
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 04 December 2023
Sec. Pediatric Pulmonology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1322360
 Meenu Singh1*
Meenu Singh1* Sneha Varkki2
Sneha Varkki2 Ilin Kinimi3
Ilin Kinimi3 Rashmi R. Das4
Rashmi R. Das4 Jagdish Prasad Goyal5
Jagdish Prasad Goyal5 Mushtaq Bhat6
Mushtaq Bhat6 Rajeshwar Dayal7
Rajeshwar Dayal7 Pawan Kalyan8
Pawan Kalyan8 Jitender Gairolla9
Jitender Gairolla9 Indu Khosla10
Indu Khosla10
Background: Currently, there are no guidelines or consensus statements about the usage of inhaled mucoactive drugs in pediatric respiratory disease conditions from an Indian perspective.
Objective: To develop a practical consensus document to help pediatricians in clinical decision-making when choosing an appropriate mucoactive drug for the management of specific respiratory disease conditions.
Methods: A committee of nine experts with significant experience in pediatric respiratory disease conditions and a microbiological expert constituted the panel. An electronic search of the PubMed/MEDLINE, Cochrane Library, Scopus, and Embase databases was undertaken to identify relevant articles. Various combinations of keywords such as inhaled, nebulized, mucoactive, mucolytic, mucokinetic, expectorants, mucoregulators, mucociliary clearance, respiratory disorders, pediatric, cystic fibrosis (CF), non-CF bronchiectasis, acute wheezing, asthma, primary ciliary dyskinesia (PCD), critically ill, mechanical ventilation, tracheomalacia, tracheobronchomalacia, esophageal atresia (EA), tracheoesophageal fistula (TEF), acute bronchiolitis, sputum induction, guideline, and management were used. Twelve questions were drafted for discussion. A roundtable meeting of experts was conducted to arrive at a consensus. The level of evidence and class of recommendation were weighed and graded.
Conclusions: Inhaled mucoactive drugs (hypertonic saline, dry powder mannitol, and dornase alfa) can enhance mucociliary clearance in children with CF. Experts opined that hypertonic saline could be beneficial in non-CF bronchiectasis, acute bronchiolitis, and PCD. The current state of evidence is inadequate to support the use of inhaled mucoactive drugs in asthma, acute wheezing, tracheomalacia, tracheobronchomalacia, and EA with TEF.
India has a high burden of acute and chronic respiratory diseases. Pediatric respiratory diseases place a substantial financial and human resource strain on our healthcare system every year. Several childhood disorders, such as primary ciliary dyskinesia (PCD), cystic fibrosis (CF), non-CF bronchiectasis, and severe asthma exhibit airway mucus hypersecretion (1). Mucoactive drugs have a long and well-established record of being an effective therapy for the management of respiratory diseases in which mucus hypersecretion is a clinical challenge (1, 2). Mucoactive drugs are classified as expectorants, mucoregulators, mucolytics, or mucokinetic drugs based on their potential mechanism of action (Figure 1) (1, 2). Inhaled mucoactive drugs are delivered directly to the airway and are used to improve mucus properties and reduce the mucus load in the lungs of patients suffering from muco obstructive pulmonary illness (1, 2). In this article, we have attempted to review the available literature and summarize recommendations on the role of inhaled mucoactive drugs in pediatric respiratory disease conditions from an Indian perspective.
A panel consisting of nine experts (mean age: 53.5 years; specialty: pediatrics) with significant experience in pediatric respiratory disease conditions and a microbiological expert participated in the development of this consensus manuscript (Supplementary Table S1). Panel members were carefully selected based on their wide clinical expertise and knowledge in the field. A minimum of 10 years of clinical expertise in the field was mandatory. A moderator was identified among the panel to drive the consensus process.
An electronic search of the PubMed/MEDLINE, Cochrane Library, Scopus, and Embase databases was undertaken to identify relevant articles between January 1980 and August 2022. Various combinations of keywords such as “inhaled,” “nebulized,” “mucoactive,” “mucolytic,” “mucokinetic,” “expectorants,” “mucoregulators,” “mucociliary clearance,” “respiratory disorders,” “pediatric,” “cystic fibrosis,” “non-cystic fibrosis bronchiectasis,” “acute wheezing,” “asthma,” “primary ciliary dyskinesia,” “critically ill,” “mechanical ventilation,” “tracheomalacia,” “tracheobronchomalacia,” “esophageal atresia,” “tracheoesophageal fistula,” “acute bronchiolitis,” “sputum induction,” “guideline,” and “management” were used. Appropriate variations in search phrases and Boolean operators (AND, OR) were used. Randomized controlled trials, case reports, practice guidelines, systematic literature reviews, and meta-analyses were included. Animal studies and studies published in a language other than English were excluded. Duplicates were removed during the screening procedure. After an extensive search, 12 clinically relevant questions (Supplementary Table S2) were drafted to facilitate discussion. A virtual meeting was conducted on 24 June 2022 to finalize the questionnaire. Key articles were shortlisted and circulated among the expert panel members.
The class of recommendation (COR) and certainty of evidence (COE) were weighed and graded according to predefined scales as outlined in Table 1 (3–5). The COR was based on the grading system used by Knuuti et al., which was suitably modified and adapted to current settings (3). A roundtable meeting of experts was held on 23 August 2022 to finalize the recommendations on the role of inhaled mucoactive drugs in pediatric respiratory disease conditions. To assess the COE, we employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) technique, as defined in the GRADE handbook (4, 5). The COE for each of the outcomes was independently evaluated by two authors. We rated the evidence from RCTs as being of high quality and downgraded it to one level for serious (or two levels for very serious) limitations based on the following considerations: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. After the group discussion, clinical consensus statements were formulated based on the opinions and agreement of the majority. During group discussions, all panelists were encouraged to participate actively. The differences in opinions were discussed and resolved. Certain recommendations are based on the collective clinical judgment from real-world practice and no grading of recommendations has been applied for the same. A draft of the clinical consensus statements and recommendations was circulated among the expert panel for review. After the second meeting, the experts discussed updating any new findings (if any). A second round of basic literature searches was conducted in PubMed/MEDLINE, Cochrane Library, Scopus, and Embase databases in June 2023 to check for any new updates/findings. The final draft of the clinical consensus statements and recommendations was circulated among the expert panel for final review and approval in the first week of July 2023.
Cystic fibrosis is a genetic illness caused by a gene defect on chromosome 7 that encodes for CF transmembrane conductance regulator (CFTR) protein (6). In patients with CF, mucociliary clearance is impaired. Evidence (Table 2) showed that inhaled hypertonic saline (HS; an expectorant) enhanced mucociliary clearance (15), improved lung clearance index (8, 16), and reduced pulmonary exacerbations (17) in children with CF as compared with isotonic saline. Salbutamol followed by inhaled 3% HS positively affected structural lung changes relative to 0.9% saline (9). Higher HS strengths (5%, 6%, or 7%) may have the same or better effect. However, more research is needed in a developing country setting like India. In children with CF, the use of inhaled mannitol, a hyperosmotic mucoactive drug, also resulted in an improvement in lung function [forced expiratory volume in 1 s (FEV1)] (12, 18, 19). Clinical evidence supports the use of recombinant human DNAase I (rhDNase; dornase alfa), a mucolytic drug as it improved lung function (FEV1) and reduced pulmonary exacerbations in children with CF (20, 21). N-acetylcysteine (NAC), a mucolytic drug, causes cleavage of disulfide bonds to two sulfhydryl groups, resulting in thinning of the mucus (1). No beneficial effect of NAC on lung function has been reported in children with CF (14). Heparin inhalation showed no significant effect on sputum clearance, FEV1, or sputum inflammatory markers in adults with CF (22). Currently, there is no evidence of the role of inhaled heparin in children with CF.
Children with CF would benefit from initiation of HS inhalation (6% or 7%; twice or thrice daily) from the time of diagnosis (COR: I, agreement, very low CoE).
The experts concurred that HS inhalation (6% or 7%; twice or thrice daily) as an adjunct to the airway clearance technique (ACT) can enhance mucociliary clearance and reduce pulmonary exacerbations in children with CF based on real-world practice (COR: II, conditional recommendation, very low CoE). As local side-effects are common after a higher strength of HS (6% and 7%) that may affect tolerability, more research on 3% HS is needed.
Inhaled dry powder mannitol (400 mg; twice daily) is useful for clearing retained airway secretions in children with CF (COR: II, conditional recommendation, very low to low CoE). However, a mannitol dry powder inhaler is currently unavailable in India for managing mucus hypersecretion in children with CF. The experts suggested that tolerability testing is needed before treatment.
Dornase alfa (rhDNAse; 2.5 mg; once or twice daily) in nebulized form is useful in reducing the risk of exacerbations of respiratory symptoms requiring parenteral antibiotics in children with CF (COR: I, agreement, moderate to high COE). However, rhDNase is currently unavailable in India and the cost associated with therapy is high for managing mucus hypersecretion in children with CF.
The use of NAC is not beneficial in children with CF. However, more data are needed (COR: III, disagreement, very low COE).
Bronchiectasis is a chronic respiratory disease associated with wet cough in children and recurrent infective exacerbations impacting the quality of life (QoL) (23, 24). Treatment for non-CF bronchiectasis consists of management of nutrition, airway clearance, and antibiotics for exacerbations (23, 24). Tarrant et al. systematically reviewed the effects of mucoactive drugs in chronic non-CF bronchiectasis. Both HS (6% or 7%) and normal saline (0.9% sodium chloride; NS) improved FEV1, forced vital capacity (FVC), and forced expiratory flow25%–75% (FEF25%–75%) in bronchiectasis after one dose and after 3–12 months (25). Mannitol failed to improve spirometry in bronchiectasis. On the contrary, rhDNase caused significant reductions in FEV1 and FVC, but increased exacerbation rate, and reduced spirometry (25). Another review of inhaled HS in bronchiectasis found that it improved expectoration, reduced sputum viscosity, improved lung function, and reduced the frequency of exacerbations (23). The British Thoracic Society guidelines for the management of non-CF bronchiectasis in adults mention that inhaled HS may be used as an adjunct to physiotherapy (26). The use of rhDNase is not advised as it worsens lung function due to an increase in exacerbation frequency. In addition, it states that there is no definitive clinical evidence to confirm its use in children or adults with bronchiectasis (26). Anuradha et al. highlighted that inhaled salbutamol (200 µg) followed by 3% HS nebulization twice daily for 8 weeks before chest physiotherapy significantly improved FEV1 in children (N = 26; 5–15 years of age) with non-CF bronchiectasis (27). In addition, improvement in FVC and reduction in the frequency of exacerbation were significant compared with conventional ACT (N = 26; inhaled salbutamol before chest physiotherapy) (27). Table 3 lists clinical studies of inhaled mucoactive drugs in chronic non-CF bronchiectasis.
Evidence on the efficacy and safety of inhaled HS in adults with non-CF bronchiectasis is available, and it is beneficial (low CoE). More research into the pediatric population is required. Based on the data available for adults, the experts proposed that the inhaled HS before chest physiotherapy can be tried in children with non-CF bronchiectasis until more data are available.
The HS inhalation is associated with the risk of bronchospasm. Based on real-world experience similar to that of children with CF, the experts suggested that inhaled salbutamol followed by HS before chest physiotherapy and postural drainage can be helpful in children with non-CF bronchiectasis. Multicenter RCTs are required to evaluate the efficacy and safety of inhaled HS in children with non-CF bronchiectasis.
Evidence on the efficacy and safety of inhaled mannitol in adolescents and adults with non-CF bronchiectasis is available (low CoE). However, inhaled mannitol may not be readily available in India, and data on children are required.
There are no pediatric studies that assessed the efficacy and safety of rhDNase in non-CF bronchiectasis. The data in adults show that it worsens lung function (moderate CoE). Thus, the experts agreed that, currently rhDNase should not be used in children with non-CF bronchiectasis.
Ater et al. studied the effectiveness of 5% HS on acute wheezing (Table 4A) in children after salbutamol inhalation relative to NS (35). Inhaled HS substantially shortened the stay and admission rate (35). In children with acute viral wheeze, HS/salbutamol significantly reduced hospital stay and oxygen therapy time, and improved asthma clinical severity score quicker than NS/salbutamol (36).
HS alone has never been used to treat children with acute wheezing as it can provoke bronchospasm.
HS (5% and 3%), when used along with salbutamol, has been shown in two studies by Ater D et al. (35) and Kanjanapradap T et al. (36) to have a positive effect (shorter length of hospital stay) in preschool wheeze relative to NS. However, the experts unanimously agreed that the current state of evidence is inadequate to recommend the routine use of HS in clinical practice in children with acute wheezing. (COR: III, disagreement, low CoE).
Asthma is an inflammatory chronic airway disease characterized by bronchial hyperresponsiveness and airflow obstruction. Wheezing, mucus hypersecretion, and mucus plugging are reported in patients with asthma, especially during exacerbations (37). Short-acting beta2-agonist bronchodilators, such as salbutamol and systemic corticosteroids, are usually advised for asthma exacerbations (37). It has also been seen that salbutamol produced a greater bronchodilator response (FEV1 and maximum mid-expiratory flow) when inhaled with 3% HS vs. NS in asthmatic children with mild or moderate bronchial obstruction (Table 4B) (37).
HS alone has never been used to treat children with asthma as it can provoke bronchospasm.
The current state of evidence is inadequate to recommend the routine use of HS in clinical practice for asthma. There is a need for well-designed multicenter RCTs to assess the role of HS in children with asthma. (COR: III, disagreement, very low CoE).
Primary ciliary dyskinesia is a rare disorder characterized by motile ciliary dysfunction. This leads to an array of clinical manifestations, including neonatal respiratory distress (in term infants), persistent wet cough, rhinitis without remission, chronic sinusitis (in childhood), and bronchiectasis (in adolescence) (38). Currently, there is a lack of RCTs that have assessed the effectiveness of mucoactive drugs in children with PCD. Few case studies have highlighted the use of inhaled HS and rhDNase in the management of PCD in children (39, 40). The European Respiratory Society (ERS) consensus statement suggests: (i) inhaled NS or HS to increase mucus clearance (low-quality evidence, weak recommendation); or (ii) inhaled rhDNase in selected patients with PCD (low-quality evidence, weak recommendation) (38). Pediatric PCD patients require mucus hypersecretion management when they develop bronchiectasis, also known as non-CF bronchiectasis. Table 5 lists clinical studies of inhaled mucoactive drugs in the management of PCD.
There is a lack of RCTs that have assessed the effectiveness of inhaled HS in children with PCD. Adult studies of inhaled HS or NS on non-CF bronchiectasis show beneficial effects. Thus, the evidence on adults can be extrapolated to pediatrics. In line with the ERS consensus statement, the experts agreed that the use of inhaled NS or HS should possibly be considered to increase mucus clearance in patients with PCD (COR: II, conditional agreement) (38).
The use of NAC is not recommended in children with PCD (COR: III, disagreement, very low CoE).
Ventilator-associated pneumonia (VAP) is a serious complication related to mechanical ventilation in the neonatal period in pediatric intensive care units. Ezzeldin et al. found a significant reduction in the incidence density of VAP (Table 6) with a 3% HS group as an adjunct to VAP prevention protocol in intubated and mechanically ventilated premature infants (46).. In mechanically ventilated children after cardiac surgery, inhaled rhDNase resulted in a reduction in the duration of ventilator support by approximately 1 day and lowered the incidence of atelectasis vs. NS (47).
Inhaled HS (3%) has been shown to reduce the incidence density of VAP in intubated and mechanically ventilated premature infants. However, further research is needed in children (COR: III, disagreement, very low CoE).
Dornase alfa has been shown to reduce the length of stay and duration of ventilation in intubated and mechanically ventilated infants. However, further research is needed in children (COR: III, disagreement, very low CoE). It is also not available in India.
In adults, NAC has not been shown to be more effective than NS (COR: III, disagreement, very low CoE). Further data are needed on the pediatric population.
Tracheomalacia and tracheobronchomalacia have been increasingly recognized in children in recent years. Depending on the site and severity of the lesion, clinical presentation includes early onset stridor or fixed wheeze, recurrent infections, and cough (51). Isotonic saline or HS can aid in mucus clearance (52). Boogaard et al. found that in children with airway Tracheobronchomalacia and lower respiratory tract infections (Table 7A), the use of inhaled rhDNase did not enhance recovery from respiratory symptoms markedly cough, dyspnea, and difficulty in sputum expectoration (56).
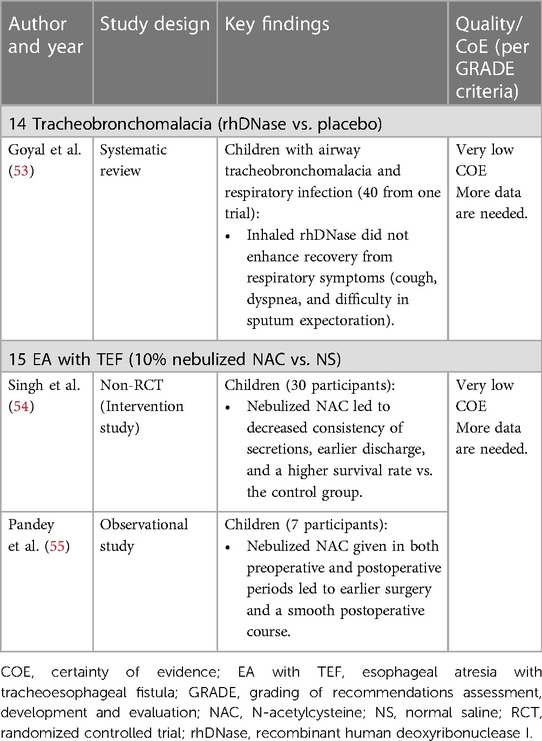
Table 7. Clinical studies of inhaled mucoactive drugs in children with tracheobronchomalacia and EA with TEF.
There is limited evidence regarding the role of inhaled mucoactive drugs in patients with tracheomalacia and tracheobronchomalacia. Further research evaluating the efficacy and safety of inhaled mucoactive drugs in tracheomalacia and tracheobronchomalacia is needed in pediatric patients. (COR: III, disagreement, very low CoE).
Congenital esophageal atresia (EA with tracheoesophageal fistula (TEF is a rare condition that occurs in 1 per 3,000 live births. Recurrent pneumonia, wheezing, and persistent cough are noted in these children (57). Inhaled NAC (Table 7B) has shown promising results in liquefying the airway secretions in EA with TEF and patients were discharged earlier than when treated with NS (54).
There is limited evidence regarding the role of inhaled NAC in patients with EA with TEF. More prospective RCTs are required to make strong recommendations. (COR: III, disagreement, very low CoE).
Acute bronchiolitis is a common cause of hospitalization and morbidity in infancy (58). The mainstay of therapy for acute bronchiolitis includes airway support, gentle nasal suctioning, fluid administration, and adequate nutrition (58, 59). Evidence (Table 8) suggests that inhaled HS shortened the length of hospital stay and improved the clinical severity score (in the first 3 days) in children with acute bronchiolitis (58, 63–65). Furthermore, treatment with inhaled HS may also substantially reduce the risk of hospitalization among outpatients and emergency department patients (58). Only one study assessed the effectiveness of inhaled NAC solution in children with acute bronchiolitis. In children with acute viral bronchiolitis, inhaled NAC in NS displayed an improvement in clinical severity score and resulted in early discharge from the hospital in children relative to salbutamol (62).
Inhaled HS therapy offers benefits in terms of reduced rate of hospitalization and readmission rates in infants and children with acute bronchiolitis. The experts suggested that inhaled salbutamol followed by HS (3%; every 6–8 h until discharge) may be considered in children with acute bronchiolitis (COR: II, conditional agreement, low CoE). The experts suggested that in certain phenotypes of bronchiolitis (history of atopy or wheezing), salbutamol may be considered.
Inhaled NAC is not studied well in acute bronchiolitis and is not commonly used in children with acute bronchiolitis. Multicenter RCTs are required to evaluate the efficacy and safety of inhaled NAC in children with acute bronchiolitis (very low CoE).
Suri R et al. assessed the effectiveness (Table 9) of sputum induction (SI) with 7% inhaled HS on airway inflammation in children with CF (67). Sputum induction was found to be safe with no evidence of a proinflammatory effect. Furthermore, SI helped in the identification of organisms in culture-negative symptomatic children, circumventing the need for bronchoscopy (67). Ferreira et al. found that SI capacity was significantly increased in children with CF after 7% HS inhalation (68). Pathogen yield from SI was shown to be superior to cough swabs, and the technique can be used as a substitute for bronchoalveolar lavage in children with CF (69). Ultrasonic nebulizers are more successful in inducing sputum relative to jet nebulizers, and pretreatment with salbutamol can inhibit bronchoconstriction induced by HS inhalation (70). The usefulness of SI has also been studied in adult patients with pulmonary tuberculosis with 7% HS for improving bacteriological yield (71). The use of induced sputum samples was more sensitive than gastric washing specimens for the diagnosis of tuberculosis in patients who could not expectorate spontaneously (72).
Nebulization with HS (7%) may be considered to facilitate sputum expectoration even in patients who usually do not expectorate (COR: II; conditional agreement, very low CoE). This method has been applied in patients with CF to enhance mucus clearance, for the identification of infectious agents, and for cytological examination in inflammatory airway disorders. It may avoid invasive interventions, such as bronchoscopy, to obtain better samples for pathogen detection (69).
A comparison of the efficacy and safety data of various strengths of HS for SI is required.
Currently, there are no country-specific guidelines/recommendations for the treatment of pediatric respiratory diseases with inhaled mucoactive drugs from an Indian perspective. Studies have shown that Indian children differ in the etiology and clinical presentation of certain pediatric respiratory conditions (e.g., CF, non-CF bronchiectasis) compared to Western populations (73–75). Furthermore, due to a lack of well-designed RCTs in this field of study in India, medical practitioners rely on data from the Western world. To the best of our knowledge, this is the first practical consensus document to assist pediatricians in clinical decision-making when selecting an appropriate mucoactive medication for the management of certain respiratory illnesses based on the most recent available information. Experts recommended inhaled mucoactive drugs (HS, mannitol, and dornase alfa) in children with CF. Inhaled HS was conditionally recommended for CF, acute bronchiolitis, and PCD. Experts agreed that inhaled HS before chest physiotherapy may be helpful in children with non-CF bronchiectasis, although more research into the pediatric population is required. The current state of evidence is inadequate to support the use of mucoactive drugs in asthma, wheezing, tracheomalacia tracheobronchomalacia, and EA with TEF. Currently, dornase alfa and mannitol dry powder are not available for use in India. Dornase alfa therapy is expensive, but the drug can be imported and is certainly useful in patients who can afford it. An alternative lower-cost therapy is inhaled HS, which has shown benefits in CF, non-CF bronchiectasis, PCD, and acute bronchiolitis. Currently, 3% and 7% HS concentrations of HS are accessible in India.
The panelists were chosen from across India based on their level of clinical expertise, academic distinctions, and involvement in relevant clinical research. The expert committee was formed without any bias in terms of selection.
The patient's voice was not included in the consensus process.
In this article, we have summarized clinical consensus statements/recommendations on the role of inhaled mucoactive drugs in pediatric respiratory disease conditions from an Indian perspective. Children with CF would benefit from the initiation of HS inhalation (as an adjunct to ACT) from the time of diagnosis. Clinical evidence supports the benefits of inhalation of rhDNase and mannitol dry powder in patients with CF; however, these drugs are currently not available in India. Experts suggested that inhaled salbutamol followed by HS in non-CF bronchiectasis and acute bronchiolitis may be beneficial. Inhaled salbutamol followed by inhaled HS can increase mucus clearance in children with PCD with underlying bronchiectasis and persistent weight cough similar to other non-CF bronchiectasis. Dornase alfa has been shown to reduce the length of stay and duration of ventilation in intubated and mechanically ventilated infants; however, more data are needed in this regard. The current state of evidence is inadequate to support the use of mucoactive drugs in asthma, wheezing, tracheomalacia, tracheobronchomalacia, and EA with TEF. Further, prospective RCTs are required to make a strong recommendation. Lastly, population-based studies are required to validate the effectiveness of inhaled mucoactive drugs in Indian children with specific respiratory conditions where mucus hypersecretion is a clinical challenge.
MS: Conceptualization, Data curation, Writing – review & editing, Visualization. SV: Data curation, Visualization, Writing – review & editing. IK: Data curation, Visualization, Writing – review & editing. RD: Conceptualization, Data curation, Visualization, Writing – review & editing. JG: Data curation, Visualization, Writing – review & editing. MB: Data curation, Visualization, Writing – review & editing. RD: Data curation, Visualization, Writing – review & editing. PK: Data curation, Visualization, Writing – review & editing. JG: Data curation, Visualization, Writing – review & editing. IK: Data curation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors acknowledge the role of ICMR Advanced Centre for Research in Evidence-based Child Health, PGIMER, Chandigarh. We would like to thank BioQuest Solutions for their editorial support. BioQuest Solutions received the unrestricted educational grant and logistical support from Cipla Ltd. for the consensus meeting.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1322360/full#supplementary-material
1. Linssen RSN, Ma J, Bem RA, Rubin BK. Rational use of mucoactive medications to treat pediatric airway disease. Paediatr Respir Rev. (2020) 36:8–14. doi: 10.1016/j.prrv.2020.06.007
2. Shen Y, Huang S, Kang J, Lin J, Lai K, Sun Y, et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int J Chron Obstruct Pulmon Dis. (2018) 13:399–407. doi: 10.2147/COPD.S144312
3. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2019) 41(3):407–77. Published correction appears in Eur Heart J. 2020 November 21;41(44):4242. doi: 10.1093/eurheartj/ehz425
4. Schünemann H, Broek J, Guyatt G, Oxman AD, Grade Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available at: https://gdt.gradepro.org/app/handbook/handbook.html (Cited Jul 17 2023).
5. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Br Med J. (2004) 328(7454):1490. doi: 10.1136/bmj.328.7454.1490
6. Chen Q, Shen Y, Zheng J. A review of cystic fibrosis: basic and clinical aspects. Anim Models Exp Med. (2021) 4(3):220–32. doi: 10.1002/ame2.12180
7. Wark P, McDonald VM, Smith S. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. (2023) 6(6):CD001506. doi: 10.1002/14651858.CD001506.pub5
8. Ratjen F, Davis SD, Stanojevic S, Kronmal RA, Hinckley Stukovsky KD, Jorgensen N, et al. Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2019) 7(9):802–9. doi: 10.1016/S2213-2600(19)30187-0
9. Tiddens HAWM, Chen Y, Andrinopoulou ER, Davis SD, Rosenfeld M, Ratjen F, et al. The effect of inhaled hypertonic saline on lung structure in children aged 3-6 years with cystic fibrosis (SHIP-CT): a multicentre, randomised, double-blind, controlled trial. Lancet Respir Med. (2022) 10(7):669–78. doi: 10.1016/S2213-2600(21)00546-4
10. Elkins M, Dentice R. Timing of hypertonic saline inhalation for cystic fibrosis. Cochrane Database Syst Rev. (2020) 2(2):CD008816. doi: 10.1002/14651858.CD008816.pub4
11. Nevitt SJ, Thornton J, Murray CS, Dwyer T. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev. (2020) 5(5):CD008649. doi: 10.1002/14651858.CD008649.pub4
12. Sadr S, Kiani M, Rezaei M, Khanbabaee G, Tabatabaee SA, Hosseini A. The efficacy of nebulized soluble mannitol and comparison with 5% hypertonic saline on pulmonary function of children with cystic fibrosis. J Compr Pediatr. (2019) 10(3):e85616. doi: 10.5812/compreped.85616
13. Yang C, Montgomery M. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. (2021) 3(3):CD001127. doi: 10.1002/14651858.CD001127.pub5
14. Duijvestijn YC, Brand PL. Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatr. (1999) 88(1):38–41. doi: 10.1111/j.1651-2227.1999.tb01265.x
15. Donaldson SH, Danielle Samulski T, LaFave C, Zeman K, Wu J, Trimble A, et al. A four-week trial of hypertonic saline in children with mild cystic fibrosis lung disease: effect on mucociliary clearance and clinical outcomes. J Cyst Fibros. (2020) 19(6):942–8. doi: 10.1016/j.jcf.2020.07.009
16. Stahl M, Wielpütz MO, Ricklefs I, Dopfer C, Barth S, Schlegtendal A, et al. Preventive inhalation of hypertonic saline in infants with cystic fibrosis (PRESIS). A randomized, double-blind, controlled study. Am J Respir Crit Care Med. (2019) 199(10):1238–48. doi: 10.1164/rccm.201807-1203OC
17. Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. (2006) 354(3):229–40. doi: 10.1056/NEJMoa043900
18. De Boeck K, Haarman E, Hull J, Lands LC, Moeller A, Munck A, et al. Inhaled dry powder mannitol in children with cystic fibrosis: a randomised efficacy and safety trial. J Cyst Fibros. (2017) 16(3):380–7. doi: 10.1016/j.jcf.2017.02.003
19. Aitken ML, Bellon G, De Boeck K, Flume PA, Fox HG, Geller DE, et al. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med. (2012) 185(6):645–52. doi: 10.1164/rccm.201109-1666OC
20. Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The pulmozyme study group. N Engl J Med. (1994) 331(10):637–42. doi: 10.1056/NEJM199409083311003
21. Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. (2001) 139(6):813–20. doi: 10.1067/mpd.2001.118570
22. Serisier DJ, Shute JK, Hockey PM, Higgins B, Conway J, Carroll MP. Inhaled heparin in cystic fibrosis. Eur Respir J. (2006) 27(2):354–8. doi: 10.1183/09031936.06.00069005
23. Máiz Carro L, Martínez-García MA. Nebulized hypertonic saline in noncystic fibrosis bronchiectasis: a comprehensive review. Ther Adv Respir Dis. (2019) 13(13):1753466619866102. doi: 10.1177/1753466619866102
24. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of cochrane systematic reviews. Cochrane Database Syst Rev. (2015) 2015(7):CD010337. doi: 10.1002/14651858.CD010337.pub2
25. Tarrant BJ, Le Maitre C, Romero L, Steward R, Button BM, Thompson BR, et al. Mucoactive agents for chronic, non-cystic fibrosis lung disease: a systematic review and meta-analysis. Respirology. (2017) 22(6):1084–92. doi: 10.1111/resp.13047
26. British Thoracic Society bronchiectasis guideline group-guideline for non-CF bronchiectasis in adults. (2019). Available at: https://www.brit-thoracic.org.uk/document-library/guidelines/bronchiectasis/bts-guideline-for-non-cf-bronchiectasis/
27. Anuradha KWDA, Gunathilaka PKG, Wickramasinghe VP. Effectiveness of hypertonic saline nebulization in airway clearance in children with non-cystic fibrosis bronchiectasis: a randomized control trial. Pediatr Pulmonol. (2021) 56(2):509–15. doi: 10.1002/ppul.25206
28. Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir Med. (2005) 99(1):27–31. doi: 10.1016/j.rmed.2004.05.006
29. Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med. (2011) 105(12):1831–5. doi: 10.1016/j.rmed.2011.07.019
30. Nicolson CH, Stirling RG, Borg BM, Button BM, Wilson JW, Holland AE. The long-term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med. (2012) 106(5):661–7. doi: 10.1016/j.rmed.2011.12.021
31. Bilton D, Daviskas E, Anderson SD, Kolbe J, King G, Stirling RG, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest. (2013) 144(1):215–25. doi: 10.1378/chest.12-1763
32. Bilton D, Tino G, Barker AF, Chambers DC, De Soyza A, Dupont LJ, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax. (2014) 69(12):1073–9. doi: 10.1136/thoraxjnl-2014-205587
33. Wills PJ, Wodehouse T, Corkery K, Mallon K, Wilson R, Cole PJ. Short-term recombinant human DNase in bronchiectasis. Effect on clinical state and in vitro sputum transportability. Am J Respir Crit Care Med. (1996) 154(2 Pt 1):413–7. doi: 10.1164/ajrccm.154.2.8756815
34. O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. RhDNase study group. Chest. (1998) 113(5):1329–34. doi: 10.1378/chest.113.5.1329
35. Ater D, Shai H, Bar BE, Fireman N, Tasher D, Dalal I, et al. Hypertonic saline and acute wheezing in preschool children. Pediatrics. (2012) 129(6):e1397–403. doi: 10.1542/peds.2011-3376
36. Kanjanapradap T, Deerojanawong J, Sritippayawan S, Prapphal N. Does nebulized hypertonic saline shorten hospitalization in young children with acute viral wheezing? Pediatr Pulmonol. (2018) 53(2):138–44. doi: 10.1002/ppul.23924
37. Teper A, Kofman C, Alchundia Moreira J, Köhler T, García Bournissen F. Bronchodilator response to albuterol nebulized with hypertonic saline in asthmatic children. Pediatr Pulmonol. (2021) 56(12):3714–9. doi: 10.1002/ppul.25653
38. Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. (2009) 34(6):1264–76. doi: 10.1183/09031936.00176608
39. Kumar A, Walker WT. Management of a child with primary ciliary dyskinesia. Oxf Med Case Rep. (2020) 2020(2):omz135. doi: 10.1093/omcr/omz135
40. El-Abiad NM, Clifton S, Nasr SZ. Long-term use of nebulized human recombinant DNase1 in two siblings with primary ciliary dyskinesia. Respir Med. (2007) 101(10):2224–6. doi: 10.1016/j.rmed.2007.05.014
41. Paff T, Daniels JM, Weersink EJ, Lutter R, Vonk Noordegraaf A, Haarman EG. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur Respir J. (2017) 49(2):1601770. doi: 10.1183/13993003.01770-2016
42. Kaspy K, Alarie N, Vallee-Smedja S, Adam J, Shapiro DF. Initiation of nebulized hypertonic saline in infants with primary ciliary dyskinesia. Am J Respir Crit Care Med. (2020) et al;201:A7768. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A7768.
43. Stafanger G, Garne S, Howitz P, Morkassel E, Koch C. The clinical effect and the effect on the ciliary motility of oral N-acetylcysteine in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J. (1988) 1(2):161–7. doi: 10.1183/09031936.93.01020161
44. Anand R, McAuley DF, Blackwood B, Yap C, ONeill B, Connolly B, et al. Mucoactive agents for acute respiratory failure in the critically ill: a systematic review and meta-analysis. Thorax. (2020) 75(8):623–31. doi: 10.1136/thoraxjnl-2019-214355
45. Shein SL, Gallagher JT, Deakins KM, Weinert DM. Prophylactic use of nebulized hypertonic saline in mechanically ventilated children: a randomized blinded pilot study. Respir Care. (2016) 61(5):586–92. doi: 10.4187/respcare.04418
46. Ezzeldin Z, Mansi Y, Gaber M, Zakaria R, Fawzy R, Mohamed MA. Nebulized hypertonic saline to prevent ventilator associated pneumonia in premature infants, a randomized trial. J Matern Fetal Neonatal Med. (2018) 31(22):2947–52. doi: 10.1080/14767058.2017.1359826
47. Riethmueller J, Borth-Bruhns T, Kumpf M, Vonthein R, Wiskirchen J, Stern M, et al. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children. Pediatr Pulmonol. (2006) 41(1):61–6. Published correction appears in Pediatr Pulmonol. 2006;41(4):388.doi: 10.1002/ppul.20298
48. Youness HA, Mathews K, Elya MK, Kinasewitz GT, Keddissi JI. Dornase alpha compared to hypertonic saline for lung atelectasis in critically ill patients. J Aerosol Med Pulm Drug Deliv. (2012) 25(6):342–8. doi: 10.1089/jamp.2011.0954
49. Zitter JN, Maldjian P, Brimacombe M, Fennelly KP. Inhaled dornase alfa (Pulmozyme) as a noninvasive treatment of atelectasis in mechanically ventilated patients. J Crit Care. (2013) 28(2):218.e1–e7. doi: 10.1016/j.jcrc.2012.09.015
50. Masoompour SM, Anushiravani A, Tafaroj Norouz A. Evaluation of the effect of nebulized N-acetylcysteine on respiratory secretions in mechanically ventilated patients: randomized clinical trial. Iran J Med Sci. (2015) 40(4):309–15.26170516
51. Wallis C, Alexopoulou E, Antón-Pacheco JL, Bhatt JM, Bush A, Chang AB, et al. ERS Statement on tracheomalacia and bronchomalacia in children. Eur Respir J. (2019) 54(3):1900382. doi: 10.1183/13993003.00382-2019
52. Kamran A, Jennings RW. Tracheomalacia and tracheobronchomalacia in pediatrics: an overview of evaluation, medical management, and surgical treatment. Front Pediatr. (2019) 7:512. doi: 10.3389/fped.2019.00512
53. Goyal V, Masters IB, Chang AB. Interventions for primary (intrinsic) tracheomalacia in children. Cochrane Database Syst Rev. (2012) 10:CD005304. doi: 10.1002/14651858.CD005304.pub3
54. Singh G, Pandey A, Shandilya G, Gupta A, Rawat JD, Wakhlu A, et al. Evaluation of nebulized N-acetyl cysteine in outcome of esophageal atresia with tracheoesophegeal fistula. J Pediatr Surg. (2020) 55(12):2635–9. doi: 10.1016/j.jpedsurg.2020.04.013
55. Pandey A, Gangopadhyay AN, Sharma SP, Kumar V. Esophageal atresia with tracheo-esophageal fistula: role of nebulized N-acetylcysteine in the outcome. J Indian Assoc Pediatr Surg. (2009) 14(4):232. doi: 10.4103/0971-9261.59612
56. Boogaard R, de Jongste JC, Vaessen-Verberne AA, Hop WC, Merkus PJ. Recombinant human DNase in children with airway malacia and lower respiratory tract infection. Pediatr Pulmonol. (2009) 44(10):962–9. doi: 10.1002/ppul.21073
57. Porcaro F, Valfré L, Aufiero LR, Dall’Oglio L, De Angelis P, Villani A, et al. Respiratory problems in children with esophageal atresia and tracheoesophageal fistula. Ital J Pediatr. (2017) 43(1):77. doi: 10.1186/s13052-017-0396-2
58. Zhang L, Mendoza-Sassi RA, Wainwright C, Aregbesola A, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. (2017) 12:CD006458. doi: 10.1002/14651858.CD006458.pub4
59. Joshi K, Parihar AS, Singh J. Effect of nasal suction on reliving feeding difficulty in children affected with bronchiolitis. Int J Contemp Pediatr. (2020) 7(1):168–72. doi: 10.18203/2349-3291.ijcp20195748
60. Zhang L, Mendoza-Sassi RA, Wainwright CE, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. (2023) 4(4):CD006458. doi: 10.1002/14651858.CD006458.pub5
61. Enriquez A, Chu IW, Mellis C, Lin WY. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database Syst Rev. (2012) 11(11):CD008395. doi: 10.1002/14651858.CD008395.pub2
62. Naz F, Raza AB, Ijaz I, Kazi MY. Effectiveness of nebulized N-acetylcysteine solution in children with acute bronchiolitis. J Coll Physicians Surg Pak. (2014) 24(6):408–11.24953914
63. Zhang L, Gunther CB, Franco OS, Klassen TP. Impact of hypertonic saline on hospitalization rate in infants with acute bronchiolitis: a meta-analysis. Pediatr Pulmonol. (2018) 53(8):1089–95. doi: 10.1002/ppul.24066
64. Islam MS, Mollah MAH, Khanam R, Chowdhury AS, Rahman MM, Al Baqui SAA, et al. Comparative efficacy of nebulized 7% hypertonic saline versus 0.9% normal saline with salbutamol in children with acute bronchiolitis. Bangladesh J Child Health. (2019) 43(2):80–4. doi: 10.3329/bjch.v43i2.42550
65. Yu JF, Zhang Y, Liu ZB, Wang J, Bai LP. 3% Nebulized hypertonic saline versus normal saline for infants with acute bronchiolitis: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltim). (2022) 101(43):e31270. doi: 10.1097/MD.0000000000031270
66. Gibson PG, Grootendor DC, Henry RL, Pin I, Rytila PH, Wark P, et al. Sputum induction in children. Eur Respir J Suppl. (2002) 37:44s–6s. doi: 10.1183/09031936.02.00004402
67. Suri R, Marshall LJ, Wallis C, Metcalfe C, Shute JK, Bush A. Safety and use of sputum induction in children with cystic fibrosis. Pediatr Pulmonol. (2003) 35(4):309–13. doi: 10.1002/ppul.10226
68. Ferreira ACM, Marson FAL, Cohen MA, Bertuzzo CS, Levy CE, Ribeiro AF, et al. Hypertonic saline as a useful tool for sputum induction and pathogen detection in cystic fibrosis. Lung. (2017) 195(4):431–9. doi: 10.1007/s00408-017-0008-3
69. Ronchetti K, Tame JD, Paisey C, Thia LP, Doull I, Howe R, et al. The CF-sputum induction trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respir Med. (2018) 6(6):461–71. doi: 10.1016/S2213-2600(18)30171-1
70. Popov TA, Pizzichini MM, Pizzichini E, Kolendowicz R, Punthakee Z, Dolovich J, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J. (1995) 8(4):559–65. doi: 10.1183/09031936.95.08040559
71. Seong GM, Lee J, Lee JH, Kim JH, Kim M. Usefulness of sputum induction with hypertonic saline in a real clinical practice for bacteriological yields of active pulmonary tuberculosis. Tuberc Respir Dis (Seoul). (2014) 76(4):163–8. doi: 10.4046/trd.2014.76.4.163
72. Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis. (2007) 44(11):1415–20. doi: 10.1086/516782
73. Prasad R, Sharma H, Kaur G. Molecular basis of cystic fibrosis disease: an Indian perspective. Indian J Clin Biochem. (2010) 25(4):335–41. doi: 10.1007/s12291-010-0091-1
74. Kumar A, Lodha R, Kumar P, Kabra SK. Non-cystic fibrosis bronchiectasis in children: clinical profile, etiology and outcome. Indian Pediatr. (2015) 52(1):35–7. doi: 10.1007/s13312-015-0563-8
Keywords: mucoactive, inhaled, pediatric, respiratory disorders, consensus, India
Citation: Singh M, Varkki S, Kinimi I, Das RR, Goyal JP, Bhat M, Dayal R, Kalyan P, Gairolla J and Khosla I (2023) Expert group recommendation on inhaled mucoactive drugs in pediatric respiratory diseases: an Indian perspective. Front. Pediatr. 11:1322360. doi: 10.3389/fped.2023.1322360
Received: 16 October 2023; Accepted: 20 November 2023;
Published: 4 December 2023.
Edited by:
Bülent Taner Karadağ, Marmara University, TürkiyeReviewed by:
Predrag B. Minić, University of Belgrade, Serbia© 2023 Singh, Varkki, Kinimi, Das, Goyal, Bhat, Dayal, Kalyan, Gairolla and Khosla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meenu Singh bWVlbnVzaW5naDRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.