
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 10 January 2024
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1338126
 Muhammed Majeed1,2
Muhammed Majeed1,2 Kalyanam Nagabhushanam2
Kalyanam Nagabhushanam2 Sivakumar Arumugam1
Sivakumar Arumugam1 Nagarjuna Chadalavada3
Nagarjuna Chadalavada3 Jyotsna Seepana4
Jyotsna Seepana4 Thumjaa Annamalai5
Thumjaa Annamalai5 Avinash Murali1
Avinash Murali1 Priji Prakasan1
Priji Prakasan1 Lakshmi Mundkur1*
Lakshmi Mundkur1*
Objectives: Acute diarrhea in children is generally managed by replacing the lost fluid with oral rehydration solution (ORS). Probiotic supplementation has been reported to reduce the severity of diarrhea. In the present study, we investigated the effect of Weizmannia coagulans (Bacillus coagulans) MTCC 5856, along with ORS on acute diarrhea of all causes in non-hospitalized children.
Methods: A total of 110 children of ages between 1 and 10 were enrolled in a double-blind placebo-controlled study and were randomly allocated to receive W. coagulans MTCC 5856 (4 × 108 spores, N = 54) + ORS and zinc (Zn) or a placebo (N = 56) + ORS and (Zn) for 5 days. The consistency of the stool, mean duration of diarrhea in hours, mean diarrhea frequency per day, and the dehydration status were collected as efficacy endpoints. Safety was evaluated by the occurrence of adverse events.
Results: The mean age of the children was 5.55 ± 2.57 years (61 boys and 49 girls). The mean duration of diarrhea was 51.31 ± 20.99 h in the W. coagulans MTCC 5856 group and 62.74 ± 24.51 h in the placebo (p = 0.011) group. The frequency of diarrhea was lower in children supplemented with the probiotic, but the difference was not statistically significant. The perceived efficacy score and dehydration status improved significantly in the W. coagulans MTCC 5856 group compared with the placebo group. No adverse events were recorded.
Conclusion: The results of the study suggest that W. coagulans MTCC 5856 could be supplemented along with ORS and zinc to reduce the duration of diarrhea in non-hospitalized children.
Clinical Trial Registration: ClinicalTrials.gov, identifier CTRI/2022/06/043239.
The World Health Organization (WHO) defines diarrhea as three or more watery stools in a 24-hour period and the condition is termed as acute if the illness lasts for less than 14 days (1). Acute diarrhea is commonly due to viral and bacterial infections, and rarely parasitic infections (2, 3). As per the WHO reports, more than 1.7 billion cases of diarrhea are reported globally and more than 525,000 children under 5 years die every year, despite a mortality reduction by more than 30% between 2000 and 2015 (4, 5). Diarrhea is characterized by watery stool, increased frequency of defecation, often accompanied by fever, vomiting, and electrolyte imbalances. It is one of the main causes of dehydration, which may be life-threatening for children with malnutrition or impaired immunity (6). Oral rehydration solution (ORS), containing salts and sugar, is used as fluid and electrolytes replacement therapy for the management of acute diarrhea (1). Zinc is a vital micronutrient that gets drained during diarrhea and its supplementation along with ORS reduces the duration and severity of the illness (7, 8).
Normal bacteria in the intestine promote nutrient absorption, regulate immunity, and play an essential role in protecting the intestinal barrier. An imbalance in the gut microbiota is reported in conditions like diarrhea (9). Probiotics are microorganisms that can promote the growth of beneficial intestinal microbiota, improve intestinal environment, and promote increased immunity and resistance to pathogens (10). Recently, probiotics have gained considerable interest as they promote speedy recovery from acute diarrhea and reduce the severity of diseases (11). Several probiotic strains of Bifidobacteria, Lactobacillus, Enterococcus faecium SF68, Saccharomyces boulardii, and the combinations of different probiotics were reported to be effective in reducing the severity and duration of acute diarrhea in children (12–17). In a review of 82 clinical studies on the effects of probiotics on acute diarrhea, Collison et al. observed that the duration of hospitalization on average was shorter in probiotic groups than in control groups, although the results were highly heterogeneous (18). Recently, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Special Interest Group on Gut Microbiota and Modifications has recommended the use of S. boulardii or L. rhamnosus GG for the management of antibiotic associated diarrhea in children (19).
Weizmannia coagulans (Bacillus coagulans) is a Gram-positive, aerobic, or facultative anaerobic, spore-forming, lactic acid-producing bacterium (20). Several strains of W. coagulans are isolated from various sources and are available as probiotic supplements (21). W. coagulans MTCC 5856 is one such strain, identified as generally regarded as safe (GRAS) by the United States Food and Drug Administration for human consumption, and marketed as a dietary supplement for over three decades (22). The probiotic has excellent stability and is widely used in medicine, food, and the chemical industry (23).
W. coagulans MTCC 5856 showed significant antidiarrheal activities in preclinical studies (24). Furthermore, oral administration of W. coagulans MTCC 5856 safely improved overall gut health in diarrhea predominant irritable bowel syndrome (IBS) patients (25). Recent studies have shown that it has therapeutic effects on intestinal diseases, such as acute diarrhea, antibiotic-related diarrhea, constipation, and colitis, via modulation of the microbiota composition, host immunity, and metabolism (25–29). Additionally, toxicological experiments and many clinical observations have shown that W. coagulans is safe and has no effect of mutagenicity, teratogenicity, or genotoxicity (28, 30).
Some clinical studies have evaluated the safety of a few strains of W. coagulans in children and reported they are well tolerated (31, 32). Strain specificity is considered an important factor that determines the efficacy of probiotics in diarrhea (33). Therefore, in the present study, W. coagulans MTCC 5856 was supplemented along with the standard treatment of Zn and ORS to assess its safety and efficacy in children with acute diarrhea.
A randomized, double-blind, placebo-controlled parallel group trial was conducted in three Government centers as well as some private sectors, including Aditya Multi-Specialty Hospital, Guntur; Government Medical College and General Hospital, Srikakulam; and Aarupadai Veedu Medical College & Hospital (AVMCH), Pondicherry, from July 2022 to December 2022. The study was carried out in accordance with Good Clinical Practices as required by the International Conference on Harmonization. A written, informed consent was provided by the parents or legal guardians of all children participating in the study at the time of the child's enrolment. The study protocol (CW/97/BCG_ADRH/II/MAR/21) was approved by the institutional ethics committee (IEC) of each hospital and the trial was registered prospectively with the Clinical Trial Registry of India (CTRI) with the registration number CTRI/2022/06/043239 registered on 14/06/2022.
The sample size was determined based on the baseline stool frequencies reported in an earlier publication (34). Considering the mean stool frequency value of 8.62 ± 3.17 with 80% power, alpha = 0.05, significance level and correlation of 0.4, the total sample size was determined to be 96 participants. Allowing a drop-out rate of 15%, the total sample size for recruitment was finalized as 110 children, in a 1:1 ratio between the two treatment groups (55 per treatment group).
The randomization sequence table was prepared by an independent statistician using randomization software (SAS-9.4). The randomization was performed as per a computer-generated randomization schedule using block randomization method with a block size of 6.
To improve the blinding and concealment of allocation, the placebo and probiotic were coded centrally with randomization numbers as per the computer-generated randomization schedule. The participants, clinical staff, and data analysts were unaware of trial group assignments. The children enrolled in this study were randomly assigned to receive either the probiotic or the placebo. Sealed envelopes with the randomization codes were provided to the principal investigator to be opened in case of emergency.
Children between the ages of 1 and 10 years with clinical symptoms of acute diarrhea, as per the WHO 2005 definition, were enrolled into the study. Acute moderate diarrhea was defined as the passage of unusually loose or watery stools at least three times in the previous 24-h period, with mild or moderate signs of dehydration (the children had moderate dehydration with symptoms of restlessness and irritability, sunken eyes, and feeling thirsty, as per the WHO classification) (35, 36). The children/parents were informed about the purpose of the study by the principal investigator and the study protocol was also well explained to the parents or legal guardians who were willing to comply with all its procedures.
Exclusion criteria included the requirement for antibiotics or intravenous fluid treatment, and clinical signs of severe dehydration or severe diarrhea that requires treatment other than ORS. The children who were immunodeficient, malnourished, suffering from severe chronic illness or consuming any commercially available probiotics, or had undergone recent surgeries were also excluded from the study. In addition, children hospitalized for pneumonia, acute gastroenteritis, or having bilious vomiting, hematochezia, pancreatic dysfunction, or insufficiency as judged by medical history were also excluded from the study.
The probiotic and placebo were provided by Sami-Sabinsa group of companies. Each sachet of the probiotic contained 4 × 108 spores of W. coagulans MTCC 5856, while the placebo contained maltodextrin. Investigational products were manufactured, handled, and stored in accordance with applicable good manufacturing practice (GMP) and with the approved protocol.
The study participants were instructed to consume a one gram sachet containing W. coagulans MTCC 5856 (4 × 108 spores) or a matching placebo dissolved in approximately 10 ml of freshly boiled and cooled drinking water twice daily along with ORS and Zn supplementation therapy. The probiotic sachets contained 0.03 g of W. coagulans MTCC 5856 equivalent to 4 × 108 spores and 0.97 g of maltodextrin, while placebo contained 1 g of maltodextrin. The appearance of the probiotic and placebo sachets and their contents were indistinguishable. The subjects were monitored for a duration of 5 days, which involved three visits (days 1, 3, and 5) followed by telephonic contact with the parent/guardian, 3 days after the final visit to enquire about the overall well-being of the study participant. All the participants were supplied with a diary for the parent or legal guardian to complete. They were required to fill in the diary relating to study treatment, perceived efficacy scales, and stool record. Empty vials and sachets were recorded for compliance with the study.
The primary efficacy endpoints were the mean duration of diarrhea (expressed in hours), as counted from the time of randomization up to recovery (the first normal stool as recorded as <5 according to Bristol score as normalization of stool) and mean diarrhea frequency per day after the initiation of treatment. Frequency of stools was defined as the sum of the frequency of stools and the frequency of diapers with stools. The secondary end points were the perceived efficacy of the treatment, recorded every day by caregivers as frequency of diarrhea and dehydration status (Table 1).
The Bristol stool chart was assessed for each stool passing based on the entries made by parents in the subject diaries given to them to record diarrhea frequency and duration of diarrhea as well as investigational product compliance daily. The criteria for the evaluation of stool were explained to the parents and the children. The Bristol stool chart scale is a 7-point scale that ranges from type 1 for the hardest to type 7 for the softest stool. The stool consistency ≥5 on the Bristol stool scale is considered to be loose or liquid stool, which is indicative of diarrhea along with other symptoms (37). The caregivers were also asked to assess the signs of dehydration including pinch analysis on abdomen skin and were instructed to record their score on the perceived efficacy scale questionnaire. As an exploratory end point, Rota virus and Adenovirus were screened at baseline to evaluate the efficacy of the study treatment in children with confirmed viral diarrhea.
The study participants were monitored for safety data throughout the course of the study. All the children underwent general physical examination including recording of vitals such as temperature, pulse, and respiratory rate at baseline and on day 5. The occurrences of any adverse events and/or serious adverse events were monitored throughout the study period.
The normality of the quantitative variables was analyzed using the Shapiro–Wilks test, and the data are represented as means, standard deviation (SD), or median, and range based on their distribution. The differences within the group at different time points were compared by repeated measures analysis of variance (ANOVA) and Dunnett's multiple comparison. For categorical variables, the frequency and percentage of the population were presented. A descriptive comparison was provided to differentiate the treatment effect between the treatment groups. For continuous variables, the Mann–Whitney U test was performed for the comparative analysis between treatment groups for non-normally distributed data and the results were presented as mean and p-value. Comparative analysis was performed using the Wilcoxon signed rank test for the non-normally distributed data within the group and the results were presented as mean and p-value. All the statistical analysis was performed by STATA Software version 16.0 by an independent statistician blinded to the study groups. The level of significance was defined as a p-value less than 0.05.
A total of 210 children diagnosed with acute diarrhea were prescreened and 110 children, who met the inclusion criteria, were randomized to receive W. coagulans MTCC 5856 (n = 54) or a placebo (n = 56) (Figure 1). The baseline demographic characteristics were well balanced between the two groups. The mean age of the children was 5.55 ± 2.57 years (5.78 ± 2.76 in the probiotic and 5.34 ± 2.38 in the placebo groups) and the mean weight was 21.44 ± 8.34 kg (22.19 ± 8.41and 20.71 ± 8.28) with an average BMI of 17.83 ± 8.37 (18.89 ± 11.36 and 16.80 ± 3.52) in W. coagulans MTCC 5856 and placebo, respectively. Among the study participants, 55.45% were boys and 44.54% were girls. A total of 34 (30.90%) children were positive for adenoviruses in stool (18 in the probiotic group and 16 in the placebo group). Rotavirus was found in the stools of 23 (20.90%) children; 10 in the active group and 13 in the placebo group, while 9 children were positive for both viruses (5 in the probiotic group and 4 in the placebo group). The details of the demographic characteristics are presented in Table 2.
The primary outcome parameters were the analysis of mean duration and frequency of diarrhea from randomization to recovery. The children in the probiotic group showed a mean recovery (normal stool with Bristol stool chart score <5) after 51.43 ± 20.76 h, whereas the children in the placebo group showed recovery after 62.74 ± 24.51 h of treatment, which was significantly better (p = 0.012) in the probiotic supplemented children (Figure 2A). The median recovery and confidence interval (CI) were 50.43 (45.76, 57.09) h for the W. coagulans MTCC 5856 group and 67.65 (56.17, 69.30) h for the placebo group. The net reduction in diarrhea duration was 17.22 h (95% CI: 1.3–21.40, p = 0.012), calculated as 67.65–50.43 h. A Kaplan–Meier curve, generated to compare the total duration of diarrhea between groups, showed a statistically significant difference between treatment groups with a log rank p-value of 0.0024 and hazard ratio of 0.55 (95% CI: 0.37, 0.82) (Figure 2B). In the probiotic supplemented group, 53/54 (98.14%) children recovered from diarrhea in 5 days, while 50/56 (89.28%) children recovered in the placebo group. The cumulative recovery was better in the W. coagulans MTCC 5856 group compared with the placebo group.
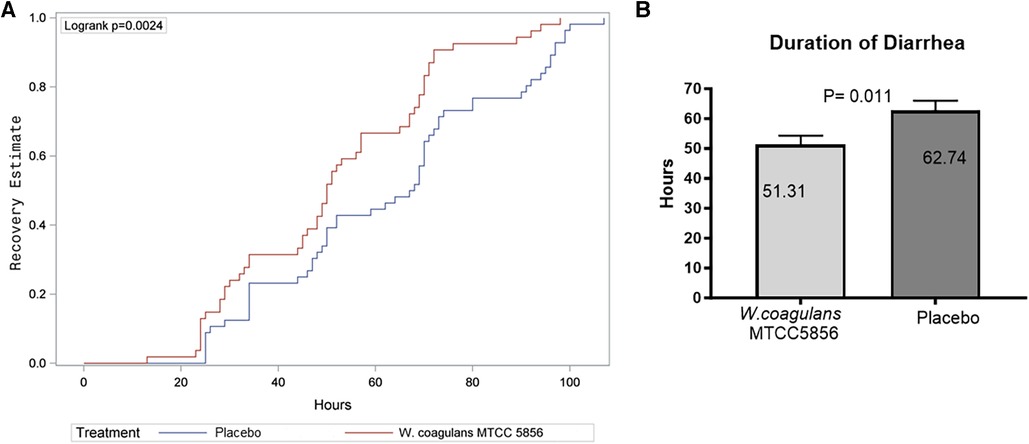
Figure 2. Recovery from diarrhea. (A) Kaplan–Meier estimate of recovery from diarrhea. (B) Duration of recovery in placebo and W. coagulans MTCC5856. Recovery was defined as the first normal stool recorded according to Bristol score; a score <5.
The mean frequency of diarrhea per day did not differ significantly between groups. A gradual decrease in the frequency was observed in both treatment groups. (Table 3). Repeated measure ANOVA within the group showed a significant improvement in both W. coagulans and the placebo groups from day 3 onward.
The difference between the dehydration status was evaluated as per WHO classification. The dehydration status improved (reduction in score) in all children with time and was found to be statistically significant in children supplemented with W. coagulans MTCC 5856 (2.37 ± 1.56 on day 1–0.00 on day 5) compared with the placebo (2.16 ± 1.58 to 0.09 ± 0.29, p = 0.026 on day 5) (Table 4). The perceived efficacy score as recorded by the caregivers also improved in both groups and was significantly better in the probiotic group on day 5 (3.69 ± 0.75 to 0.00 in the probiotic group and 3.73 ± 0.59 to 0.23 ± 0.54 in the placebo group, p < 0.001).
The children who were positive for rotavirus or adenovirus at baseline took a longer duration for recovery in both groups, although the difference did not reach statistical significance. The median duration of diarrhea was shorter in the W. coagulans MTCC 5856 supplemented group (59.95 ± 18.88 h in rotavirus and 58.82 ± 21.42 h in adenovirus positive children) compared with the placebo (70.11 ± 17.57 h and 62.86 ± 26.89 h in rotavirus and adenovirus positive children, respectively), but the difference was not significant. The median recovery and confidence interval were 48.50 (43.15, 55.84) h for the W. coagulans MTCC 5856 group and 62.00 (52.50, 68.51) h for the placebo group. The net reduction in diarrhea duration was 13.50 h (95% CI: 0.2–22.30, p = 0.03), calculated as 62.0–48.50 h in rotavirus negative children. The median recovery and confidence interval in adenovirus negative children was 48.50 (41.07, 54.39) h for the W. coagulans MTCC 5856 group and 66.50 (54.26, 71.46) h for the placebo group. The net reduction in diarrhea duration was 18.00 h (95% CI: 1.9–26.60, p = 0.007), calculated as 66.50–48.50 h. Probiotic supplementation reduces the recovery time in children positive for the virus, but it was not significant most likely due to the small numbers in the present study (Table 5).
Vital signs were also measured as a part of the safety analysis, and no abnormal or out-of-range values were observed. Further, no changes associated with the treatment in safety measurements including adverse events and/or serious adverse events were reported in both groups (Table 6). The vital signs like diastolic and systolic blood pressures (67.28 ± 7.95 /102.15 ± 10.90 and 70.21 ± 9.05/ 102.77 ± 10.82), pulse rate (94.80 ± 9.68 and 95.29 ± 9.69), and body temperature (97.99 ± 0.73 and 98.17 ± 0.58) in the probiotic and placebo groups, respectively, were normal in both groups at the end of the study (Table 7).
In the present study, we compared the efficacy of ORS and zinc therapy with probiotic supplementation against acute diarrhea in children. W. coagulans MTCC 5856 +ORS, zinc supplementation resulted in significantly faster recovery from diarrhea in children in the age group of 2–10 years. The perceived efficacy score and dehydration improvements were better with the addition of probiotics compared with the zinc ORS therapy. The frequency of diarrheal episodes decreased significantly in both groups from day 3 onward. We could not observe any differential effect of probiotic in viral diarrhea, which could be due to the low number of positive cases in our study.
The WHO recommends treating diarrhea with ORS containing sugar and salt in clean water with 20 mg zinc to improve the outcomes (35). Oral hydration therapy with ORS and Zn supplementation is reported to have beneficial effects on the absorption of water and electrolytes by the intestine, regeneration of gut epithelium, and increased levels of enterocyte brush-border enzymes (38). Clinical evidence has shown that reducing the duration of diarrhea is crucial in reducing the risks of severe dehydration in children (39, 40).
Probiotics have also been introduced as adjuvant therapy for the management of diarrhea since the last few decades. However, clinical studies investigating the efficacy of probiotics for the treatment of diarrheal diseases reported contentious results with both efficacious and not-so-positive effects (14, 18). Probiotic treatment was reported to significantly reduce the duration of diarrhea in children with mild diarrhea in earlier studies (16, 41). Children with acute gastroenteritis were reported to benefit with supplementation with probiotic strains like L. reuteri ATCC 55730, L. rhamnosus 19070-2, and L. reuteri DSM 12246 (17, 42). A recent study in children with infectious diarrhea claimed that inclusion of a probiotic mixture was safe and effective in shortening the duration of watery diarrhea and faster discharge form hospital (43). In an earlier study, Lactobacillus sporogenes (B. coagulans) was reported to show no therapeutic impact on management of acute diarrhea as an adjunct to ORS (31). A meta-analysis suggested that the duration of diarrhea was shorter in the probiotic group and the supplementation with combination of probiotics had a better effect than a single probiotic (44). Supplementation of the probiotic bacteria such as Lactobacillus reuteri, Saccharomyces boulardii, Lactobacillus rhamnosus, and Lactobacillus acidophilus increased the treatment efficacy and reduced the duration of hospital stay (45–47). In contrast to these studies, L. sporogenes (B. coagulans) was reported to show no therapeutic impact on management of acute diarrhea as an adjunct to ORS (31). Similarly, supplementation with B.clausii and ORS + zinc showed no significant improvement in acute diarrhea in infants (48). In corroboration with some of the studies with probiotics, we observed a reduction in the duration of diarrhea, by the addition of W. coagulans MTCC 5856 to ORS and zinc mixture.
The mechanism of action of W. coagulans MTCC 5856 in acute diarrhea is not fully understood. However, it has been shown that W. coagulans can interact with several pathways to produce beneficial effects. W. coagulans MTCC 5856 is known to possess a broad spectrum of antimicrobial activity, which may help to maintain microbial homeostasis and resist the growth of pathogens. In addition, the strain produces organic acid (lactic acid) and short-chain fatty acids (SCFAs), which may lower the pH of the gut cavity, thereby inhibiting the growth of pathogens. These short-chain fatty acids play a pivotal role in the maintenance of overall gut health, intestinal morphology, and function (27, 30, 49). Moreover, W. coagulans MTCC 5856 is reported to have immunomodulatory properties in human colonic cells, which make the spores efficacious in the management of infectious diarrhea (50). In vivo studies also reported that W. coagulans MTCC 5856 increased the expression of tight junction proteins and reduced the damage caused by inflammation in gut tissues (51). W. coagulans MTCC 5856 also elicited antidiarrheal activity and inhibited the gastrointestinal motility in fasted rats (24). Similar effects were translated to clinical studies also where W. coagulans MTCC 5856 significantly reduced the diarrhea, abdominal pain, and stool frequency in diarrhea predominant IBS patients (25).
Stringent quality control and safety procedures are mandatory for probiotics, when considering the vulnerability of pediatric population. Supplementation with W. coagulans MTCC 5856 was well tolerated and there were no unexpected adverse events during the study. Minor adverse events reported were similar between the placebo and the probiotic groups. The present study reported no significant changes in the vital signs and no adverse effects were reported by any of the participating children, reiterating the safety of W. coagulans MTCC 5856.
The major limitation of the study was the relatively smaller number of recruited children. All the children were recruited from the southern part of India, restricting the ethnic diversity. We did not analyze the effect of the probiotic on gut microbiome, which could have added value to the mechanism of action of the strain. However, the study was well designed and conducted as a double-blind, placebo-controlled trial, comparing ORS with zinc treatment with the same enriched with W. coagulans MTCC 5856 in children with acute diarrhea.
The present randomized, double-blind, placebo-controlled trial showed that W. coagulans MTCC 5856 supplemented with ORS and Zn had comparatively more efficacy than ORS and Zn treatment alone in managing acute diarrhea in children without malnutrition. Further, this is the first time the specific strain of W. coagulans MTCC 5856 is being tested for the safety and efficacy in children with acute diarrhea, which make the results clinically important for health professionals, including general practitioners, general pediatricians, pediatric gastroenterologists, and nutritionists.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Ethics committee—Aditya Multi Speciality Hospital, EC No. ECR/1347/Inst/AP/2020, The Institutional Ethics committee—Government Medical College and Government General Hospital, EC No. ECR/492/Inst/AP/2013/RR-16, and the Institutional Ethics committee—Aarupadai Veedu Medical College & Hospital (AVMCH), EC No. ECR/847/Inst/PY/2016/RR-20. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
MM: Conceptualization, Resources, Writing – review & editing. KN: Conceptualization, Methodology, Project administration, Writing – review & editing. SA: Methodology, Writing – review & editing. NC: Investigation, Supervision, Writing – review & editing. JS: Investigation, Supervision, Writing – review & editing. TA: Investigation, Supervision, Writing – review & editing. AM: Methodology, Project administration, Writing – review & editing. PP: Writing – original draft, Writing – review & editing. LM: Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The study was supported by Sami-Sabinsa Group Limited/Sabinsa Corporation. The funder was involved in conceptualizing the project and providing resources. The funder was not involved in study design, data collection, and analysis of results, but was part of reviewing the manuscript and the decision to publish.
The authors thank Mr. Kamal Kammili and his team at Sanjeevani BioSolutions, Hyderabad, for the statistical analysis, the clinical team in the three hospitals, and the ClinWorld team for their help in conducting the study.
MM, KN, SA, AM, PP, and LM are employees of Sami-Sabinsa Group Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. Clinical Management of Acute Diarrhoea. WHO/UNICEF Joint Statement. Geneva: World Health Organization (2004).
2. Elliott EJ. Acute gastroenteritis in children. BMJ. (2007) 334(7583):35–40. doi: 10.1136/bmj.39036.406169.80
3. Radlović N, Leković Z, Vuletić B, Radlović V, Simić D. Acute diarrhea in children. Srp Arh Celok Lek. (2015) 143(11–12):755–62. doi: 10.2298/SARH1512755R
4. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388(10063):3027–35. doi: 10.1016/S0140-6736(16)31593-8
5. WHO. Diarrheal diseases (fact sheet). WHO. (2017). Available at: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (Accessed October 10, 2023).
6. Koletzko S, Osterrieder S. Acute infectious diarrhea in children. Dtsch Arztebl Int. (2009) 106(33):539–47. doi: 10.3238/arztebl.2009.0539
7. Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. (2016) 12(12):Cd005436. doi: 10.1002/14651858.CD005436.pub5
8. Bajait C, Thawani V. Role of zinc in pediatric diarrhea. Indian J Pharmacol. (2011) 43(3):232–5. doi: 10.4103/0253-7613.81495
9. Li Y, Xia S, Jiang X, Feng C, Gong S, Ma J, et al. Gut microbiota and diarrhea: an updated review. Front Cell Infect Microbiol. (2021) 11:625210. doi: 10.3389/fcimb.2021.625210
10. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. (2013) 6(1):39–51. doi: 10.1177/1756283X12459294
11. Yang B, Lu P, Li M-X, Cai X-L, Xiong W-Y, Hou H-J, et al. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine (Baltimore). (2019) 98(37):e16618. doi: 10.1097/MD.0000000000016618
12. Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea–a double-blind study. Aliment Pharmacol Ther. (2012) 36(4):363–9. doi: 10.1111/j.1365-2036.2012.05180.x
13. Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048]. BMC Pediatr. (2004) 4:18. doi: 10.1186/1471-2431-4-18
14. Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. (2001) 33:S17–25. doi: 10.1097/00005176-200110002-00004
15. Tremblay A, Xu X, Colee J, Tompkins TA. Efficacy of a multi-strain probiotic formulation in pediatric populations: a comprehensive review of clinical studies. Nutrients. (2021) 13(6):1908. doi: 10.3390/nu13061908
16. Szajewska H, Kołodziej M, Gieruszczak-Białek D, Skorka A, Ruszczyński M, Shamir R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children–a 2019 update. Aliment Pharmacol Ther. (2019) 49(11):1376–84. doi: 10.1111/apt.15267
17. Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. (2010) 2010(11):CD003048. doi: 10.1002/14651858.CD003048.pub3
18. Collinson S, Deans A, Padua-Zamora A, Gregorio GV, Li C, Dans LF, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. (2020) 12(12):CD003048. doi: 10.1002/14651858.CD003048.pub4
19. Szajewska H, Berni Canani R, Domellöf M, Guarino A, Hojsak I, Indrio F, et al. Probiotics for the management of pediatric gastrointestinal disorders: position paper of the ESPGHAN special interest group on gut microbiota and modifications. J Pediatr Gastroenterol Nutr. (2023) 76(2):232–47. doi: 10.1097/MPG.0000000000003633
20. Kim LS, Hilli L, Orlowski J, Kupperman JL, Baral M, Waters RF. Efficacy of probiotics and nutrients in functional gastrointestinal disorders: a preliminary clinical trial. Dig Dis Sci. (2006) 51(12):2134–44. doi: 10.1007/s10620-006-9297-8
21. Cao J, Yu Z, Liu W, Zhao J, Zhang H, Zhai Q, et al. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J Funct Foods. (2020) 64:103643. doi: 10.1016/j.jff.2019.103643
22. Majeed M, Prakash L. Lactospore®-Lactic Acid Bacillus-Lactobacillus Sporogenes. Piscataway, NJ: Nutri science publishers Piscataway (1998). p. 1–56.
23. Majeed M, Majeed S, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F. Evaluation of the stability of Bacillus coagulans MTCC 5856 during processing and storage of functional foods. Int J Food Sci Technol. (2016) 51(4):894–901. doi: 10.1111/ijfs.13044
24. Majeed M, Natarajan S, Sivakumar A, Ali F, Pande A, Majeed S, et al. Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats. Int J Pharma Bio Sci. (2016) 7(1):311–6.
25. Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. (2015) 15(1):1–10. doi: 10.1186/s12937-016-0140-6
26. Majeed M, Nagabhushanam K, Arumugam S, Majeed S, Ali F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res. (2018) 62. doi: 10.29219/fnr.v62.1218
27. Majeed M, Nagabhushanam K, Mundkur L, Paulose S, Divakar H, Rao S, et al. Probiotic modulation of gut microbiota by Bacillus coagulans MTCC 5856 in healthy subjects: a randomized, double-blind, placebo-control study. Medicine (Baltimore). (2023) 102(20):e33751. doi: 10.1097/MD.0000000000033751
28. Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Pande A, Majeed S, et al. A double-blind, placebo-controlled, parallel study evaluating the safety of Bacillus coagulans MTCC 5856 in healthy individuals. J Clin Toxicol. (2016) 6(283):2161–0495.
29. Majeed M, Nagabhushanam K, Paulose S, Arumugam S, Mundkur L. The effects of Bacillus coagulans MTCC 5856 on functional gas and bloating in adults: a randomized, double-blind, placebo-controlled study. Medicine (Baltimore). (2023) 102(9):e33109. doi: 10.1097/MD.0000000000033109
30. Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Eshuis-de Ruiter T, Booij-Veurink J, et al. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J Microbiol Biotechnol. (2016) 32:1–12. doi: 10.1007/s11274-016-2027-2
31. Dutta P, Mitra U, Dutta S, Rajendran K, Saha TK, Chatterjee MK. Randomised controlled clinical trial of Lactobacillus sporogenes (Bacillus coagulans), used as probiotic in clinical practice, on acute watery diarrhoea in children. Trop Med Int Health. (2011) 16(5):555–61. doi: 10.1111/j.1365-3156.2011.02745.x
32. Sudha M R, Neelamraju J, Surendra Reddy M, Kumar M. Evaluation of the effect of probiotic Bacillus coagulans unique IS2 on Mutans streptococci and Lactobacilli levels in saliva and plaque: a double-blind, randomized, placebo-controlled study in children. Int J Dent. (2020) 2020:8891708. doi: 10.1155/2020/8891708
33. Lee Y-Y, Leow AH-R, Chai P-F, Raja Ali RA, Lee W-S, Goh K-L. Use of probiotics in clinical practice with special reference to diarrheal diseases: a position statement of the Malaysian Society of Gastroenterology and Hepatology. JGH Open. (2021) 5(1):11–9. doi: 10.1002/jgh3.12469
34. Sudha MR, Jayanthi N, Pandey D, Verma A. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: a double blind placebo controlled study. Benef Microbes. (2019) 10(2):149–54. doi: 10.3920/BM2018.0094
35. WHO. The treatment of diarrhoea. A manual for physicians and other senior health workers, 4th Revision. World Health Organization (2005). Available at: https://www.who.int/publications/i/item/9241593180 (Accessed June 22, 2023).
36. Brandt KG, Antunes MM dC, da Silva GAP. Acute diarrhea: evidence-based management. J Pediatr (Rio J). (2015) 91:S36–43. doi: 10.1016/j.jped.2015.06.002
37. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. (1997) 32(9):920–4. doi: 10.3109/00365529709011203
38. Galvao TF, e Silva Thees MFR, Pontes RF, Silva MT, Pereira MG. Zinc supplementation for treating diarrhea in children: a systematic review and meta-analysis. Revista Panamericana de Salud Publica. (2013) 33(5):370–7. doi: 10.1590/S1020-49892013000500009
39. Vecchio AL, Dias JA, Berkley JA, Boey C, Cohen MB, Cruchet S, et al. Comparison of recommendations in clinical practice guidelines for acute gastroenteritis in children. J Pediatr Gastroenterol Nutr. (2016) 63(2):226. doi: 10.1097/MPG.0000000000001133
40. Okubo Y, Miyairi I, Michihata N, Morisaki N, Kinoshita N, Urayama KY, et al. Recent prescription patterns for children with acute infectious diarrhea. J Pediatr Gastroenterol Nutr. (2019) 68(1):13–6. doi: 10.1097/MPG.0000000000002115
41. Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Møller PL, Pedersen P, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. J Pediatr Infect Dis. (2002) 21(5):411–6. doi: 10.1097/00006454-200205000-00012
42. Shornikova A-V, Casas IA, Mykkänen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. J Pediatr Infect Dis. (1997) 16(12):1103–7. doi: 10.1097/00006454-199712000-00002
43. Mosaddek ASM. Effect of probiotics in the treatment of acute watery diarrhoea in children admitted to a tertiary care hospital in Bangladesh: a non-randomized prospective clinical trial. Int J Collab Res Intern Med Public Health. (2022) 14(2):1–7.
44. Huang R, Xing H-Y, Liu H-J, Chen Z-F, Tang B-B. Efficacy of probiotics in the treatment of acute diarrhea in children: a systematic review and meta-analysis of clinical trials. Transl Pediatr. (2021) 10(12):3248. doi: 10.21037/tp-21-511
45. Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). (2015) 91:392–6. doi: 10.1016/j.jped.2014.10.009
46. Mourey F, Sureja V, Kheni D, Shah P, Parikh D, Upadhyay U, et al. A multicenter, randomized, double-blind, placebo-controlled trial of Saccharomyces boulardii in infants and children with acute diarrhea. J Pediatr Infect Dis. (2020) 39(11):e347. doi: 10.1097/INF.0000000000002849
47. Lai H-H, Chiu C-H, Kong M-S, Chang C-J, Chen C-C. Probiotic Lactobacillus casei: effective for managing childhood diarrhea by altering gut microbiota and attenuating fecal inflammatory markers. Nutrients. (2019) 11(5):1150. doi: 10.3390/nu11051150
48. Lahiri KR, Singh R, Apte M, Patil M, Taksande A, Varona R, et al. Efficacy and safety of Bacillus clausii (O/C, N/R, SIN, T) probiotic combined with oral rehydration therapy (ORT) and zinc in acute diarrhea in children: a randomized, double-blind, placebo-controlled study in India. Trop Dis Travel Med Vaccines. (2022) 8(1):9. doi: 10.1186/s40794-022-00166-6
49. Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Natarajan S, Beede K, et al. Galactomannan from Trigonella foenum-graecum L. seed: prebiotic application and its fermentation by the probiotic Bacillus coagulans strain MTCC 5856. Food Sci Nutr. (2018) 6(3):666–73. doi: 10.1002/fsn3.606
50. Shinde T, Vemuri R, Shastri MD, Perera AP, Tristram S, Stanley R, et al. Probiotic Bacillus coagulans MTCC 5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation. J Funct Foods. (2019) 52:100–8. doi: 10.1016/j.jff.2018.10.031
Keywords: Weizmannia coagulans (Bacillus coagulans) MTCC 5856, acute diarrhea, dehydration, oral rehydration solution, children, probiotics, randomized trial
Citation: Majeed M, Nagabhushanam K, Arumugam S, Chadalavada N, Seepana J, Annamalai T, Murali A, Prakasan P and Mundkur L (2024) Probiotic Weizmannia coagulans MTCC 5856 as adjunct therapy in children's acute diarrhea—a randomized, double-blind, placebo-controlled study. Front. Pediatr. 11:1338126. doi: 10.3389/fped.2023.1338126
Received: 14 November 2023; Accepted: 20 December 2023;
Published: 10 January 2024.
Edited by:
Pedro Gutierrez-Castrellon, International Scientific Council for Probiotics A.C., MexicoReviewed by:
Dimas Rosa, Grupo de Investigación del Caribe y Centroamérica para la Microbiota, Probióticos y Prebióticos GICCAMPP, Dominican Republic© 2024 Majeed, Nagabhushanam, Arumugam, Chadalavada, Seepana, Annamalai, Murali, Prakasan and Mundkur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lakshmi Mundkur bGFrc2htaUBzYW1pLXNhYmluc2Fncm91cC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.