteroceles are focal cystic dilations of the intravesical portion of the distal ureter caused by congenital obstruction and may exist solitary or associated with a complete ureteral duplication. When occurring in the latter circumstance, they are associated with the ectopic insertion of the upper moiety ureter (109). According to the Glassberg classification system, ureteroceles are classified in two categories: the orthotopic type and the ectopic type. In the former case, ureteroceles are entirely located intravesical on the trigone and usually associated with the single system, while in the latter case, ureteroceles are extravesical or ectopic with a portion situated near the bladder neck or urethra and associated with the complete duplication. Ureterocele-associated pathophysiologic features (i.e., upper moiety obstruction caused by intravesical ureteral obstruction and VUR to the ipsilateral lower moiety ureter) can be perceived by ceVUS.
Bladder diverticulum
Bladder diverticulum is rare in the pediatric populations, described as a protrusion of the bladder mucosa and submucosa due to the weakness of the muscularis propria in the detrusor musculature of the bladder wall, resulting in poor emptying function (Figure 5). It can be congenital or acquired. The similarities of both types are that they have the same pathologies and histological examinations of “hypoplasia of the muscularis layer”.
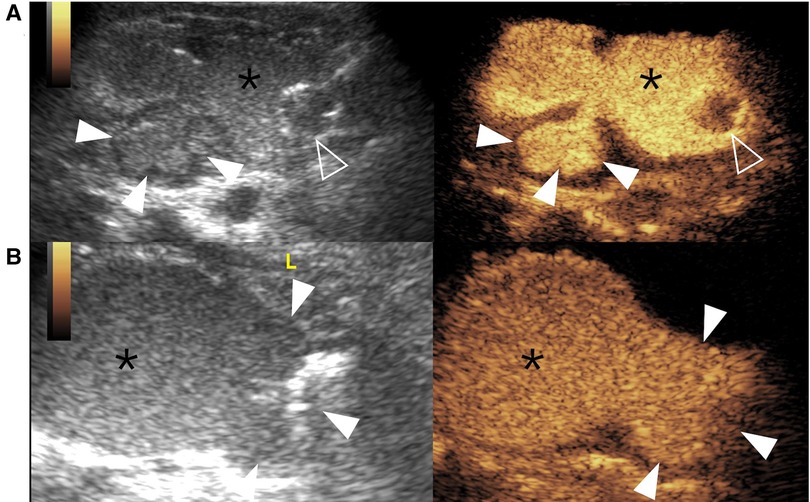
Figure 5. Bladder diverticulum on contrast-enhanced voiding urosonography of the dual-screen mode in a 3-year-old boy (A) and a 1-year-old boy (B). (A) The sagittal plane of the bladder (asterisks) shows a contrast-filled saccular-like diverticulum (solid arrowheads) behind the bladder. The open arrowhead indicates the echogenic balloon. (B) The transverse plane of the bladder (asterisks) shows a large contrast-filled diverticulum (solid arrowheads) on the left of the bladder.
Congenital or primary diverticulum occurs infrequently and can be usually single and unilateral. It is usually present during childhood with normal intravesical pressures in the absence of a bladder outlet obstruction and mainly caused by congenital connective tissue diseases, such as Ehlers–Danlos syndrome, Williams–Beuren syndrome, or Menkes kinky hair syndrome (110, 111). On cystoscopy, it reveals a smooth wall and is usually seen next to the vesicoureteric junction. Acquired or secondary diverticula are more common than the congenital types, usually multiple and resulting from any part of the bladder wall. On cystoscopy, they display multiple bladder trabecular changes. This type can be a result of high intravesical pressure caused by bladder outlet obstruction or detrusor-sphincter dyssynergia during micturition with the typical representative of neurogenic bladder.
Hutch diverticulum, also called as periureteral diverticulum, is a rare special type of congenital diverticula, located posterior-lateral to the ureteral orifice apart from the trigone, and developing to incorporate it from a small herniation (112). Ipsilateral long-standing VUR is one of the most common complications associated with it, and ceVUS is a valuable method to demonstrate VUR.
Posterior urethral valves
PUVs, only occurring in males, are the most common urethral obstructed anomaly and have high risks to result in end-stage renal failure. Its incidence is approximately 1/5,000–8,000. It is well known that PUVs are classified into three types according to the anatomic structure characteristics of the obstructed location firstly proposed by Young et al. (113) in 1919 and later precisely explained by Douglas Stephens in 1996. Clinically, type 1 and type 2 are the two common main types. Thereinto, type 2 is considered to be a normal anatomic variant and seems to be overestimated. Type 1 has a valvular shape oblique to the urethral axis, originating in abnormally located Wolffian duct orifices. Its verumontanum is larger than normal and continuous to the lesion, with the inferior crest thick like a fin. Type 3 has a membrane or diaphragm transverse to the urethral axis with a central hole, occurring from a persistent urogenital membrane. It has a relatively small verumontanum, which is discontinuous to the lesion, and the inferior is very thin. Early diagnosis, accurate discrimination of type 1 from type 3, and prompt treatment can prevent the progress of urethral obstructions and are helpful for improvements of future prognosis.
The findings of PUVs at ceVUS demonstrate bladder thickening and irregularity with diverticula formation, dilatation of the posterior urethra with a diameter of at least 7 mm, lumen narrowing of the valve area, decreased caliber of the anterior urethra, and difficulties of the contrast agents getting through the valve region (99). PUV is one common cause resulting in VUR. Some authors can also diagnose PUVs on B-mode ultrasonography (114).
Anterior urethral valves
AUVs are rarer and less frequent than PUVs in congenital lower urinary obstruction but are the most common cause of anterior urethral obstruction. Similar to PUV, AUV can also lead to renal failure. The valvular mucosa folds of AUV arise from the ventral part of the urethra and uprise during voiding, causing obstruction of the urine flow. The shape of the valves can be mostly semilunar (70%) and fractionally iris-like (30%). The location of AUV is variable, from the most common location the bulbar urethra (40%) to the distal portion of the urethra (e.g., fossa navicularis) (115). By and large, AUVs are usually located near the bulbar urethra or the penoscrotal junction. Clinical manifestations vary depending on visiting ages, obstruction severity, and related complications; presentations mainly include infectious-related symptoms primarily in the newborn and infant and voiding-related symptoms chiefly in the elderly child. VUR, one of the various complications, is highly predictive of poor outcomes, including renal failure and/or death. Sometimes longitudinal trans-penile B-mode ultrasonography can be used to diagnose AUVs during micturition. It is equivocal between the relationship of AUV and the anterior urethral diverticula as they often occur simultaneously (33%) (116). Normally, the discrepancy between the posterior and anterior urethral distention ranges from 0 to 2 mm. But in patients with AUV, we can observe the dilatation of the proximal urethra, the valve structure shown as a linear filling defect, and the prominent narrowing of the distal urethra.
Diverticula of the prostatic utricle
The normal prostatic utricle is a small, blind-opening pouch located in the midline of the verumontanum (i.e., the floor of the prostatic urethra), with columnar or cuboidal epithelium lining, communicating with the urethra. When enlarged to at least 1 cm, it is considered pathological, which is lined by squamous epithelium. The most common symptoms of diverticula of the prostatic utricle include UTI, postvoid dribbling, and epididymitis. Enlarged prostatic utricles are frequently seen in boys with intersex, prune belly syndrome, Down syndrome, cryptorchidism, or hypospadias (11%–14%) (117, 118).
Stricture of the bulbar urethra
The causes leading to stricture of the bulbar urethra can be inflammatory, traumatic, or congenital. While most urethral bulbar strictures are the result of infectious urethritis, straddle injury, or other iatrogenic instrumentation causes, congenital stricture of the bulbar urethra is rare and referred to as obstructive Cobb's collar, Moormann's ring, or Young's type III valve, which is caused by disabling of canalization of the cloacal membrane in the development of fetuses. The ceVUS technique can not only assess the length and thickness of bulbar urethral stricture but also differentiate the congenital stricture of the bulbar urethra from segmental strictures, which is important for surgery planning. Congenital stricture of the bulbar urethra at ceVUS demonstrates a focal narrowing of the bulbar urethra, a dilated urethra proximal to the stricture, and a normal penile urethra. The site of narrowing in the bulbous urethra is more distal to the external urethral sphincter, which is different from PUV. Patients with bulbar urethral stricture present with UTI or diurnal enuresis, and VUR takes up to 53% of the cases (119).
Spinning top urethra
Spinning top urethras are named for the markedly dilatated posterior urethra presenting a fusiform distal-end appearance and mainly in females. It is used to be considered a normal variant, but recently, some investigators have proposed that these conditions should hint at the lower urinary tract functional disorders. Spinning top urethra can be associated with bladder disturbance, recurrent UTI, and voiding dysfunction, as well as VUR. As ceVUS is a real-time tool, it can identify spinning top urethra, which is only present during the early voiding phase and resolves in the later voiding phase.
Urogenital sinus
Persistent urogenital sinus (PUGS) is one form of congenital developmental cloacal anomalies, of which the incidence is about 6/100,000 (120) and only occurs in females. Its anatomical characteristic is described as a single common channel where the urethra and vagina converge due to the failure of urethra–vaginal septum formation, while the normal orifice of the anus exists. It can be isolated or has an association with a variety of diseases including congenital adrenal hyperplasia, McKusick–Kaufman syndrome, or Bardet–Biedl syndrome. There are two types of PUGS according to the length of the common PUGS: high (>3 cm) and low (<3 cm). Accurately distinguishing from the two types and determining the level where the urethra opens are critically crucial for surgery planning strategies. The technique of ceGS combined with ceVUS can delineate the anatomical abnormalities of PUGS, reveal the common passage of urethra and vagina, and measure the length of urethra and the common route (121). As it is sometimes challenging to evaluate the anatomy of the urogenital sinus, a three-dimensional or four-dimensional ultrasound reconstruction and respective post-processing techniques could be used to improve the diagnostic accuracy (24). CeGS alone is also used to evaluate the effect of surgical treatment and follow up for complications after surgery (62).
Discussion
In this narrative review, we elaborated on the evolution history of intracavitary CEUS techniques (ceVUS, ceRUG, and ceGS) and further introduced their appropriate dosages and safety in pediatrics. Most important of all, we described related pediatric clinical applications of ceVUS, ceRUG, and ceGS in lower genitourinary diseases (e.g., VUR, megaureter, ectopic ureter, ureterocele, bladder diverticulum, PUV, AUV, diverticula of the prostatic utricle, stricture of the bulbar urethra, spinning top urethra, and urogenital sinus).
Most of the published studies and reviews were related to ceVUS in evaluating VUR (64). Only a few case reports or case series and reviews were about other genitourinary diseases (7, 24, 62). Compared with previous reviews, ours is the first comprehensive and systematic review to describe the evolution history, appropriate dosages and safety of intracavitary CEUS techniques (ceVUS, ceRUG, and ceGS), and their clinical applications in lower genitourinary diseases in pediatrics.
As the only officially approved intracavitary CEUS application in children worldwide, ceVUS has many advantages compared with fluoroscopy because it is safe, radiation-free, real-time, and bedside or intraoperatively performed (6, 122). As numerous evidence-based medical studies have shown that ceVUS is comparable with or even more sensitive in detecting VUR than VCUG or RNC in routine diagnostic scenarios, it has much potential to complement or even replace traditional radiated procedures in several conditions (107).
Although ceVUS has so many advantages, it is crucial to consider its limitations. Firstly, there is no single uniform standardization to perform this approach requiring individual case-by-case examination. In addition, some inherent limitations regarding ultrasound use should be taken into consideration (e.g., operator dependence, bowel gas interference, and difficulties obtaining some anatomical regions). Finally, the biggest limitation of ceVUS is that it cannot present panoramic views leading to the incapability of visualizing the urethra and reno-ureteral system at one view during voiding (80). Sometimes, to assess the urethra simultaneously, VCUG is reserved for evaluating some complex anatomic settings pre-surgically.
Despite intracavitary CEUS ultrasound examinations being off-label except for ceVUS, the European Society of Pediatric Radiology (ESPR) abdominal task force still recommends ceRUG in evaluating the urethra or ceGS in imaging urogenital sinus malformations as an alternative option (24, 123). As a non-irradiating, safe, and sedation-free imaging method, some promising experience of ceRUG and ceGS has been accumulated in appropriate clinical circumstances. The greatest benefit of the two techniques over fluoroscopy is their ability to not only reveal the opacified organs and abnormal connections but also highlight the structures around the organs. However, studies about the applications of ceRUG and ceGS are only limited to a few case reports or small cumulative cohorts. To further explore the value of ceRUG and ceGS in pediatric lower genitourinary anomalies, more excellent studies about ceRUG and ceGS in the future are anticipated.
Conclusion
We presented three intracavitary CEUS methods including ceVUS, ceRUG, and ceGS in pediatric lower genitourinary anomalies, which have the advantages of no radiation exposure and displaying both morphological and functional imaging. As a safe problem-solving and increasingly welcomed imaging modality, ceVUS is practical and effective and can be a feasible imaging alternative to fluoroscopy because of its high diagnostic accuracy for detecting and grading VUR in pediatrics. In addition, in the evaluation of urethral pathologies and urogenital sinus, ceRUG and ceGS have become promising techniques for their feasibility, efficacy, and no need for sedation. With the advent of 3D printing, we look forward to further significant developments of the intracavitary CEUS technique.
Author contributions
XC introduced the idea for the article, and the first draft of the manuscript was written by JR. All authors agreed to be accountable for the content of the work. All authors contributed to the article and approved the submitted version.
Funding
This work has received funding from the National Natural Science Foundation of China (grant no. 82071953) and the Medical Youth Top-notch Talent Project of Hubei Province (2020LJRC010).
Conflict of interest
The handling editor QT declared a shared parent affiliation with the authors JR, TM, XC at the time of review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nino F, Ilari M, Noviello C, Santoro L, Rätsch IM, Martino A, et al. Genetics of vesicoureteral reflux. Curr Genomics. (2016) 17(1):70–9. doi: 10.2174/1389202916666151014223507
2. Rasouly HM, Lu W. Lower urinary tract development and disease. Wiley Interdiscip Rev Syst Biol Med. (2013) 5(3):307–42. doi: 10.1002/wsbm.1212
3. Conway JJ, Kruglik GD. Effectiveness of direct and indirect radionuclide cystography in detecting vesicoureteral reflux. J Nucl Med. (1976) 17(02):81–3.1245882
4. Elsingergy MM, Bellah RD, Back SJ, Weiss DA, Darge K. Retrograde urethrography in children: a decade of experience at a children’s hospital. Pediatr Radiol. (2023) 53(5):862–74. doi: 10.1007/s00247-023-05589-7
5. Valentini AL, Giuliani M, Gui B, Laino ME, Zecchi V, Rodolfino E, et al. Persistent urogenital sinus: diagnostic imaging for clinical management. What does the radiologist need to know? Am J Perinatol. (2016) 33(5):425–32. doi: 10.1055/s-0035-1565996
6. Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr Radiol. (2013) 43(9):1063–73. doi: 10.1007/s00247-013-2746-6
7. Chow JS, Paltiel HJ, Padua HM, McNamara E, Dickie BH. Case series: comparison of contrast-enhanced genitosonography (CEGS) to fluoroscopy and cone-beam computed tomography in patients with urogenital sinus and the cloacal malformation. Clin Imaging. (2020) 60(2):204–8. doi: 10.1016/j.clinimag.2019.11.010
8. Simicic Majce A, Arapovic A, Saraga-Babic M, Vukojevic K, Benzon B, Punda A, et al. Intrarenal reflux in the light of contrast-enhanced voiding urosonography. Front Pediatr. (2021) 9:642077. doi: 10.3389/fped.2021.642077
9. Ntoulia A, Back SJ, Shellikeri S, Poznick L, Morgan T, Kerwood J, et al. Contrast-enhanced voiding urosonography (CEVUS) with the intravesical administration of the ultrasound contrast agent Optison™ for vesicoureteral reflux detection in children: a prospective clinical trial. Pediatr Radiol. (2018) 48(2):216–26. doi: 10.1007/s00247-017-4026-3
10. Cvitkovic-Roic A, Turudic D, Milosevic D, Palcic I, Roic G. Contrast-enhanced voiding urosonography in the diagnosis of intrarenal reflux. J Ultrasound. (2022) 25(1):89–95. doi: 10.1007/s40477-021-00568-w
11. Seelbach J, Krüger PC, Waginger M, Renz DM, Mentzel HJ. Safety and parents’ acceptance of ultrasound contrast agents in children and adolescents—contrast enhanced voiding urosonography and contrast enhanced ultrasound. Med Ultrason. (2022) 24(1):27–32. doi: 10.11152/mu-3196
12. Benya EC, Prendergast FM, Liu DB, Wyers MR. Assessment of distal ureteral and ureterovesical junction visualization on contrast-enhanced voiding urosonography. Pediatr Radiol. (2021) 51(8):1406–11. doi: 10.1007/s00247-021-04979-z
13. Kim D, Choi YH, Choi G, Lee S, Lee S, Cho YJ, et al. Contrast-enhanced voiding urosonography for the diagnosis of vesicoureteral reflux and intrarenal reflux: a comparison of diagnostic performance with fluoroscopic voiding cystourethrography. Ultrasonography. (2021) 40(4):530–7. doi: 10.14366/usg.20157
14. Marschner CA, Schwarze V, Stredele R, Froelich MF, Rübenthaler J, Geyer T, et al. Safety assessment and diagnostic evaluation of patients undergoing contrast-enhanced urosonography in the setting of vesicoureteral reflux confirmation. Clin Hemorheol Microcirc. (2021) 79(1):65–72. doi: 10.3233/ch-219110
15. Rubelj K, Oletić L, Valent Morić B, Trutin I. Our experience with contrast-enhanced voiding urosonography. Acta Clin Croat. (2021) 60(2):184–90. doi: 10.20471/acc.2021.60.02.03
16. Fischer KM, Bowen DK, Kovell RC, Back SJ, Darge K, Weiss DA. Intraoperative contrast enhanced sonourethrography to characterize urethral stricture in a pediatric patient. Urol Case Rep. (2020) 32:101223. doi: 10.1016/j.eucr.2020.101223
17. Rojas-Ticona J, Fernández Córdoba MS, Cabezalí Barbancho D, Marijuán Sahuquillo V, Argumosa Salazar YM, Ramírez Piqueras M, et al. Serial voiding urosonography in posterior urethral valve diagnosis and management in pediatric patients. Cir Pediatr. (2020) 33(1):36–42.32166922
18. Siomou E, Giapros V, Serbis A, Makrydimas G, Papadopoulou F. Voiding urosonography and voiding cystourethrography in primary vesicoureteral reflux associated with mild prenatal hydronephrosis: a comparative study. Pediatr Radiol. (2020) 50(9):1271–6. doi: 10.1007/s00247-020-04724-y
19. Yi H, Cui X, Cai B, Qiu L, Song P, Zhang W. A quantitative grading system of vesicoureteral reflux by contrastenhanced voiding urosonography. Med Ultrason. (2020) 22(3):287–92. doi: 10.11152/mu-2311
20. Ključevšek D, Pečanac O, Tomažič M, Glušič M. Potential causes of insufficient bladder contrast opacification and premature microbubble destruction during contrast-enhanced voiding urosonography in children. J Clin Ultrasound. (2019) 47(1):36–41. doi: 10.1002/jcu.22656
21. Velasquez M, Emerson MG, Diaz E, Kennedy W, Rubesova E, Barth RA. The learning curve of contrast-enhanced ‘microbubble’ voiding urosonography-validation study. J Pediatr Urol. (2019) 15(4):385.e1–e6. doi: 10.1016/j.jpurol.2019.04.015
22. Mane N, Sharma A, Patil A, Gadekar C, Andankar M, Pathak H. Comparison of contrast-enhanced voiding urosonography with voiding cystourethrography in pediatric vesicoureteral reflux. Turk J Urol. (2018) 44(3):261–7. doi: 10.5152/tud.2018.76702
23. Patel H, Watterson C, Chow JS. Case of urethral duplication seen by voiding urosonography. Clin Imaging. (2018) 49:106–10. doi: 10.1016/j.clinimag.2017.11.011
24. Seranio N, Darge K, Canning DA, Back SJ. Contrast enhanced genitosonography (CEGS) of urogenital sinus: a case of improved conspicuity with image inversion. Radiol Case Rep. (2018) 13(3):652–4. doi: 10.1016/j.radcr.2018.03.014
25. Woźniak MM, Osemlak P, Ntoulia A, Borzęcka H, Bieniaś B, Brodzisz A, et al. 3D/4D contrast-enhanced urosonography (ceVUS) in children - is it superior to the 2D technique? J Ultrason. (2018) 18(73):120–5. doi: 10.15557/JoU.2018.0017
26. Zhang W, Cai B, Zhang X, Zhou J, Qiu L, Yi H. Contrast-enhanced voiding urosonography with intravesical administration of ultrasound contrast agent for the diagnosis of pediatric vesicoureteral reflux. Exp Ther Med. (2018) 16(6):4546–52. doi: 10.3892/etm.2018.6793
27. Kuzmanovska D, Risteski A, Kambovska M, Trpcevski T, Sahpazova E, Petrovski M. Voiding urosonography with second-generation ultrasound contrast agent for diagnosis of vesicoureteric reflux: first local pilot study. Open Access Maced J Med Sci. (2017) 5(2):215–21. doi: 10.3889/oamjms.2017.055
28. Colleran GC, Barnewolt CE, Chow JS, Paltiel HJ. Intrarenal reflux: diagnosis at contrast-enhanced voiding urosonography. J Ultrasound Med. (2016) 35(8):1811–9. doi: 10.7863/ultra.15.09056
29. Piskunowicz M, Swieton D, Rybczynska D, Szarmach A, Szurowska E, Pruijm M. Premature destruction of microbubbles during voiding urosonography in children and possible underlying mechanisms: post hoc analysis from the prospective study. Biomed Res Int. (2016) 2016:1764692. doi: 10.1155/2016/1764692
30. Woźniak MM, Wieczorek AP, Pawelec A, Brodzisz A, Zajączkowska MM, Borzęcka H, et al. Two-dimensional (2D), three-dimensional static (3D) and real-time (4D) contrast enhanced voiding urosonography (ceVUS) versus voiding cystourethrography (VCUG) in children with vesicoureteral reflux. Eur J Radiol. (2016) 85(6):1238–45. doi: 10.1016/j.ejrad.2015.11.006
31. Babu R, Gopinath V, Sai V. Voiding urosonography: contrast-enhanced ultrasound cystography to diagnose vesico-ureteric reflux: a pilot study. J Indian Assoc Pediatr Surg. (2015) 20(1):40–1. doi: 10.4103/0971-9261.145548
32. Faizah MZ, Hamzaini AH, Kanaheswari Y, Dayang AA, Zulfiqar MA. Contrast enhanced voiding urosonography (CE-VUS) as a radiation-free technique in the diagnosis of vesicoureteric reflux: our early experience. Med J Malaysia. (2015) 70(5):269–72.26556113
33. Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol. (2014) 44(6):719–28. doi: 10.1007/s00247-013-2832-9
34. Wong LS, Tse KS, Fan TW, Kwok KY, Tsang TK, Fung HS, et al. Voiding urosonography with second-generation ultrasound contrast versus micturating cystourethrography in the diagnosis of vesicoureteric reflux. Eur J Pediatr. (2014) 173(8):1095–101. doi: 10.1007/s00431-014-2297-3
35. Woźniak MM, Osemlak P, Pawelec A, Brodzisz A, Nachulewicz P, Wieczorek AP, et al. Intraoperative contrast-enhanced urosonography during endoscopic treatment of vesicoureteral reflux in children. Pediatr Radiol. (2014) 44(9):1093–100. doi: 10.1007/s00247-014-2963-7
36. Woźniak MM, Pawelec A, Wieczorek AP, Zajączkowska MM, Borzęcka H, Nachulewicz P. 2D/3D/4D contrast-enhanced voiding urosonography in the diagnosis and monitoring of treatment of vesicoureteral reflux in children - can it replace voiding cystourethrography? J Ultrason. (2013) 13(55):394–407. doi: 10.15557/JoU.2013.0042
37. Duran C, del Riego J, Riera L, Martin C, Serrano C, Palaña P. Voiding urosonography including urethrosonography: high-quality examinations with an optimised procedure using a second-generation US contrast agent. Pediatr Radiol. (2012) 42(6):660–7. doi: 10.1007/s00247-012-2360-z
38. Ključevšek D, Battelino N, Tomažič M, Kersnik Levart T. A comparison of echo-enhanced voiding urosonography with x-ray voiding cystourethrography in the first year of life. Acta Paediatr. (2012) 101(5):e235–9. doi: 10.1111/j.1651-2227.2011.02588.x
39. Kis E, Nyitrai A, Várkonyi I, Máttyus I, Cseprekál O, Reusz G, et al. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol. (2010) 25(11):2289–93. doi: 10.1007/s00467-010-1618-7
40. Duran C, Valera A, Alguersuari A, Ballesteros E, Riera L, Martin C, et al. Voiding urosonography: the study of the urethra is no longer a limitation of the technique. Pediatr Radiol. (2009) 39(2):124–31. doi: 10.1007/s00247-008-1050-3
41. Kopac M, Riccabona M, Haim M. Contrast-enhanced voiding urosonography and genitography in a baby with ambiguous genitalia and urogenital sinus. Ultraschall Med. (2009) 30(3):299–300. doi: 10.1055/s-0028-1109353
42. Papadopoulou F, Anthopoulou A, Siomou E, Efremidis S, Tsamboulas C, Darge K. Harmonic voiding urosonography with a second-generation contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol. (2009) 39(3):239–44. doi: 10.1007/s00247-008-1080-x
43. Giordano M, Marzolla R, Puteo F, Scianaro L, Caringella DA, Depalo T. Voiding urosonography as first step in the diagnosis of vesicoureteral reflux in children: a clinical experience. Pediatr Radiol. (2007) 37(7):674–7. doi: 10.1007/s00247-007-0499-9
44. Papadopoulou F, Tsampoulas C, Siomou E, Tzovara J, Siamopoulou A, Efremidis SC. Cyclic contrast-enhanced harmonic voiding urosonography for the evaluation of reflux. Can we keep the cost of the examination low? Eur Radiol. (2006) 16(11):2521–6. doi: 10.1007/s00330-006-0253-y
45. Berrocal T, Gayá F, Arjonilla A. Vesicoureteral reflux: can the urethra be adequately assessed by using contrast-enhanced voiding US of the bladder? Radiology. (2005) 234(1):235–41. doi: 10.1148/radiol.2341031503
46. Darge K, Moeller RT, Trusen A, Butter F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteric reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. (2005) 35(1):73–8. doi: 10.1007/s00247-004-1317-2
47. Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Fede C, Visalli C, et al. Harmonic US imaging of vesicoureteric reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol. (2004) 34(6):481–7. doi: 10.1007/s00247-004-1190-z
48. Vassiou K, Vlychou M, Moisidou R, Sioka A, Fezoulidis IV. Contrast-enhanced sonographic detection of vesicoureteral reflux in children: comparison with voiding cystourethrography. Rofo. (2004) 176(10):1453–7. doi: 10.1055/s-2004-813170
49. Xhepa R, Bosio M, Manzoni G. Voiding cystourethrosonography for the diagnosis of vesicoureteral reflux in a developing country. Pediatr Nephrol. (2004) 19(6):638–43. doi: 10.1007/s00467-004-1439-7
50. Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Baldari S, Fede C. Vesicoureteral reflux: comparison between urosonography and radionuclide cystography. Pediatr Nephrol. (2003) 18(8):768–71. doi: 10.1007/s00467-003-1130-4
51. Darge K, Trusen A, Gordjani N, Riedmiller H. Intrarenal reflux: diagnosis with contrast-enhanced harmonic US. Pediatr Radiol. (2003) 33(10):729–31. doi: 10.1007/s00247-003-1050-2
52. Maté A, Bargiela A, Mosteiro S, Diaz A, Bello MJ. Contrast ultrasound of the urethra in children. Eur Radiol. (2003) 13(7):1534–7. doi: 10.1007/s00330-002-1790-7
53. Uhl M, Kromeier J, Zimmerhackl LB, Darge K. Simultaneous voiding cystourethrography and voiding urosonography. Acta Radiol. (2003) 44(3):265–8. doi: 10.1080/j.1600-0455.2003.00065.x
54. Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur J Radiol. (2002) 43(2):122–8. doi: 10.1016/s0720-048x(02)00114-6
55. Darge K, Zieger B, Rohrschneider W, Ghods S, Wunsch R, Troeger J. Contrast-enhanced harmonic imaging for the diagnosis of vesicoureteral reflux in pediatric patients. Am J Roentgenol. (2001) 177(6):1411–5. doi: 10.2214/ajr.177.6.1771411
56. Escape I, Martínez J, Bastart F, Solduga C, Sala P. Usefulness of echocystography in the study of vesicoureteral reflux. J Ultrasound Med. (2001) 20(2):145–9. doi: 10.7863/jum.2001.20.2.145
57. Valentini AL, Salvaggio E, Manzoni C, Rendeli C, Destito C, Summaria V, et al. Contrast-enhanced gray-scale and color Doppler voiding urosonography versus voiding cystourethrography in the diagnosis and grading of vesicoureteral reflux. J Clin Ultrasound. (2001) 29(2):65–71. doi: 10.1002/1097-0096(200102)29:2%3C65::Aid-jcu1000%3E3.0.Co;2-i
58. Mentzel HJ, Vogt S, Patzer L, Schubert R, John U, Misselwitz J, et al. Contrast-enhanced sonography of vesicoureterorenal reflux in children: preliminary results. Am J Roentgenol. (1999) 173(3):737–40. doi: 10.2214/ajr.173.3.10470914
59. Bosio M. Cystosonography with echocontrast: a new imaging modality to detect vesicoureteric reflux in children. Pediatr Radiol. (1998) 28(4):250–5. doi: 10.1007/s002470050343
60. Kaneko K, Kuwatsuru R, Fukuda Y, Yamataka A, Yabuta K, Katayama H, et al. Contrast sonography for detection of vesicoureteral reflux. Lancet. (1994) 344(8923):687. doi: 10.1016/S0140-6736(94)92123-7
61. Barnewolt CE, Acharya PT, Aguirre Pascual E, Back SJ, Beltrán Salazar VP, Chan PKJ, et al. Contrast-enhanced voiding urosonography part 2: urethral imaging. Pediatr Radiol. (2021) 51(12):2368–86. doi: 10.1007/s00247-021-05116-6
62. Chow JS, Bellah RD, Darge K, Ntoulia A, Phelps AS, Riccabona M, et al. Contrast-enhanced genitosonography and colosonography: emerging alternatives to fluoroscopy. Pediatr Radiol. (2021) 51(12):2387–95. doi: 10.1007/s00247-020-04770-6
63. Didier RA, Biko DM, Hwang M, Unnikrishnan S, Woźniak MM, Yusuf GT, et al. Emerging contrast-enhanced ultrasound applications in children. Pediatr Radiol. (2021) 51(12):2418–24. doi: 10.1007/s00247-021-05045-4
64. Ntoulia A, Aguirre Pascual E, Back SJ, Bellah RD, Beltrán Salazar VP, Chan PKJ, et al. Contrast-enhanced voiding urosonography, part 1: vesicoureteral reflux evaluation. Pediatr Radiol. (2021) 51(12):2351–67. doi: 10.1007/s00247-020-04906-8
65. Sofia C, Solazzo A, Cattafi A, Chimenz R, Cicero G, Marino MA, et al. Contrast-enhanced voiding urosonography in the assessment of vesical-ureteral reflux: the time has come. Radiol Med. (2021) 126(7):901–9. doi: 10.1007/s11547-021-01360-w
66. Barr RG, Wilson SR, Lyshchik A, McCarville B, Darge K, Grant E, et al. Contrast-enhanced ultrasound: state of the art in North America. Ultrasound Q. (2020) 36(3):206–17. doi: 10.1097/ruq.0000000000000514
67. Viteri B, Calle-Toro JS, Furth S, Darge K, Hartung EA, Otero H. State-of-the-art renal imaging in children. Pediatrics. (2020) 145(2):e20190829. doi: 10.1542/peds.2019-0829
68. Chua ME, Kim JK, Mendoza JS, Fernandez N, Ming JM, Marson A, et al. The evaluation of vesicoureteral reflux among children using contrast-enhanced ultrasound: a literature review. J Pediatr Urol. (2019) 15(1):12–7. doi: 10.1016/j.jpurol.2018.11.006
69. Ntoulia A, Anupindi SA, Darge K, Back SJ. Applications of contrast-enhanced ultrasound in the pediatric abdomen. Abdom Radiol. (2018) 43(4):948–59. doi: 10.1007/s00261-017-1315-0
70. Yusuf GT, Fang C, Huang DY, Sellars ME, Deganello A, Sidhu PS. Endocavitary contrast enhanced ultrasound (CEUS): a novel problem solving technique. Insights Imaging. (2018) 9(3):303–11. doi: 10.1007/s13244-018-0601-x
71. Duran C, Beltrán VP, González A, Gómez C, Riego JD. Contrast-enhanced voiding urosonography for vesicoureteral reflux diagnosis in children. Radiographics. (2017) 37(6):1854–69. doi: 10.1148/rg.2017170024
72. Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, et al. Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med. (2017) 38(1):33–43. doi: 10.1055/s-0042-110394
73. Stenzel M, Mentzel HJ. Ultrasound elastography and contrast-enhanced ultrasound in infants, children and adolescents. Eur J Radiol. (2014) 83(9):1560–9. doi: 10.1016/j.ejrad.2014.06.007
74. Schreiber-Dietrich DG, Cui XW, Piscaglia F, Gilja OH, Dietrich CF. Contrast enhanced ultrasound in pediatric patients: a real challenge. Z Gastroenterol. (2014) 52(10):1178–84. doi: 10.1055/s-0034-1366766
75. Tse KS, Wong LS, Lau HY, Fok WS, Chan YH, Tang KW, et al. Paediatric vesicoureteric reflux imaging: where are we? Novel ultrasound-based voiding urosonography. Hong Kong Med J. (2014) 20(5):437–43. doi: 10.12809/hkmj144215
76. Ignee A, Schuessler G, Cui XW, Dietrich CF. Intracavitary contrast medium ultrasound - different applications, a review of the literature ad future prospects. Ultraschall Med. (2013) 34(6):504–25. doi: 10.1055/s-0033-1335546
77. Dietrich CF, Cui XW, Barreiros AP, Hocke M, Ignee A. EFSUMB guidelines 2011: comment on emergent indications and visions. Ultraschall Med. (2012) 33(Suppl 1):S39–47. doi: 10.1055/s-0032-1312895
78. Darge K, Grattan-Smith JD, Riccabona M. Pediatric uroradiology: state of the art. Pediatr Radiol. (2011) 41(1):82–91. doi: 10.1007/s00247-010-1644-4
79. McCarville MB. Contrast-enhanced sonography in pediatrics. Pediatr Radiol. (2011) 41(Suppl 1):S238–42. doi: 10.1007/s00247-011-2005-7
80. Zimbaro G, Ascenti G, Visalli C, Bottari A, Zimbaro F, Martino N, et al. Contrast-enhanced ultrasonography (voiding urosonography) of vesicoureteral reflux: state of the art. Radiol Med. (2007) 112(8):1211–24. doi: 10.1007/s11547-007-0218-5
81. Darge K, Riedmiller H. Current status of vesicoureteral reflux diagnosis. World J Urol. (2004) 22(2):88–95. doi: 10.1007/s00345-004-0404-1
82. Darge K. Diagnosis of vesicoureteral reflux with ultrasonography. Pediatr Nephrol. (2002) 17(1):52–60. doi: 10.1007/s004670200010
83. Riccabona M. Potential of modern sonographic techniques in paediatric uroradiology. Eur J Radiol. (2002) 43(2):110–21. doi: 10.1016/s0720-048x(02)00118-3
84. Tremewan RN, Bailey RR, Little PJ, Maling TM, Peters TM, Tait JJ. Diagnosis of gross vesico-ureteric reflux using ultrasonography. Br J Urol. (1976) 48(6):431–5. doi: 10.1111/j.1464-410x.1976.tb06674.x
85. Evans ED, Meyer JS, Harty MP, Bellah RD. Assessment of increase in renal pelvic size on post-void sonography as a predictor of vesicoureteral reflux. Pediatr Radiol. (1999) 29(4):291–4. doi: 10.1007/s002470050591
86. Jequier S, Paltiel H, Lafortune M. Ureterovesical jets in infants and children: duplex and color Doppler US studies. Radiology. (1990) 175(2):349–53. doi: 10.1148/radiology.175.2.2183278
87. Hofmann V. Ultrasonic diagnosis of vesico-ureteral reflux in children. Z Urol Nephrol. (1981) 74(4):249–61.7269809
88. Egghart G, Schlickenrieder JH, Hautmann R. Diagnosis of reflux in children with CO2 and ultrasound. Technic and preliminary results. Urol A. (1986) 25(6):329–32.
89. Kessler RM, Altman DH. Real-time sonographic detection of vesicoureteral reflux in children. Am J Roentgenol. (1982) 138(6):1033–6. doi: 10.2214/ajr.138.6.1033
90. Alzen G, Wildberger J, Ferris E, Guenther R. Sonographic detection of vesicoureteral reflux with air: a new method. Eur Radiol. (1994) 4(2):142–5. doi: 10.1007/BF00231200
91. Rohden Lv, Bosse U, Wiemann D Refluxsonographie bei kindern mit einem ultraschallkontrastmittel im vergleich zur rontgenmiktionszystourethrographie. Padiatrische Praxis (1995) 49(1):49–58.
92. Goldberg BB, Liu J-B, Forsberg F. Ultrasound contrast agents: a review. Ultrasound Med Biol. (1994) 20(4):319–33. doi: 10.1016/0301-5629(94)90001-9
93. Beyer HJ, Hofmann V, Brettschneider D. The micturition sonourogram: a new possibility for determining vesicorenal reflux in children. Ultraschall Med. (1985) 6(4):182–8. doi: 10.1055/s-2007-1006053
94. Radmayr C, Klauser A, Pallwein L, Zurnedden D, Bartsch G, Frauscher F. Contrast enhanced reflux sonography in children: a comparison to standard radiological imaging. J Urol. (2002) 167(3):1428–30. doi: 10.1016/S0022-5347(05)65335-9
95. Hanbury DC, Coulden RA, Farman P, Sherwood T. Ultrasound cystography in the diagnosis of vesicoureteric reflux. Br J Urol. (1990) 65(3):250–3. doi: 10.1111/j.1464-410x.1990.tb14720.x
96. Farina R, Arena C, Pennisi F, Di Benedetto V, Politi G, Di Benedetto A. Retrograde echocystography: a new ultrasonographic technique for the diagnosis and staging of vesicoureteral reflux. Radiol Med. (1999) 97(5):360–4.10432967
97. Gilsanz V, Miller J, Reid B. Ultrasonic characteristics of posterior urethral valves. Radiology. (1982) 145(1):143–5. doi: 10.1148/radiology.145.1.7122871
98. Cremin BJ, Aaronson IA. Ultrasonic diagnosis of posterior urethral valve in neonates. Br J Radiol. (1983) 56(667):435–8. doi: 10.1259/0007-1285-56-667-435
99. Good CD, Vinnicombe SJ, Minty IL, King AD, Mather SJ, Dicks-Mireaux C. Posterior urethral valves in male infants and newborns: detection with US of the urethra before and during voiding. Radiology. (1996) 198(2):387–91. doi: 10.1148/radiology.198.2.8596837
100. Battelino N, Ključevšek D, Tomažič M, Levart TK. Vesicoureteral reflux detection in children: a comparison of the midline-to-orifice distance measurement by ultrasound and voiding urosonography. Pediatr Nephrol. (2016) 31(6):957–64. doi: 10.1007/s00467-015-3301-5
101. Ntoulia A, Anupindi SA, Back SJ, Didier RA, Hwang M, Johnson AM, et al. Contrast-enhanced ultrasound: a comprehensive review of safety in children. Pediatr Radiol. (2021) 51(12):2161–80. doi: 10.1007/s00247-021-05223-4
102. Sargent M. Opinion: what is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. (2000) 30(9):587–93. doi: 10.1007/s002470000263
103. Wiener JS, Huck N, Blais AS, Rickard M, Lorenzo A, Di Carlo HNM, et al. Challenges in pediatric urologic practice: a lifelong view. World J Urol. (2021) 39(4):981–91. doi: 10.1007/s00345-020-03203-1
104. Chua ME, Mendoza JS, Ming JM, Dy JS, Gomez O. Diagnostic accuracy of contrast-enhanced voiding urosonogram using second-generation contrast with harmonic imaging (CEVUS-HI) study for assessment of vesicoureteral reflux in children: a meta-analysis. World J Urol. (2019) 37(10):2245–55. doi: 10.1007/s00345-018-2587-x
105. Yousefifard M, Toloui A, Rafiei Alavi SN, Madani Neishaboori A, Ahmadzadeh K, Ghelichkhani P, et al. Contrast-enhanced voiding urosonography, a possible candidate for the diagnosis of vesicoureteral reflux in children and adolescents; a systematic review and meta-analysis. J Pediatr Urol. (2022) 18(1):61–74. doi: 10.1016/j.jpurol.2021.10.023
106. Wilcox D, Mouriquand P. Management of megaureter in children. Eur Urol. (1998) 34(1):73–8. doi: 10.1159/000019665
107. King LR. Megaloureter: definition, diagnosis and management. J Urol. (1980) 123(2):222–3. doi: 10.1016/S0022-5347(17)55867-X
108. Davda S, Vohra A. Adult duplex kidneys: an important differential diagnosis in patients with abdominal cysts. JRSM Short Rep. (2013) 4(2):1–3. doi: 10.1177/2042533312472126
110. Telli O, Guclu AG, Haciyev P, Burgu B, Gogus C. A rare entity in adults: bilateral Hutch diverticulum with calculi. Can Urol Assoc J. (2015) 9(5-6):E343–4. doi: 10.5489/cuaj.2327
111. Breivik N, Refsum S Jr, Oppedal BR, Vesterhus P. Ehlers–Danlos syndrome and diverticula of the bladder. Z Kinderchir. (1985) 40(4):243–6. doi: 10.1055/s-2008-1059784
112. Pace AM, Powell C. Congenital vesical diverticulum in a 38-year-old female. Int Urol Nephrol. (2005) 37(3):473–5. doi: 10.1007/s11255-004-8074-x
113. Young HH, Frontz WA, Baldwin JG. Congenital obstruction of the posterior Urethra11read at the meeting of the Association of Genito-Urinary Surgeons at Atlantic City, New Jersey, June, 1919. J Urol. (1919) 3(5):289–366. doi: 10.1016/S0022-5347(17)74179-1
114. Son JK, Taylor GA. Transperineal ultrasonography. Pediatr Radiol. (2014) 44(2):193–201. doi: 10.1007/s00247-013-2789-8
115. Arena S, Romeo C, Racchiusa S, Russo T, Santacaterina E, Arena F. Anterior urethral valves in the fossa navicularis in a newborn: report of a case and review of the literature. Pediatr Med Chir. (2011) 33(2):102–3.22111296
116. Prakash J, Dalela D, Goel A, Singh V, Kumar M, Garg M, et al. Congenital anterior urethral valve with or without diverticulum: a single-centre experience. J Pediatr Urol. (2013) 9(6):1183–7. doi: 10.1016/j.jpurol.2013.05.006
117. Berrocal T, López-Pereira P, Arjonilla A, Gutiérrez J. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics. (2002) 22(5):1139–64. doi: 10.1148/radiographics.22.5.g02se101139
118. Husmann DA, Allen TD. Endoscopic management of infected enlarged prostatic utricles and remnants of rectourethral fistula tracts of high imperforate anus. J Urol. (1997) 157(5):1902–6. doi: 10.1016/S0022-5347(01)64898-5
119. Levin TL, Han B, Little BP. Congenital anomalies of the male urethra. Pediatr Radiol. (2007) 37(9):851–62. doi: 10.1007/s00247-007-0495-0
120. Singh S, Singh P, Singh R. Persistent urogenital sinus. J Anat Soc India. (2010) 59(2):242–4. doi: 10.1016/S0003-2778(10)80034-6
121. Wang Y, Hu S, Wang H. Multimodal ultrasound imaging of persistent urogenital Sinus with uterus didelphys and double vagina malformation: a case report. Medicine. (2021) 100(52):e28477. doi: 10.1097/MD.0000000000028477
122. Darge K, Back SJ, Bulas DI, Feinstein SB, Ntoulia A, Volberg FM, et al. Pediatric contrast-enhanced ultrasound: shedding light on the pursuit of approval in the United States. Pediatr Radiol. (2021) 51(12):2128–38. doi: 10.1007/s00247-021-05102-y
123. Riccabona M, Lobo ML, Ording-Muller LS, Thomas Augdal A, Fred Avni E, Blickman J, et al. European Society of Paediatric Radiology abdominal imaging task force recommendations in paediatric uroradiology, part ix: imaging in anorectal and cloacal malformation, imaging in childhood ovarian torsion, and efforts in standardising paediatric uroradiology terminology. Pediatr Radiol. (2017) 47(10):1369–80. doi: 10.1007/s00247-017-3837-6
Keywords: children, intracavitary, ultrasound contrast agent, contrast-enhanced voiding urosonography, contrast-enhanced genitosonography, genitourinary anomaly
Citation: Ren J, Ma T, Huang S, Chen G, Dietrich CF, Peng Y and Cui X (2023) A narrative review on the applications of intracavitary contrast-enhanced ultrasonography in pediatric lower genitourinary anomalies. Front. Pediatr. 11:984643. doi: 10.3389/fped.2023.984643
Received: 2 July 2022; Accepted: 13 April 2023;
Published: 19 May 2023.
Edited by:
Qiangsong Tong, Huazhong University of Science and Technology, ChinaReviewed by:
Magdalena Wozniak, Medical University of Lublin, PolandCristian Roberto Sager, Garrahan Hospital, Argentina
© 2023 Ren, Ma, Huang, Chen, Dietrich, Peng and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinwu Cui Y3VpeGlud3VAbGl2ZS5jbg== Yuexiang Peng c3lweXg0QDE2My5jb20=
 Jiayu Ren
Jiayu Ren Ting Ma1
Ting Ma1 Xinwu Cui
Xinwu Cui