- 1Pediatric Surgery Department, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Pediatric Surgery, Henan Children's Hospital, Zhengzhou, China
- 3Thoracic Surgery Department, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been presented to be a prognostic indicator in several cancers. We were supposed to evaluate the prognostic role of such inflammatory markers in hepatoblastoma (HB).
Methods: Total of 101 children, diagnosed with hepatoblastoma between January 2010 and January 2018, were enrolled before treatment in the study. The clinicopathological parameters, and outcomes were collected through laboratory analyses and patient follow-up. The association between NLR, PLR, and clinicopathological characters were analyzed with Wilcoxon test, Chi-Squared test, Kaplan-Meier, Log-rank and Cox regression analyses.
Results: NLR and PLR were significantly elevated in HB patients (P < 0.001), and related to age (P < 0.001), risk stratification system (P < 0.001), and pretreatment extent of disease (P < 0.0001). NLR was significantly related to alpha-fetoprotein (P = 0.034) and lactate dehydrogenase (P = 0.026). The 3-year overall survival (OS) and event-free survival (EFS) were poor in the high-NLR group (OS: 44.3% vs. 90.3%, P < 0.0001, EFS: 38.6% vs. 80.6%, P = 0.0001). The 3-year OS and EFS were poor in the high-PLR group (OS: 49.1% vs. 68.8%, P = 0.016, EFS: 39.6% vs. 64.6%, P = 0.0117). The multivariate analysis suggested that NLR (HR: 11.359, 95% CI: 1.218–105.947; P = 0.033) and risk stratification (HR: 44.905, 95% CI: 2.458–820.36; P = 0.01), were independent predictors of OS.
Conclusion: Our research showed that elevated NLR and PLR were the poor prognostic factors in HB patients before treatment. The NLR was an independent prognostic factor for OS.
Introduction
Primary hepatic malignancies among children are relatively rare, accounting for approximately 1% of new cancer diagnosis in childhood. Hepatoblastoma (HB) occurs at an incidence of 0.5–1.5 per 100,000 population, which accounts for about 80% of the malignant liver tumors in children, younger than 3 years (1). Treatment of hepatoblastoma includes chemotherapy, radiotherapy, surgical resection, immunotherapy and liver transplantation (2). The survival outcomes have been improved significantly during the past four decades. Effective HB treatment requires complete tumor resection. According to the International Childhood Liver Tumors Strategy Group (SIOPEL), about 90% of patients can be treated with radical surgery and the 5-year overall survival rate for HB can reach 75%. Liver transplantation is the only way to prolong survival in the remaining 10% of children with unresectable tumors (3, 4). In the previous reports, tumor stage, alpha-fetoprotein (AFP), histological type, type of radical surgery, metastasis, and adjuvant therapy such as chemoradiation were proposed to be the prognostic indicators in hepatoblastoma (4). Because of limited patient numbers, potential risk factors were considered not to be statistically significant, the most accurate prediction of disease staging usually occurred after resection and thus there was not a better biomarker to detect HB at an early tumor stage. The application of new biomarkers to the established prognostication systems might be beneficial to the early assessment and optimal treatment of disease.
Some literature reports that the systemic inflammatory response is involved in the process of tumor occurrence, invasion and metastasis. The systemic inflammatory response is manifested by the increase of neutrophils, platelets and relative lymphopenia detected in Peripheral blood count examination (5). Activated neutrophils and platelets promote tumor cell proliferation and metastasis by activating angiogenic, epithelial, and stromal growth factors in the tumor microenvironment (5, 6). In addition, unbalanced immune response and lymphocytopenia might reflect an impaired T lymphocyte-mediated antitumor response (7). Therefore, comprehensive analysis of neutrophils, platelets, and lymphocytes can better determine the progression and prognosis of malignant tumors (8). Moreover, these indicators are easily available and affordable through routine laboratory tests. Neutrophil–lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are calculated as neutrophil and platelet counts divided by lymphocyte counts. Those combined calculations are considered to be comparatively more stable than the absolute counts, and they could reflect the changes in inflammatory factors and immune cells (9).
Several studies demonstrated that elevated ratio of peripheral NLR and PLR, in patients with different types of cancers, were associated with a poor prognosis (10–12). However, there are little data in literature regarding the prognostic significance of the NLR and PLR for HB patients. Therefore, we conducted this research to investigate the associations between pretreatment NLR/PLR and other clinical parameters, meanwhile, investigated whether they affect the event-free survival (EFS) and overall survival (OS) of patients with HB after hepatectomy and chemotherapy.
Patients and methods
Between January 2010 and January 2018, the clinical data of 101 HB patients who were diagnosed by histology, cytology, and typical imaging findings in the First Affiliated Hospital and Children's Hospital of Zhengzhou University were retrospectively collected. All of the patients were treated in accordance with the guidelines from SIOPEL and Children's Oncology Group (COG) risk stratification system for children with hepatoblastoma. Very low risk category [ie, well-differentiated fetal histology with pretreatment extent of disease (PRETEXT) I/II], recommends upfront resection with no chemotherapy. Low-risk patients receive upfront resection and then adjuvant chemotherapy. Intermediate-risk and high-risk individuals receive neoadjuvant therapy followed by resection and adjuvant chemotherapy (13). Patients who underwent surgical excision and chemotherapy were selected for the study. Patients age below 6 days or above 6 years with a history of active infection or chronic inflammation, those who were lost to follow-up after surgery, and premature infant were excluded. In the same period, 101 children with indirect inguinal hernia in our hospital were selected as the control group. The study was approved by the Ethics Committee of Research and Clinical Experiments of the First Affiliated Hospital of Zhengzhou University (No. KY-2021-0213).
Blood sample and clinical variables analysis
Blood samples obtained after diagnosis of hepatoblastoma and before initial treatment were used to measure neutrophil, lymphocyte and platelet counts. Other variables, including AFP, lactate dehydrogenase (LDH), aspartate transaminase (AST) and alanine aminotransferase (ALT) were simultaneously obtained. Blood NLR and PLR were calculated as the ratio of the total count of neutrophils and platelets divided by the total count of lymphocytes.
The following clinical variables were included for analysis: age, gender, risk stratification system, AFP, AST, ALT, histologic type. HB patients are not routinely staged by the standard TNM staging system, however other systems such as the PRETEXT and COG staging schemes are used (14).
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 and GraphPad Prism version 5.0. The Wilcoxon test was used to compare two sets of quantitative data. The comparision of categorical variables between two groups was performed using the chi-squared test. The Kaplan–Meier method was used to calculate survival curves. Survival outcomes between groups were compared with the log-rank test. Variables that proved to be significant in the univariate analysis were used for performance of the multivariate Cox regression analysis. The P-value < 0.05 was considered to be significant. EFS was defined as the period from the start with the first treatment after the diagnosis of hepatoblastoma to disease progression, death, or the occurrence of a second malignant tumor. OS was defined as the period from the start with the first treatment after the diagnosis of hepatoblastoma to death or death regardless of the cause.
Results
Optimum cutoffs of the PLR and NLR
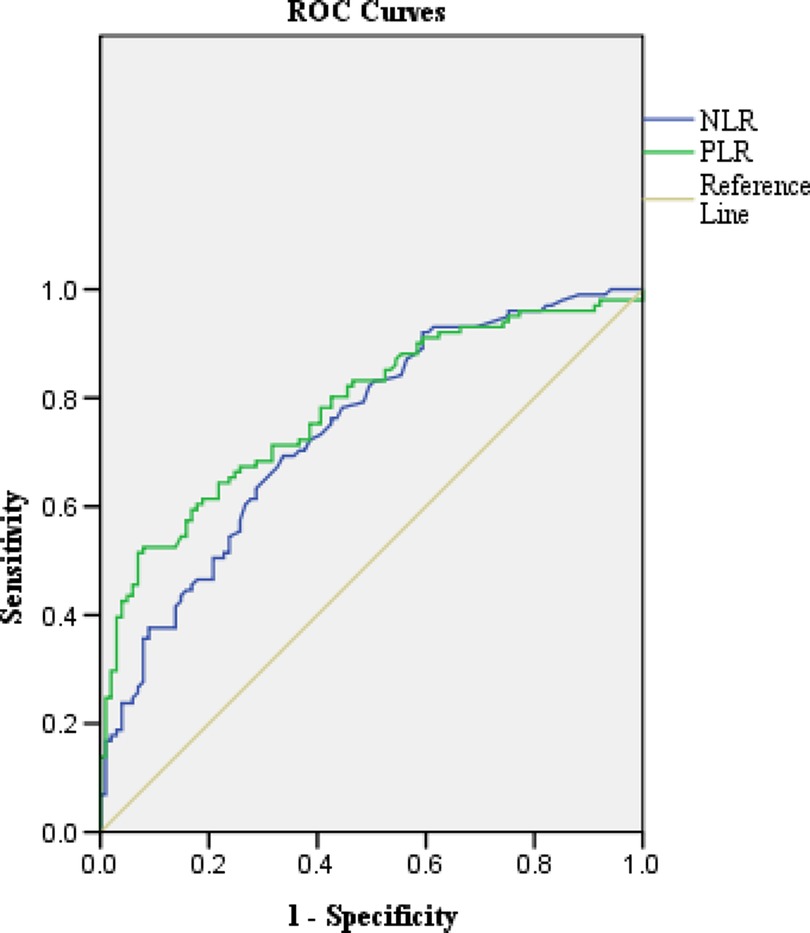
The optimal cut-off values for NLR and PLR were estimated by receiver operative curve (ROC) analysis (15). Based on the AUROC curve, the NLR and PLR cut-off points were defined as 0.59 and 106.94 (AUROC: 0.739 and 0.78, respectively, Figure 1). NLR ≥ 0.59 and PLR ≥ 106.94 were considered to be elevated (High-NLR and High-PLR), while NLR < 0.59 and PLR < 106.94 were reduced (Low-NLR and Low-PLR).
Comparisons of NLR and PLR between case group and control group
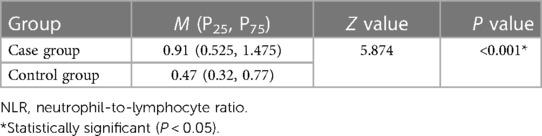
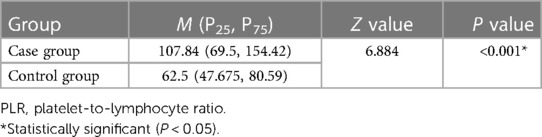
The level of NLR before treatment was higher in case group than those in the control group. There were significant differences between two groups (P < 0.001, Table 1). The PLR in the case group was significantly higher than that in the control group before treatment (P < 0.001, Table 2).
Clinicopathologic characteristics
This study was conducted in a retrospective cohort of 101 patients with HB. The clinical characteristics of patients classified as high/low NLR and PLR are presented in Table 3. The median age of the patients in this series was 12 (range 0.3–63) months. 60 (59.41%) patients were male, and the pathological type in 62.38% of patients was epithelioid. The PRETEXT stage I, II, III and IV patients were accounted for 10.89%, 29.70%, 39.60% and 19.80%, respectively. There were 73 (72.28%) patients with low and extremely high levels of AFP (<100 or >10,000 ng/ml). More than half (64.36%) of the patients had a LDH > 300 U/L, while the patients with AST and ALT > 40 U/L were accounted for 82.18% and 13.86%, respectively.
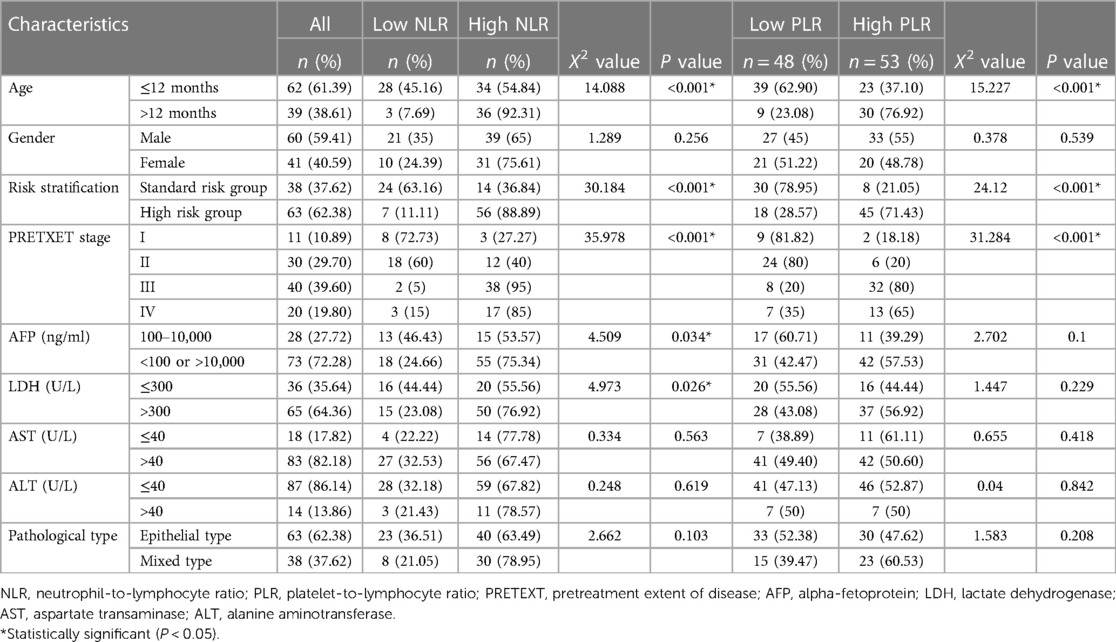
Table 3. Association of the patients’ characteristics with the platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios.
Standard- vs. high-risk tumors
Based on the risk stratification criteria of the SIOPEL, the children were divided into the standard risk group and the high-risk group. High risk HB criteria include patients with preoperative PRETEXT IV, serum AFP < 100 ng/ml, distant metastasis at diagnosis, invasion of the portal vein, inferior vena cava or hepatic vein and tumor rupture or intraperitoneal hemorrhage (4, 16). There were 38 standard risk patients with 1 death (mortality, 2.6%). At least one high-risk factor in 63 patients, and 37 died (mortality, 58.7%).
Relationship between clinicopathologic variables and NLR and PLR
The numbers of low NLR and high NLR patients were 31 and 70, respectively. The numbers of low PLR and high PLR patients were 48 and 53, respectively (Table 3). Age, risk stratification and PRETEXT stage were significantly associated with the high NLR and PLR groups (P < 0.001, respectively). AFP and LDH were significantly related with the high NLR group (P = 0.034 and P = 0.026 respectively).
Prognosis analysis of NLR and PLR
Patients were followed up for at least 3 years by telephone or outpatient review after discharge. The final follow-up time was January 2021. During follow-up, 49 patients (48.5%) developed tumor recurrence and 42 (41.6%) patients died due to progressive disease. For the entire study population, 3-year EFS rates was 51.5%, while 3-year OS rates was 58.4%. The 3-year OS and EFS rates of the patients with an NLR ≥ 0.59 (44.3% and 38.6%, respectively) were significantly lower than those of the patients with an NLR < 0.59 (90.3% and 80.6%, P < 0.0001 and P = 0.0001, respectively. Figures 2A,C). The 3-year OS and EFS rates of the patients with PLR ≥ 106.94 (49.1% and 39.6%, respectively) was significantly lower than those of the patients with an PLR < 106.94 (68.8% and 64.6%, P = 0.016 and P = 0.012, Figures 2B,D).
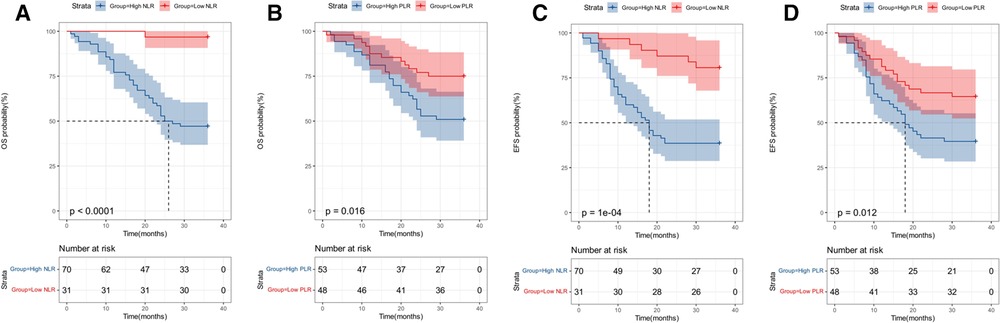
Figure 2. Survival analysis. (A) Kaplan–Meier survival curves of overall survival according to neutrophil-to-lymphocyte ratio (NLR). (B) Kaplan–Meier survival curves of overall survival according to platelet-to-lymphocyte ratio (PLR). (C) Kaplan–Meier survival curves of event-free survival according to neutrophil-to-lymphocyte ratio (NLR). (D) Kaplan–Meier survival curves of event-free survival according to platelet-to-lymphocyte ratio (PLR).
Prognostic factors for OS
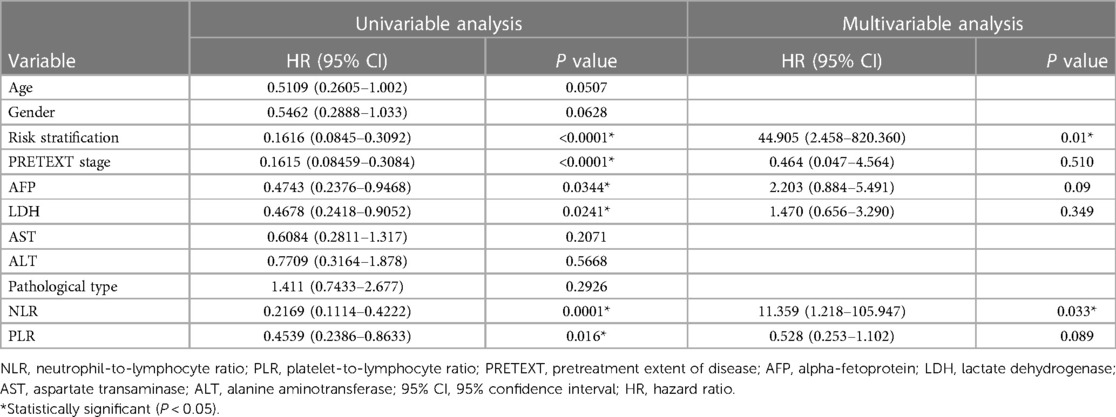
The results of the univariate analysis of different variables on 3-year OS are shown in Table 4. The 3-year OS rates of the standard risk patients (97.4%) were significantly higher than those of the high risk patients (41.3%, P < 0.0001). The 3-year OS rates of the patients in PRETEXT stage I and II (95.1%) were significantly higher than those of the patients in PRETEXT stage III and IV (40%, P < 0.0001). The 3-year OS rates of the patients with an AFP 100–10,000 ng/ml (78.6%) were significantly higher than those of the patients with an AFP < 100 or >10,000 ng/ml (56.2%, P = 0.0344). The 3-year OS rates of the patients with an LDH ≤ 300 U/L (80.6%) were significantly higher than those of the patients with an LDH > 300 U/L (53.8%, P = 0.0241).
The multivariate analysis revealed that a pretherapy NLR < 0.96 [hazard ratio (HR): 11.359, 95% CI: 1.218–105.947; P = 0.033] was associated with longer OS than an NLR ≥ 0.96. Compared with standard risk patients, the high risk patients showed a trend towards having worse OS (HR: 44.905, 95% CI: 2.458–820.360; P = 0.01). The multivariate analysis confirmed that NLR, risk stratification were independent predictors of OS. AFP, LDH, PRETEXT stage, PLR were not the independent prognostic factors (Table 4).
Discussion
The inflammation response plays an important role in the occurrence and development of tumors (5). Inflammatory cells such as neutrophils, which was attracted by chemokines and cytokines secreted from tumor cells, could infiltrate the tumor microenvironment and its interaction with tumor necrosis factor-alpha (TNF-α) and interleukins could enhance tumor immune response leading to tumor cell proliferation (17–19). Additionally, neutrophils could promote tumor progression via the transforming growth factor-beta (TGF-β) signaling pathway (20, 21). The lymphocyte response is an important factor in tumor immune responses, and it plays a key role in inhibiting tumor growth and progression. In the early stage of tumorigenesis, an imbalanced immune system manifests as lymphocytopenia and impaired immune response which compromises the effectiveness of host-tumor immune responses and promotes the further proliferation, diffusion and metastasis of tumor cells (22). Platelets might participate in tumor growth and metastasis through inflammatory and non-inflammatory mechanisms. Activated platelets not only regulate the function of neutrophils, lymphocytes and endothelial cells, but also promote the movement of neutrophils to inflammatory areas to induce tumor immune response (23). Platelets may also contribute to the growth and spread of malignant tumors through noninflammatory mechanisms such as promoting neo angiogenesis and attachment of tumor cells to endothelial cells, increasing microvessel permeability and extravasation of tumor cells (24, 25). In addition, the early metastatic niches formed by platelets, neutrophils and disseminated tumor cells could accelerate tumor invasion and metastasis (26). Therefore, estimated ratios such as NLR and PLR rather than absolute neutrophil, platelets and lymphocyte counts are more responsive to changes in the tumor microenvironment and tumor immune response. The high NLR and PLR indicated an imbalance immune response, which disrupted the normal anti-tumor functions and led to tumor occurrence and metastasis (27). We found that the ratio of NLR and PLR in peripheral blood of children with HB was significantly higher than that of the control group (Tables 1, 2).
NLR and PLR as an evaluation index has been widely applied in various types of solid tumors. Prabawa et al. reported that the NLR and PLR could be used as a potential biomarker for predicting the cervical cancer invasiveness (28). Xia et al. first revealed NLR was found to be an independent prognostic factor for OS and EFS in patients with osteosarcoma (29). Neofytou et al. showed that the PLR, as an adverse prognostic factor, was superior to the NLR in patients with liver-only colorectal metastases (30). In this study, we found that pretreatment NLR and PLR were significantly associated with poor OS and EFS in HB patients, which was similar to other foundings in various cancers. We also identified NLR as an independent prognostic factor for OS in multivariate analysis and we supposed that NLR might have more predictive value than PLR. To our knowledge, this is the first study to demonstrate the prognostic value of both pretreatment NLR and PLR in predicting HB.
The traditional factors: AFP, PRETEXT stage, risk stratification and the presence of metastases have long been identified as major prognostic factors for hepatoblastoma (31). The univariate analysis revealed that those factors, such as high- risk group, low or high AFP level and PRETEXT III and IV, were negative prognostic factors in patients and risk stratification was an independent prognostic factor for OS in our study. The SIOPEL identifies standard and high-risk HB with stratification to different chemotherapy groups (32, 33). In this study, we indicated that the mortality of patients with high-risk tumors was almost 20 times as high as the standard risk group, which indicating that patients with high-risk stratification would get the worse prognosis and lower survival rate. We found that 88.9% patients in the high-risk group had NLR greater than 0.59 and 71.43% patients with PLR greater than 106.94. At the same time, only 36.84% and 21.05% of low-risk patients had high NLR and high PLR. These findings suggest that NLR, PLR can be considered an indicator of risk stratification for HB. Also, we found that patients in high-risk group with higher NLR and PLR had poorer OS than standard risk patients with low NLR and PLR. This suggests that serum biomarkers, such as NLR and PLR, may be important biomarkers to further stratify patients within conventional risk stratification.
The PRETEXT system, a preoperative surgical staging system, which was based on the anatomy of the liver, was a relatively objective diagnostic method to assess the extent of the tumor (32, 33). LDH is a sensitive indicator of tumor burden and metabolic status in vivo, and its increase can indirectly reflect the size of tumor volume and metabolic activity in patients, often indicating that children are in advanced stage and have poor prognosis (34). Moon et al. showed that the survival of patients with HB is usually correlated with the PRETEXT classification (35). Chen et al. found that the increase of LDH has been associated with poor OS in patients with colorectal cancer (36). Zhang et al. reported that the high NLR has been associated with poorer prognosis and larger tumors in patients with gastric cancer (37). A similar finding was observed in our study showing that HB patients with high LDH and PRETEXT III/IV disease had a poor prognosis and the high NLR was significantly related with risk stratification and LDH. It is suggested that patients with high NLR might have larger tumor volume, burden and increasing metabolic status. Therefore, NLR before treatment has a certain reference value for evaluating whether to surgery or chemotherapy first for new hepatoblastoma.
AFP has been widely used to monitor the recurrence and treatment effect of hepatoblastoma (38). Patients with extreme levels of AFP should always be regarded as a high-risk group from the beginning of treatment and receive a suitable treatment for high-risk patients. The high-risk patients, which with extreme levels of AFP and a poor prognosis, should receive an intensive treatment at the beginning (4). In our study, there were significant differences in the survival rates of patients with different levels of AFP with HB. This is similar to the retrospective study showing that AFP < 100 and >1.2 × 106 μg/L were an indicator of poor prognosis in HB patients (16). We found that patients with high NLR might have extreme levels of AFP. There was a significant correlation between high NLR and extreme level of AFP (AFP < 100 and >10,000 ng/ml). Similar to the function of AFP, we speculate whether NLR can be used for the detection of therapeutic efficacy of hepatoblastoma and the monitoring of tumor recurrence, which still needs further research.
This study is associated with several limitations. First, this is a retrospective study, which may have led selection bias. Second, due to the low incidence of HB, the sample size that could be used was small. Patients with a raised PLR had a significantly lower OS and EFS, confirming that elevation of the PLR indicates a more aggressive clinical phenotype, while the Cox regression showed that the PLR was not an independent prognostic factor. Age as a poor prognostic factor has been proved in various malignant tumors, such as neuroblastoma and Wilms’ tumor (39, 40). This study found that age was positively correlated with NLR and PLR, but not a prognostic factor for HB. So, we speculate that the low sensitivity value of PLR and age may have been related to the small sample size. Third, our study only included age above 6 days and below 6 years. Further research is needed on children of other ages.
Conclusion
The pretreatment NLR and PLR are associated with survival in patients with HB. NLR was identified as an independent prognostic factor for HB patients. Measuring and monitoring the NLR value of HB patients may help in assessing the treatment response and predicting recurrence. More prospective and randomized studies are needed to confirm these findings and clarify the underlying mechanism between systemic inflammation and tumor cells.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Committee of scientific research project of the First Affiliated Hospital of Zhengzhou University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study conception and design: TX. Data acquisition: TX, DH. Analysis and data interpretation: TX, JW. Drafting of the manuscript: TX, SZ. Critical revision: JW, DH, SZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Research Project of Henan Provincial Colleges and Universities (Grant No. 19B320033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB. (2013) 15(10):741–6. doi: 10.1111/hpb.12112
2. Wu PV, Rangaswami A. Current approaches in hepatoblastoma-new biological insights to inform therapy. Curr Oncol Rep. (2022) 24(9):1209–18. doi: 10.1007/s11912-022-01230-2
3. Feng TC, Zai HY, Jiang W, Zhu Q, Jiang B, Yao L, et al. Survival and analysis of prognostic factors for hepatoblastoma: based on SEER database. Ann Transl Med. (2019) 7(20):555. doi: 10.21037/atm.2019.09.76
4. Ismail H, Broniszczak D, Kaliciński P, Dembowska-Bagińska B, Perek D, Teisseyre J, et al. Changing treatment and outcome of children with hepatoblastoma: analysis of a single center experience over the last 20 years. J Pediatr Surg. (2012) 47(7):1331–9. doi: 10.1016/j.jpedsurg.2011.11.073
5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
7. Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. (2007) 13(3):902–11. doi: 10.1158/1078-0432.CCR-06-2363
8. Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. (2013) 13:350. doi: 10.1186/1471-2407-13-350
9. Dirican A, Kucukzeybek BB, Alacacioglu A, Kucukzeybek Y, Erten C, Varol U, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. (2015) 20(1):70–81. doi: 10.1007/s10147-014-0672-8
10. Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. (2014) 23(1):31–9. doi: 10.1016/j.suronc.2013.12.001
11. Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. (2014) 12:58. doi: 10.1186/1477-7819-12-58
12. Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol. (2015) 22(13):4363–70. doi: 10.1245/s10434-015-4518-z
13. Trobaugh-Lotrario AD, Meyers RL, O'Neill AF, Feusner JH. Unresectable hepatoblastoma: current perspectives. Hepat Med. (2017) 9:1–6. doi: 10.2147/HMER.S89997
14. von Schweinitz D. Management of liver tumors in childhood. Semin Pediatr Surg. (2006) 15(1):17–24. doi: 10.1053/j.sempedsurg.2005.11.004
15. Liu X. Classification accuracy and cut point selection. Stat Med. (2012) 31(23):2676–86. doi: 10.1002/sim.4509
16. Maibach R, Roebuck D, Brugieres L, Capra M, Brock P, Dall'Igna P, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer. (2012) 48(10):1543–9. doi: 10.1016/j.ejca.2011.12.011
17. Kos FT, Hocazade C, Kos M, Uncu D, Karakas E, Dogan M, et al. Assessment of prognostic value of “neutrophil to lymphocyte ratio” and “prognostic nutritional Index” as a sytemic inflammatory marker in non-small cell lung cancer. Asian Pac J Cancer Prev. (2015) 16(9):3997–4002. doi: 10.7314/APJCP.2015.16.9.3997
18. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. (2002) 28(4):396–400. doi: 10.1053/ejso.2002.1257
19. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. (2012) 106(8):1439–45. doi: 10.1038/bjc.2012.92
20. Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J BUON. (2013) 18(3):728–32. doi: 10.7314/apjcp.2013.14.9.5473
21. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420(6917):860–7. doi: 10.1038/nature01322
22. Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. (2005) 92(4):651–4. doi: 10.1038/sj.bjc.6602419
23. Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. (2013) 35(3):254–61. doi: 10.1111/ijlh.12084
24. Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PloS One. (2011) 6(10):e26125. doi: 10.1371/journal.pone.0026125
25. Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. (2004) 29(2):132–40. doi: 10.1097/00006676-200408000-00008
26. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci USA. (2014) 111(30):E3053–3061. doi: 10.1073/pnas.1411082111
27. Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. (2015) 2015:983698. doi: 10.1155/2015/983698
28. Prabawa IPY, Bhargah A, Liwang F, Tandio DA, Tandio AL, Lestari AAW, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev. (2019) 20(3):863–8. doi: 10.31557/APJCP.2019.20.3.863
29. Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. (2016) 14:127. doi: 10.1186/s12957-016-0889-2
30. Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. (2014) 31(10):239. doi: 10.1007/s12032-014-0239-6
31. Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the children's hepatic tumors international collaboration. Lancet Oncol. (2017) 18(1):122–31. doi: 10.1016/S1470-2045(16)30598-8
32. Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, et al. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the international society of paediatric oncology–SIOPEL 2. Eur J Cancer. (2004) 40(3):411–21. doi: 10.1016/j.ejca.2003.06.003
33. Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. (2007) 37(2):123–32; 249-50. doi: 10.1007/s00247-006-0361-5
34. Parodi F, Passoni L, Massimo L, Luksch R, Gambini C, Rossi E, et al. Identification of novel prognostic markers in relapsing localized resectable neuroblastoma. OMICS. (2011) 15(3):113–21. doi: 10.1089/omi.2010.0085
35. Moon SB, Shin HB, Seo JM, Lee SK. Hepatoblastoma: 15-year experience and role of surgical treatment. J Korean Surg Soc. (2011) 81(2):134–40. doi: 10.4174/jkss.2011.81.2.134
36. Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. (2015) 112(6):1088–97. doi: 10.1038/bjc.2015.61
37. Zhang X, Li S, Guo Z, Xue Y. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in the elderly patients over 75 years old with gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. (2016) 19(5):526–9. doi: 10.3760/cma.j.issn.1671-0274.2016.05.011
38. Rojas Y, Guillerman RP, Zhang W, Vasudevan SA, Nuchtern JG, Thompson PA. Relapse surveillance in AFP-positive hepatoblastoma: re-evaluating the role of imaging. Pediatr Radiol. (2014) 44(10):1275–80. doi: 10.1007/s00247-014-3000-6
39. Tolbert VP, Matthay KK. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell Tissue Res. (2018) 372(2):195–209. doi: 10.1007/s00441-018-2821-2
Keywords: hepatoblastoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, prognostic, markers
Citation: Xie T, Hou D, Wang J and Zhao S (2023) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictive markers in hepatoblastoma. Front. Pediatr. 11:904730. doi: 10.3389/fped.2023.904730
Received: 25 March 2022; Accepted: 24 March 2023;
Published: 12 April 2023.
Edited by:
Jaume Mora, Sant Joan de Déu Hospital, SpainReviewed by:
Shaotao Tang, Huazhong University of Science and Technology, ChinaChaoqi Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
© 2023 Xie, Hou, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaxiang Wang d2ppYXhpYW5nQHp6dS5lZHUuY24= Song Zhao emhhb3NvbmdAenp1LmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Oncology, a section of the journal Frontiers in Pediatrics
 Tan Xie1
Tan Xie1 Jiaxiang Wang
Jiaxiang Wang Song Zhao
Song Zhao