- Department of Hepatobiliary Surgery, the First Affiliated Hospital of Chengdu Medical College, Xindu District, Chengdu, Sichuan Province, China
Objective: There has been a gradual increase in the prevalence of cesarean section deliveries and more healthcare professionals are considering the prophylactic use of corticosteroids before planned full-term cesarean sections. However, the association between dexamethasone administration before full-term cesarean delivery and short-term adverse neonatal outcomes is unclear. This study analyzed the disparities in short-term adverse neonatal effects in neonates born via full-term elective cesarean delivery with or without antenatal dexamethasone treatment.
Study design: This single-center retrospective cohort study involved neonates aged 37–39 weeks. The primary neonatal outcomes included various short-term adverse events, including neonatal admission to the neonatal intensive care unit, neonatal access to the special care baby unit, transient neonatal respiratory distress, respiratory distress syndrome, and the requirement of intravenous antibiotics or ventilatory support. Multiple logistic regression analysis was used to assess the association between these outcomes and dexamethasone exposure while adjusting for covariates.
Results: Of the 543 neonates included in the study, 121 (22.2%) had been exposed to prenatal dexamethasone. When compared with the control group, the dexamethasone-exposed group exhibited significantly higher rates of transient neonatal respiratory distress, respiratory distress syndrome, administration of intravenous antibiotics, the need for ventilatory support, and longer duration of neonatal hospitalization (P < 0.05). The association between dexamethasone exposure and short-term adverse neonatal outcomes remained significant after adjusting for potential confounders (odds ratio: 12.76, 95% confidence interval: 6.9–23.62, P < 0.001).
Conclusion: The dexamethasone-exposed group had a higher likelihood of experiencing short-term adverse outcomes when compared with non-exposed neonates, suggesting that dexamethasone may have detrimental effects on infants delivered at full term. This implies the importance of exercising caution when contemplating the use of antenatal corticosteroids.
1 Introduction
In recent decades, the rate of cesarean section deliveries has markedly increased worldwide, especially in high and middle-income countries (1–3). However, cesarean delivery independently contributes to the risk of neonatal respiratory complications, mainly respiratory distress syndrome and transient neonatal tachypnea (4, 5). Because the risk associated with elective cesarean delivery diminishes as gestational age progresses (6), it is recommended to postpone the procedure until the pregnancy reaches 39 weeks (7–10). However, approximately 10%–15% of women who choose cesarean section may deliver before the recommended gestational age (11). The Antenatal Steroid Trial for Full-Term Elective Cesarean Sections (12) and the Cochrane Review on the use of corticosteroids to prevent respiratory disorders in neonates after full-term elective cesarean deliveries (13) have led to widespread clinical acceptance of the use of prophylactic corticosteroids before scheduled full-term cesarean sections as a mitigation for potential neonatal respiratory system risks.
The Royal College of Obstetricians and Gynaecologists (RCOG) Green Top Guideline No. 7 (RCOG 2010) (14) recommended the prophylactic use of corticosteroids before full-term planned cesarean sections. However, this recommendation is not included in the current guidelines by the National Institute for Health and Care Excellence (NICE) for cesarean sections (NICE 2021) (15).
Dexamethasone, a synthetic glucocorticoid, is widely used to manage preterm labor and promote fetal pulmonary maturation, especially in low-resource settings (16, 17). The administration of prenatal corticosteroids promotes the maturation of the fetal pulmonary system in premature infants, thereby decreasing the occurrence of respiratory distress syndrome, the need for respiratory support, the duration of intensive care hospitalization, and the prevalence of various premature neonate complications, such as intraventricular hemorrhage, necrotizing enterocolitis, and neonatal mortality (18). The Maternal and Child Health Survey, a comprehensive investigation by the World Health Organization involving 359 institutions in 29 countries found that the prevalence of synthetic glucocorticoid administration was 54%, although some countries have rates of up to 91% (19).
The use of dexamethasone during late preterm birth is controversial, especially in low-resource settings (17). A thorough assessment was conducted to determine the efficacy of administering corticosteroids before full-term, scheduled cesarean deliveries and if it provides significant advantages while avoiding unnecessary harm. This harm is attributable to a limited understanding of the cellular and molecular mechanisms governing fetal lung maturation (20), coupled with inherent limitations of currently available markers (21). Evidence indicates that neonates exposed to prenatal corticosteroids may have a higher risk of unfavorable outcomes (22–25). Thus, the use of prenatal corticosteroids in the context of term deliveries warrants careful consideration.
2 Materials and methods
2.1 Data source and study cohort
The data used underlying this study are from the Dryad Digital Repository (https://doi.org/10.5061/dryad.g79cnp5qs). This retrospective single-center cohort study was carried out from December 2016 to February 2019 at the Professorial Unit, Department of Obstetrics and Gynecology, Colombo South Teaching Hospital University. The study population was described previously (26). The study participants were divided into the experimental and control groups. The experimental group comprised of mothers who were administered two intramuscular injections of dexamethasone (12 mg) at 12-hour intervals, commencing from one week to 24 h prior to delivery. The control group was made up of mothers who did not receive corticosteroid treatment before delivery.
This study involved maternal–fetal dyads who underwent elective cesarean sections at the gestational age of 37–39 weeks and who met the inclusion criteria. The cesarean sections analyzed in this study were categorized as elective, defined as cesarean sections scheduled in advance and not conducted under emergency circumstances. Information on cesarean section indications, such as elective factors like maternal request, large fetus, malpresentation/breech, and repeat cesarean section, was obtained from medical records. Neonatal care was provided within a single unit, ensuring uniform diagnostic and admission prerequisites.
The study cohort was subjected to exclusionary criteria, which included symptoms of severe maternal hypertension, severe fetal rhesus alloimmunization, or intrauterine infection characterized by maternal pyrexia, tachycardia, fetal distress, and meconium-stained liquor at delivery. Pregnant women who were concurrently receiving steroids for reasons unrelated to the study protocol, those with multiple gestations, those with emergency conditions necessitating mandated cesarean section, and cases with insufficient covariate data, were also excluded.
The requirement for ethical approval was waived because all data were meticulously anonymized and the study strictly adherence to the protocols and regulations established by the Dryad Digital Repository.
2.2 Data acquisition
Relevant patient bedside records were retrieved from archival records and subsequently transcribed onto a designated data collection template by an assistant researcher. To mitigate observer bias, a second assistant researcher not involved in developing the study protocol or in patient management, compiled the maternal demographic data, including data on maternal age, diversity of gestation, previous and current medical conditions, gestational age at the time of cesarean section, intricate surgical particulars, and postoperative complications. Moreover, data on the administration of corticosteroids to the mothers was meticulously documented. Relevant neonatal data, including birth weight and Apgar scores, were recorded. The primary outcomes under investigation included neonatal admission to the neonatal intensive care unit (NICU), assignment to the special care baby unit, transient neonatal tachypnea, respiratory distress syndrome, intravenous antibiotic administration, need for ventilatory support, and duration of neonatal hospitalization. Any of the first six above-mentioned criteria indicated short-term adverse neonatal effects.
2.3 Statistical analysis
First, we summarized the study cohort's baseline characteristics and categorized them based on dexamethasone exposure status. For continuous data, descriptive statistics involved the use of either the mean and standard deviation or the median and interquartile range depending on data distribution. Categorical data were presented as frequencies and corresponding percentages. Categorical and non-normally distributed continuous data were analyzed using the Pearson χ2 test, the Fisher exact test, or the Kruskal–Wallis test, as deemed appropriate. P < 0.05 indicated statistically significant differences. Covariate adjustments were applied where any of the following criteria were met: (1) confounders reported in the literature, (2) univariate analysis yielding a P-value of <0.1, or (3) a change in effect size of >10% upon covariate inclusion. Comprehensive univariate regression analysis was conducted on all variables to ascertain the potential factors that predict primary outcomes (adverse short-term neonatal effects). Univariate analysis was used to reveal the trends associated with adverse short-term neonatal outcomes. Logistic regression analysis was used to assess the independent association between dexamethasone exposure and adverse short-term neonatal effects. Subgroup analyses by age, parity, gravidity, and common comorbidities, such as pregnancy-induced hypertension (PIH) and gestational diabetes mellitus (GDM), were used to examine the stability of the association between dexamethasone exposure and adverse short-term neonatal effects. Smooth curve-fitting and threshold saturation effect analyses were used to assess the probability of short-term adverse neonatal effects. The likelihood of these effects was quantified using odds ratios (OR) and standard error with 95% confidence intervals (CI). Statistical analyses were done on R (http://www.R-project.org) and Free Statistics software version 1.8. A two-tailed test was employed, P < 0.05 indicating statistically significant differences.
3 Results
3.1 Baseline characteristics
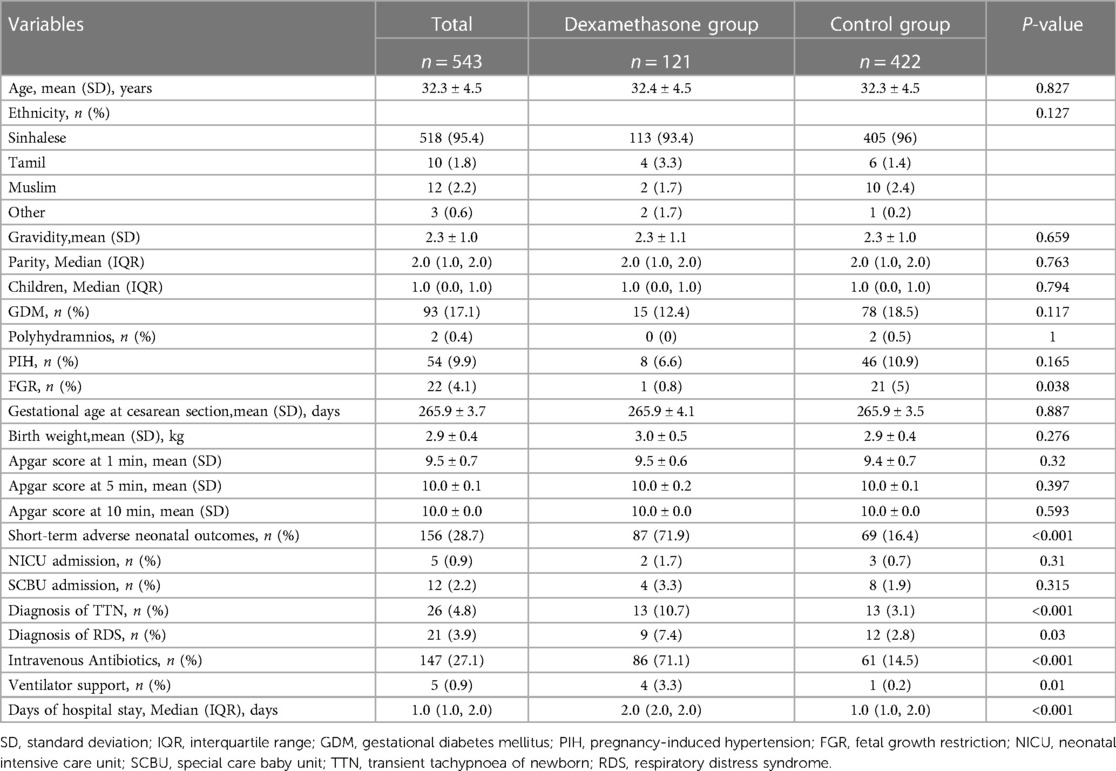
The baseline characteristics of the study's participants based on dexamethasone treatment status are shown in Table 1. From the original dataset of 560 observations, 17 entries were excluded because of a lack of crucial covariate information. Of these, 12 were excluded because of missing Apgar score data at one minute, one because of missing Apgar score data at 10 min, two because of missing gravidity data, and two because of missing GDM and PIH data. Hence, the final analysis involved 543 women and their neonates. The women had an average age of 32.3 ± 4.5 years and the majority (95.4%) identified as Sinhalese. Of the participants, 121 (22.2%) underwent planned cesarean section and received dexamethasone before the procedure (Table 1). The fetal growth restriction (FGR) rate was 5% more prevalent in the non-dexamethasone-treated group compared to a 0.8% lower incidence in the dexamethasone-treated group (P = 0.038). However, various factors, such as reproductive history (gravidity, parity, children), pregnancy comorbidities (GDM, polyhydramnios, and PIH), gestational age at cesarean section, and important neonatal characteristics like neonatal weight and Apgar scores at 1, 5, and 10 min) did not differ significantly between the two groups (P > 0.05).
The dexamethasone-treated cohort had significantly higher rates of primary outcome measures, such as transient neonatal tachypnea, respiratory distress syndrome, intravenous antibiotic administration, ventilatory support, and the duration of neonatal hospitalization when compared with the control group (P < 0.05). Moreover, in the dexamethasone group, the probabilities of neonatal admission into the NICU or placement in the special care baby unit were 1.7% and 3.3%, respectively, which was higher than in the control group.
This study observed a notable disparity in the utilization of antibiotics between the groups exposed to dexamethasone and those not exposed. A review of existing literature indicated varying rates of antibiotic usage for newborns admitted to neonatal intensive care units (NICUs), ranging from 2.4% to 97.1% (27). In order to address this issue, logistic single-factor analysis was conducted, and adjustments were made to the effect size if it exceeded a 10% change upon the inclusion of covariates. Potential confounding factors that were taken into consideration included variations in Admission to Neonatal Intensive Care Unit, Documented Transient Tachypnea of Newborn, and Documented Respiratory Distress Syndrome between the two groups (Supplementary Table S3). Furthermore, owing to the higher occurrence of short-term adverse outcomes in the dexamethasone-treated group, there was an increased likelihood of admission to the Neonatal Intensive Care Unit and diagnoses of Documented Transient Tachypnea of Newborn and Documented Respiratory Distress Syndrome, thereby contributing to the escalated usage of antibiotics in the dexamethasone group.
3.2 Association between dexamethasone and short-term adverse neonatal outcomes in full-term elective cesarean delivery
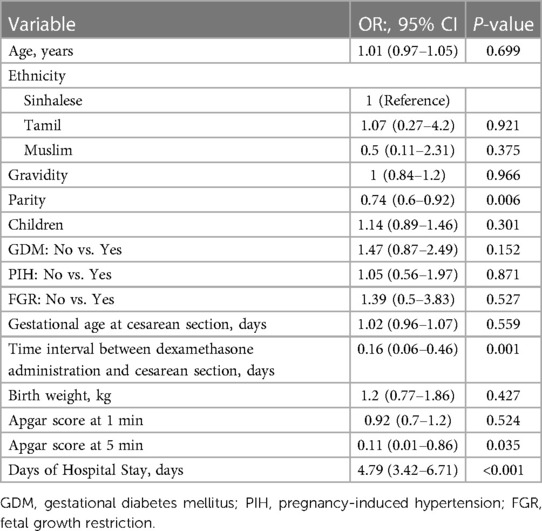
Univariate analysis revealed statistically significant associations between short-term adverse neonatal outcomes and parity (OR: 0.74, 95% CI: 0.60–0.92, P = 0.006), 5-minute Apgar scores (OR: 0.11, 95% CI: 0.01–0.86, P = 0.035), time interval between dexamethasone administration and cesarean section (OR: 0.16, 95% CI: 0.06–0.46, P = 0.001) and neonatal duration of hospital stay (OR: 4.79, 95% CI: 3.42–6.71, P < 0.001) (Table 2). Multiple logistic regression results of the analysis of the association between dexamethasone and short-term adverse neonatal outcomes are presented in Table 3. The unadjusted model (Model I) showed that dexamethasone was significantly associated with an increased risk of short-term adverse neonatal outcomes (OR: 13.09, 95% CI: 8.16–21.01, P < 0.001), and the association remained significant after adjusting for age, ethnicity, gravidity, parity, and children (Model II; OR: 16.46, 95% CI: 9.81–27.64, P < 0.001). Moreover, this association did not change significantly after adjustment for GDM, PIH, and FGR (Model III; OR: 17.34, 95% CI: 10.21–29.47, P < 0.001), or all other relevant comorbidities, including birth weight, 1- and 5-minute Apgar scores, duration of hospital stay, and gestational age at cesarean section (Model IV; OR: 12.76, 95% CI: 6.9–23.62, P < 0.001).
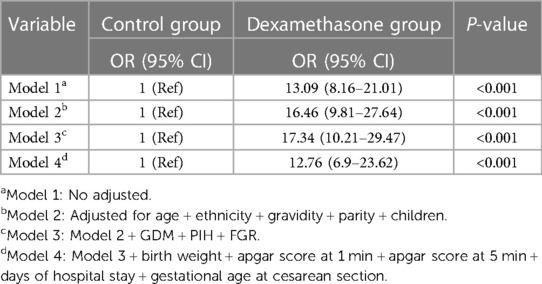
Table 3. Multivariate regression analysis of the association between dexamethasone and short-term adverse neonatal outcomes.
3.3 Sensitivity and subgroup analysis
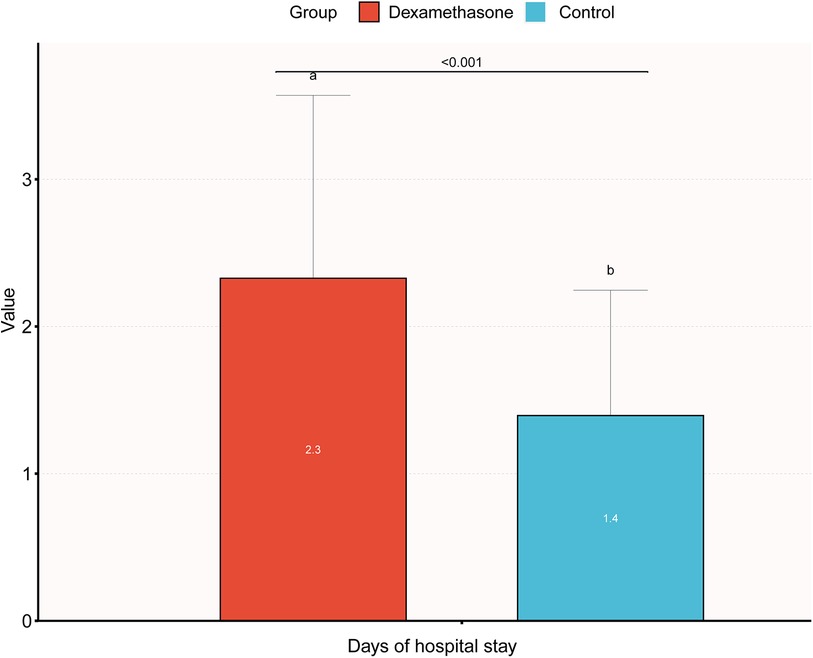
The results of stratified and interaction analyses of dexamethasone and short-term adverse neonatal outcomes subgroups of key factors were analyzed in stratified and interaction analyses. The stratified analysis showed that PIH, age, parity, and gravidity were not statistically significant after stratification (P > 0.05), indicating that the effect of dexamethasone on short-term adverse neonatal outcomes was stable and it was not affected by changes in covariates (Figure 1). The association between GDM and dexamethasone was examined for short-term adverse neonatal outcomes (P = 0.003). There is an increased likelihood that dexamethasone is linked to short-term adverse pregnancy outcomes in neonates whose mothers did not have gestational diabetes before pregnancy when compared with the control group. A comprehensive analysis of the correlation between the duration of neonatal hospitalization and dexamethasone, focusing on immediate negative consequences revealed a significant increase in the average length of hospitalization for neonates in the dexamethasone group when compared with the control group, indicating a comparatively more critical condition. Notably, the difference in the mean hospital stay between the two groups was only 0.9 days, with the maximum hospital stay being 12 days and there were no neonatal mortalities. This suggests that dexamethasone may have a transient effect on short-term adverse neonatal outcomes with a better overall prognosis (Figure 2).
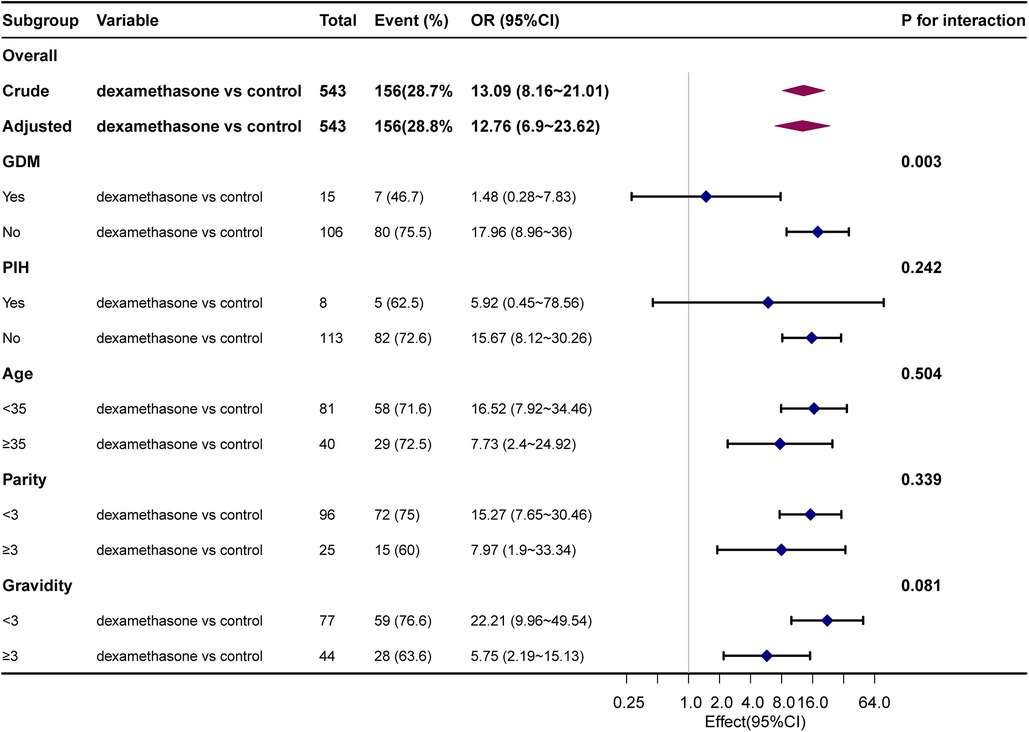
Figure 1. Association between dexamethasone and short-term adverse neonatal outcomes. Adjusted for ethnicity, children, GDM, birth weight, Apgar at one and five minutes, days of hospital stay, and gestational age at cesarean section.
4 Discussion
This study explored the association between dexamethasone usage during full-term elective cesarean deliveries and short-term adverse neonatal outcomes. This single-center retrospective cohort study made the following key findings: (1) there was a significantly elevated likelihood of short-term adverse neonatal outcomes in the cohort that received dexamethasone before full-term planned cesarean sections, (2) dexamethasone was significantly associated with short-term adverse neonatal outcomes, even after adjusting for baseline characteristics and other comorbidities, and (3) the possible effects of dexamethasone on short-term adverse neonatal outcomes are transient. Prenatal corticosteroid therapy, a fundamental component of perinatal care, warrants comprehensive evaluation because of its potential impact on fetal development and programming, given its influence on up to 20% of the transcriptome (28, 29). To avert adverse short-term neonatal outcomes it is important to minimize fetal exposure to such medications. Notably, full-term and late preterm infants are inherently exposed to elevated levels of endogenous steroids and may additionally receive prenatal corticosteroids (30). A recent meta-analysis involving 1.6 million infants found that early prenatal exposure to corticosteroids, when compared to no direction, was associated with an increased risk of neonatal intensive care unit admissions among full-term infants (OR: 1.49, 95% CI: 1.19–1.86) (23). This highlights the pressing need for informed, evidence-based use of corticosteroids to minimize the potential for over-treatment and subsequent neonatal mortality.
Furthermore, previous studies indicate that dexamethasone administration during pregnancy has potential adverse consequences, including an increased risk of cardiovascular disease in the offspring and neurotoxicity (31–35).
Our study revealed a significant correlation between dexamethasone usage and short-term adverse neonatal outcomes in full-term elective cesarean deliveries, which persisted even after adjusting for covariates linked to short-term neonatal adverse effects. Specifically, neonates in the dexamethasone-treated group exhibited a higher risk of various unfavorable outcomes, including respiratory distress syndrome, intravenous antibiotic use, ventilatory support, and a greater likelihood of admission into neonatal intensive care. These findings raise concerns about the potential risks associated with dexamethasone, particularly during full-term elective cesarean deliveries. Countries must exercise prudence when incorporating interventions into healthcare policies and ensure that decisions are rooted in evidence of efficacy and a thorough risk assessment (36).
However several studies with similar aims found that prenatal corticosteroid treatment did not clearly establish a significant association with adverse neonatal outcomes (11, 12, 37). Although these studies are relevant to our topic, they differ in their primary outcome indicators. Specifically, Stutchfield et al. (12) focused on the incidence of neonates admitted to the intensive care nursery for respiratory distress, whereas our study focused on neonates requiring specialized care as the outcome indicator. This disparity could potentially serve as a significant determinant influencing the disparate findings observed in our study. Concurrently, it is worth acknowledging that the systematic review conducted by Sotiriadis et al. (11) exhibits certain uncertainties regarding the precision of its findings, owing to the limitations associated with the certainty of evidence available in the existing literature. Conversely, our study primarily classified unfavorable outcomes based on the requirement for specialized medical attention, with a specific emphasis on the potential detrimental impacts of medications on neonatal outcomes. While acknowledging the potential divergence from existing literature, it is our firm conviction that our study contributes significant insights into distinct neonatal outcomes.
Our study found that the effect of dexamethasone on short-term adverse neonatal outcomes was transient. Specifically, although the dexamethasone group was associated with an increased risk of adverse neonatal outcomes, this effect was mainly manifested in the short term and did not persist over an extended period. No neonatal deaths were observed despite a prolonged duration (days) of hospitalization, and the mean difference was relatively small. A retrospective cohort study involving data from 588,077 live births found that antenatal corticosteroid exposure was associated with significantly lower odds of neonatal mortality and 5-minute Apgar scores of <7 in 121,151 women (38). However, there was an increased incidence of some adverse neonatal outcomes, such as surfactant replacement therapy, prolonged mechanical ventilation, antibiotics for suspected neonatal sepsis, and NICU admissions. The causes may include lung maturation and the anti-inflammatory effects of prenatal corticosteroids, which may enhance alveolar complexity (39), and the immunosuppressive effects of corticosteroids, which may cause or worsen infections (40). However, further studies are needed to confirm the long-term neuropsychiatric and cardiac risks.
4.1 Limitations of the study and suggestions for future research
(1) Data Source and Confounders: The present study relied on secondary data obtained from previous research, thereby imposing inherent constraints on the availability of information about potential confounding variables. (2) Study design: Being retrospective, this investigation is susceptible to inherent limitations, such as the potential existence of unmeasured confounders. Furthermore, the observational nature of the study does not establish causality but only offers evidence of association. The single-center nature of the study may limit the generalizability of its findings. (3) Based on the admission records, this retrospective cohort study identified one case of neonatal hypoglycemia among the hospitalized newborns. However, postnatal neonatal blood glucose data were not collected.
To address these limitations and advance our understanding of dexamethasone usage during full-term elective cesarean deliveries, future research should consider the following: (1) performing multicenter prospective studies, which can enable a more comprehensive and robust examination of the role of dexamethasone in full-term elective cesarean deliveries. These studies should include a wider range of patient characteristics and factors to better evaluate the impact of dexamethasone. (2) Future research should delve deeper into the timing of dexamethasone administration and how patient characteristics may influence its outcomes. This will help elucidate the nuances of dexamethasone use in different clinical scenarios. (3) The impact of prenatal dexamethasone on maternal well-being, including conditions like hyperglycemia and hypertension also warrants investigation. Furthermore, it is important to examine the potential of enduring consequences of prenatal steroid exposure on childhood development (41), including neurodevelopmental outcomes and related factors.
Addressing these limitations and conducting further research can improve our understanding of the benefits and potential risks of dexamethasone in the context of full-term elective cesarean deliveries.
5 Conclusion
Despite our study's limitations, our findings indicate a possible correlation between dexamethasone administration during elective cesarean delivery at full term and negative neonatal outcomes in the short term. However, these findings require further validation through thorough investigations. Nonetheless, healthcare professionals should exercise caution when considering dexamethasone therapy and carefully evaluate the trade-off between potential advantages and risks, while engaging in shared decision-making with patients to determine the most appropriate treatment strategy.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5061/dryad.g79cnp5qs.
Ethics statement
We have referenced Dryad packets in this study. The author of the original study has waived all copyright and related ownership of the data. Therefore, we can use these data for secondary analysis without infringing upon the rights of the author. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Madura Jayawardane and collaborators for their contributions in preparing and making the data publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1323097/full#supplementary-material
Supplementary Figure S1
Graphs showing smooth curve fittings of the gestation period at cesarean section and short-term adverse neonatal outcomes. Abbreviations: The solid and dashed lines represent the estimated values and their corresponding 95% confidence intervals. Analysis was adjusted for age, ethnicity, gravidity, parity, children, GDM, PIH, FGR, birth weight, and Apgar at one and five minutes. The solid and dashed lines represent the predicted value and corresponding 95% confidence intervals. The density curve of gestational age distribution during cesarean section is illustrated by the blue region. The median gestational age at cesarean section is represented by the vertical dashed line at 266 days in the legend.
Supplementary Table S1
Threshold effect analysis.
Supplementary Table S2
Collinear diagnosis.
References
1. Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One. (2016) 11(2):e0148343. doi: 10.1371/journal.pone.0148343
2. Sorrentino F, Greco F, Palieri T, Vasciaveo L, Stabile G, Carlucci S, et al. Caesarean section on maternal request-ethical and juridic issues: a narrative review. Medicina. (2022) 58(9):1255. doi: 10.3390/medicina58091255
3. Grant D. The “quiet revolution” and the cesarean section in the United States. Econ Hum Biol. (2022) 47:101192. doi: 10.1016/j.ehb.2022.101192
4. Indraccolo U, Pace M, Corona G, Bonito M, Indraccolo SR, Di Iorio R. Cesarean section in the absence of labor and risk of respiratory complications in newborns: a case-control study. J Matern Fetal Neonatal Med. (2019) 32(7):1160–6. doi: 10.1080/14767058.2017.1401999
5. Stylianou-Riga P, Boutsikou T, Kouis P, Kinni P, Krokou M, Ioannou A, et al. Maternal and neonatal risk factors for neonatal respiratory distress syndrome in term neonates in cyprus: a prospective case-control study. Ital J Pediatr. (2021) 47(1):129. doi: 10.1186/s13052-021-01086-5
6. Grobman WA, Caughey AB. Elective induction of labor at 39 weeks compared with expectant management: a meta-analysis of cohort studies. Am J Obstet Gynecol. (2019) 221(4):304–10. doi: 10.1016/j.ajog.2019.02.046
7. Bick D. Caesarean section. Clinical guideline. National collaborating centre for women’s and children’s health: commissioned by the national institute for clinical excellence. Worldviews Evid Based Nurs. (2004) 1(3):198–9. doi: 10.1111/j.1524-475X.2004.04060.x
8. Pirjani R, Afrakhteh M, Sepidarkish M, Nariman S, Shirazi M, Moini A, et al. ‘Elective caesarean section at 38–39 weeks gestation compared to >39 weeks on neonatal outcomes: a prospective cohort study. BMC Pregnancy Childbirth. (2018) 18(1):140. doi: 10.1186/s12884-018-1785-2
9. Prediger B, Polus S, Mathes T, Bühn S, Louwen F, Neugebauer E, et al. (Update of a) systematic review on the impact of elective early term (<39th gestational week) caesarean sections on maternal and neonatal health - a protocol. Syst Rev. (2018) 7(1):119. doi: 10.1186/s13643-018-0787-5
10. Wilmink FA, Pham CT, Edge N, Hukkelhoven C, Steegers E, Mol BW. Timing of elective pre-labour caesarean section: a decision analysis. Aust N Z J Obstet Gynaecol. (2019) 59(2):221–7. doi: 10.1111/ajo.12821
11. Sotiriadis A, McGoldrick E, Makrydimas G, Papatheodorou S, Ioannidis JP, Stewart F, et al. Antenatal corticosteroids prior to planned caesarean at term for improving neonatal outcomes. Cochrane Database Syst Rev. (2021) 12(12):CD006614.34935127
12. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. Br Med J. (2005) 331(7518):662. doi: 10.1136/bmj.38547.416493.06
13. Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP, McGoldrick E. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database Syst Rev. (2018) 8(4):CD006614. doi: 10.1002/14651858.CD006614.pub3
14. Badreldin N, DiTosto JD, Leziak K, Niznik CM, Yee LM. Understanding the postpartum cesarean pain experience among individuals with publicly funded insurance: a qualitative investigation. J Midwifery Womens Health. (2023). doi: 10.1111/jmwh.13540. [Epub ahead of print].
15. Cho H, Lee K, Choi E, Cho HN, Park B, Suh M, et al. Association between social support and postpartum depression. Sci Rep. (2022) 12(1):3128. doi: 10.1038/s41598-022-07248-7
16. Oladapo OT, Vogel JP, Piaggio G, Nguyen MH, Althabe F, Gülmezoglu AM, et al. Antenatal dexamethasone for early preterm birth in low-resource countries. N Engl J Med. (2020) 383(26):2514–25. doi: 10.1056/NEJMoa2022398
17. WHO ACTION Trials Collaborators. Antenatal dexamethasone for late preterm birth: a multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial. EClinicalMedicine. (2022) 44:101285. doi: 10.1016/j.eclinm.2022.101285
18. McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2020) 12(12):CD004454. doi: 10.1002/14651858.CD004454.pub4
19. Vogel JP, Souza JP, Gülmezoglu AM, Mori R, Lumbiganon P, Qureshi Z, et al. Use of antenatal corticosteroids and tocolytic drugs in preterm births in 29 countries: an analysis of the WHO multicountry survey on maternal and newborn health. Lancet. (2014) 384(9957):1869–77. doi: 10.1016/S0140-6736(14)60580-8
20. Lim K, Donovan A, Tang W, Sun D, He P, Pett JP, et al. Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell. (2023) 30(1):20–37.e9. doi: 10.1016/j.stem.2022.11.013
21. Danopoulos S, Bhattacharya S, Mariani TJ, Al Alam D. Transcriptional characterisation of human lung cells identifies novel mesenchymal lineage markers. Eur Respir J. (2020) 55(1):1900746. doi: 10.1183/13993003.00746-2019
22. Nixon PA, Washburn LK, O’Shea TM. Antenatal steroid exposure and pulmonary outcomes in adolescents born with very low birth weight. J Perinatol. (2013) 33(10):806–10. doi: 10.1038/jp.2013.69
23. Ninan K, Gojic A, Wang Y, Asztalos EV, Beltempo M, Murphy KE, et al. The proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes: systematic review and meta-analysis of 1.6 million infants. BMJ. (2023) 382:e076035. doi: 10.1136/bmj-2023-076035
24. Bandyopadhyay A, Slaven JE, Evrard C, Tiller C, Haas DM, Tepper RS. Antenatal corticosteriods decrease forced vital capacity in infants born fullterm. Pediatr Pulmonol. (2020) 55(10):2630–4. doi: 10.1002/ppul.24941
25. McKinzie AH, Yang Z, Teal E, Daggy JK, Tepper RS, Quinney SK, et al. Are newborn outcomes different for term babies who were exposed to antenatal corticosteroids. Am J Obstet Gynecol. (2021) 225(5):536.e1–e7. doi: 10.1016/j.ajog.2021.04.251
26. Nolvi S, Karlsson L, Bridgett DJ, Pajulo M, Tolvanen M, Karlsson H. Maternal postnatal psychiatric symptoms and infant temperament affect early mother-infant bonding. Infant Behav Dev. (2016) 43:13–23. doi: 10.1016/j.infbeh.2016.03.003
27. Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. (2015) 135(5):826–33. doi: 10.1542/peds.2014-3409
28. Jobe AH, Kemp M, Schmidt A, Takahashi T, Newnham J, Milad M. Antenatal corticosteroids: a reappraisal of the drug formulation and dose. Pediatr Res. (2021) 89(2):318–25. doi: 10.1038/s41390-020-01249-w
29. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. (2013) 132(5):1033–44. doi: 10.1016/j.jaci.2013.09.007
30. Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update. (2016) 22(2):240–59. doi: 10.1093/humupd/dmv047
31. Althabe F, Belizán JM, McClure EM, Hemingway-Foday J, Berrueta M, Mazzoni A, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. (2015) 385(9968):629–39. doi: 10.1016/S0140-6736(14)61651-2
32. Xu T, Zhao M, Li H, Zhou X, Liu B, Sun M, et al. Antenatal dexamethasone exposure impairs the high-conductance Ca(2+)-activated K(+) channels via epigenetic alteration at gene promoter in male offspring. Arterioscler Thromb Vasc Biol. (2020) 40(11):e284–95. doi: 10.1161/ATVBAHA.120.314905
33. Lanshakov DA, Sukhareva EV, Kalinina TS, Dygalo NN. Dexamethasone-induced acute excitotoxic cell death in the developing brain. Neurobiol Dis. (2016) 91:1–9. doi: 10.1016/j.nbd.2016.02.009
34. Costa G, Spulber S, Paci E, Casu MA, Ceccatelli S, Simola N, et al. In utero exposure to dexamethasone causes a persistent and age-dependent exacerbation of the neurotoxic effects and glia activation induced by MDMA in dopaminergic brain regions of C57BL/6J mice. Neurotoxicology. (2021) 83:1–13. doi: 10.1016/j.neuro.2020.12.005
35. Zhang S, Hu S, Dong W, Huang S, Jiao Z, Hu Z, et al. Prenatal dexamethasone exposure induces anxiety- and depressive-like behavior of male offspring rats through intrauterine programming of the activation of NRG1-ErbB4 signaling in hippocampal PV interneurons. Cell Biol Toxicol. (2023) 39(3):657–78. doi: 10.1007/s10565-021-09621-0
36. Antenatal dexamethasone for improving preterm newborn outcomes in low-resource countries: a cost-effectiveness analysis of the WHO ACTION-I trial. Lancet Glob Health. (2022) 10(10):e1523–33. doi: 10.1016/S2214-109X(22)00340-0
37. Krispin E, Borovich A, Hochberg A, Salman L, Chen R, Wiznitzer A, et al. Neonatal outcomes in term pregnancies treated with antenatal corticosteroids for suspected pre-term labor. Arch Gynecol Obstet. (2019) 299(2):403–9. doi: 10.1007/s00404-018-4976-1
38. Gulersen M, Grunebaum A, Lenchner E, Chervenak FA, Bornstein E. Antenatal corticosteroids and neonatal outcomes in preterm birth in the United States. J Perinat Med. (2022) 50(5):573–80. doi: 10.1515/jpm-2022-0002
39. Reyburn B, Li M, Metcalfe DB, Kroll NJ, Alvord J, Wint A, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. (2008) 178(4):407–18. doi: 10.1164/rccm.200802-359OC
40. Behbodi E, Villamor-Martínez E, Degraeuwe PL, Villamor E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Sci Rep. (2016) 6:37967. doi: 10.1038/srep37967
Keywords: antenatal corticosteroids, planned cesarean, newborn outcomes, termination of pregnancy, retrospective cohort study
Citation: Pei J and Chen J (2024) The influence of prenatal dexamethasone administration before scheduled full-term cesarean delivery on short-term adverse neonatal outcomes: a retrospective single-center cohort study. Front. Pediatr. 11:1323097. doi: 10.3389/fped.2023.1323097
Received: 17 October 2023; Accepted: 13 December 2023;
Published: 11 January 2024.
Edited by:
Jonathan Michael Davis, Tufts University, United StatesReviewed by:
Ryan Kilpatrick, Tufts Medical Center, United StatesElizabeth Yen, Tufts University, United States
© 2024 Pei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Chen Y3FtdWNoZW5qaWFvNTIwQGdtYWlsLmNvbQ==
 Jiaojiao Pei
Jiaojiao Pei Jiao Chen
Jiao Chen