- 1Department of Pediatrics, School of Medicine, Wakayama Medical University, Wakayama, Japan
- 2Department of Pediatrics, Graduate School of Medicine, Chiba University, Chiba, Japan
- 3Clinical Research Centre, Chiba University Hospital, Chiba, Japan
- 4Department of Public Health, Graduate School of Medicine, Chiba University, Chiba, Japan
- 5Department of Health Research, Chiba Foundation for Health Promotion and Disease Prevention, Chiba, Japan
- 6Division of Pediatrics, Tsukushi Medical and Welfare Center, Iwade, Japan
Background: To investigate risk factors for coronary arterial abnormalities (CAAs) and resistance to treatment in patients with Kawasaki disease (KD) receiving intravenous immunoglobulin (IVIG) plus ciclosporin A (CsA) as the first-line treatment, we performed a subanalysis of baseline data of participants in the KAICA trial, a phase 3, randomized study (JMA-ILA00174).
Methods: All data of the patients enrolled in the KAICA trial, who had a Gunma score ≥5 at diagnosis and had been randomly assigned to either IVIG (2 g/kg/24 h) plus CsA (5 mg/kg/day for 5 days) (n = 86) or IVIG alone (n = 87), were subjected to this study. CAA was defined by a Z score ≥2.5 observed within 4 weeks after treatment initiation. Baseline data including genotypes of KD susceptibility genes were compared between subgroups of patients for CAA or treatment response for each treatment group. Backword-forward stepwise logistic regression analyses were performed.
Results: Pre-Z-max, defined as the maximum among Z scores on four coronary artery branches before treatment, was higher in patients with CAA in both treatment groups and was associated with CAA in IVIG plus CsA treatment group [odds ratio (OR) = 17.0]. High serum total bilirubin level was relevant to treatment resistance only in the IVIG plus CsA group (OR = 2.34).
Conclusions: Coronary artery enlargement before treatment is a major determinant of CAA even in KD patients treated with initial IVIG treatment intensified by addition of CsA. Baseline serum total bilirubin level was a risk factor associated with resistance to IVIG plus CsA.
1. Introduction
Kawasaki disease (KD) is an acute systemic vasculitis of childhood that can cause dilation or aneurysmal change in the coronary arteries in 20%–25% of untreated patients (1). Coronary artery abnormalities (CAAs) in KD are now the leading cause of acquired heart diseases in developed countries (2). The aim of the acute phase treatment of KD is to prevent CAAs (3). Proven effective in the 1980s for the early resolution of inflammation and prevention of CAA development, the combination of intravenous immunoglobulin (IVIG) and oral aspirin is now the standard first-line treatment given to most patients with KD. However, resistance to this treatment is seen in around 20% of KD patients and is recognized as one of the major risk factors for CAAs.
CAAs that cannot be prevented by adequate administration of the first-line treatment have become a major clinical issue (3, 4). Recently, in an attempt to develop intensified treatment regimens that can more efficiently prevent CAAs, a series of prospective randomized clinical trials of drugs administered as adjuncts to the standard IVIG treatment has been carried out (5–7). Ciclosporin A (CsA), an inhibitor of the calcineurin-NFAT pathway, is one such drug. It was shown in our previous clinical trial (the KAICA trial, a controlled, phase 3, randomised, open-label, blinded-endpoints trial) to reduce the incidence of CAAs when administered to KD patients with high predictive scores for IVIG unresponsiveness (Gunma score) in combination with the standard IVIG therapy (5, 8–10). Thus, there are now four options that can be combined with the standard IVIG therapy for treating severe KD patients in the Japanese treatment guidelines, two that are recommended (prednisolone and CsA) and two that can be considered (infliximab and ulinastatin) (3, 11). The efficacy of IL-1 blocking therapies has been initiated in Europe and US (12, 13). However, none of the intensified regimens can eradicate CAAs. Although the incidence of CAAs was significantly reduced when compared with those receiving the conventional IVIG treatment (31%), 14% of the patients receiving IVIG plus CsA in the KAICA trial still had CAAs (5).
To investigate risk factors for CAAs and resistance to IVIG plus CsA as the first-line treatment in patients with KD, we performed a subanalysis of baseline data of participants in the KAICA trial.
2. Methods
2.1. Patients
This study was performed as a subanalysis of the KAICA trial (JMA-ILA00174, date on registration: April 2, 2014), which was a phase 3, randomized, open-label, blinded endpoint study (5). In this clinical study, 173 patients who were diagnosed with KD according to Japanese diagnostic guidelines and predicted to be non-responders to IVIG by Gunma score were randomly assigned 1:1 to IVIG plus CsA or IVIG alone (5, 10, 14). There were 86 patients in the study treatment group who received CsA 5 mg/kg per day orally divided into two daily doses for 5 days in addition to IVIG and aspirin and 87 patients in the conventional treatment group who received IVIG 2 g/kg for 24 h and aspirin 30 mg/kg per day. These 173 patients were enrolled in the present analysis.
2.2. Definition of CAAs
In the KAICA trial, absolute internal diameters of the right coronary artery, left main coronary artery, left anterior descending coronary artery, and left circumflex coronary artery were measured by three pediatric cardiologists using video-recorded data at six time points—once before the first-line treatment and at five time points after treatment initiation, namely, on day 3 and at week 1, week 2, week 4, and week 12—without any clinical information and centrally reviewed. In this subanalysis, we did not include the week 12 data in this study.
Coronary artery Z scores were calculated using the Z scores calculator (version 4.0 full, LMS_Z_Score) (15). A Z score of 2.5 was set as the threshold for the diagnosis of coronary artery dilation according to the 2017 American Heart Association guidelines (3). Patients with one or more Z scores ≥2.5 at any time point up to 4 weeks after treatment initiation were defined as having a CAA in this study. The maximum Z score of the four branches measured and calculated before the first-line treatment was defined as the “pre-Z-max”.
2.3. Definition of response to the first-line treatment
In both treatment groups, patients whose body temperature dropped below 37.5°C within 48 h after the initiation of the first-line treatment and who remained afebrile thereafter were defined as the responders to the first-line treatment. The other patients, including those with persistent fever beyond 48 h after the initiation of the first-line treatment or those becoming febrile again after an afebrile period during the 48 h, were defined to be resistant to the first-line treatment.
2.4. Baseline data and analysis plan
We extracted data of registered patients regarding basic characteristics from the KAICA trial database, such as height, weight, age, days of illness at diagnosis, days of illness at treatment initiation, single-nucleotide polymorphisms (SNPs) in inositol 1,4,5-trisphosphate 3-kinase C (ITPKC; rs28493229) and caspase-3 (CASP3; rs113420705), family history of KD, vital signs (blood pressure, heart rate, body temperature), baseline clinical data before any treatments (white blood cell [WBC] counts, WBC analysis [%], red blood cell [RBC] count, hemoglobin [Hgb], hematocrit [Hct], platelet count, aspartate aminotransferase [AST], alanine aminotransferase [ALT], total bilirubin [TB], total protein [TP], albumin [Alb], sodium [Na], potassium [K], magnesium [Mg], blood urea nitrogen [BUN], creatinine [Cre], C-reactive protein [CRP], and pre-Z-max).
Data were compared separately between patients with and without CAAs in the two treatment groups. Of the 87 patients in the IVIG group, 2 had no pre-Z-max data, so the analysis regarding CAAs was performed on 85 patients in the IVIG group.
We compared data between patients with and without a treatment response for 86 patients who received IVIG plus CsA. We also extracted the baseline data of 87 registered patients treated with IVIG alone and compared these data between patients with and without a treatment response.
2.5. Statistical analysis
Categorical variables are expressed as absolute numbers and percentages and were compared using Fisher's exact test, whereas continuous variables are expressed as mean and standard deviation and were compared using Student's t-test. Multivariate logistic regression analysis with backward-forward stepwise variable selection method was performed to identify the independent variables associated with CAAs or treatment resistance. All statistical analyses were performed using the SAS statistical software package (version 9.4; SAS Institute), with p-values < 0.05 indicating statistical significance.
This study is approved by Chiba University Ethical committee review board (No. 843).
3. Results
3.1. Risk factors for CAA
The baseline data of the two treatment groups in the KAICA trial are shown in Table 1. In both treatment groups, 19 patients had CAAs defined by a Z score ≥2.5, and the mean pre-Z-max was significantly higher in those with CAA (p < 0.0001). In the IVIG plus CsA group, baseline RBC, Hgb, and Hct levels were significantly lower in the patients with CAAs (p = 0.004, 0.041, and 0.028, respectively). For these hematological markers related to RBCs, a similar trend was seen in the IVIG group; however, only the difference in the Hct was significant (p = 0.045). There were no significant differences in the other demographic, physical, hematological, and biochemical data or in the frequencies of risk allele carriers of ITPKC and CASP3 SNPs between those with and without CAAs in either group. Backward-forward stepwise multivariate logistic regression analyses revealed that pre-Z-max was the only significant predicted factor in IVIG + CsA group (Table 2).
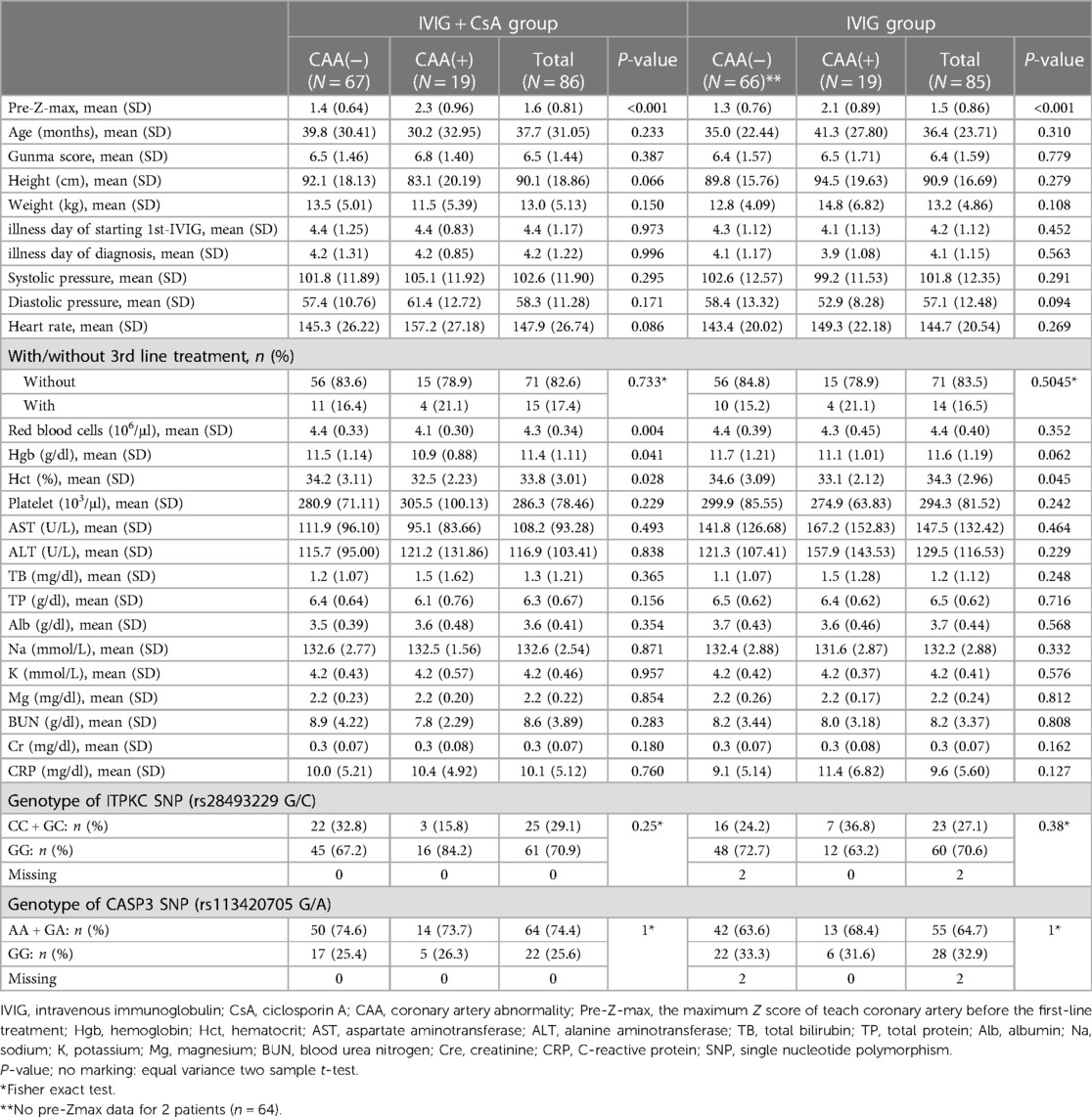
Table 1. Difference in baseline data between KD patients with or without CAA in IVIG plus CsA and IVIG groups.
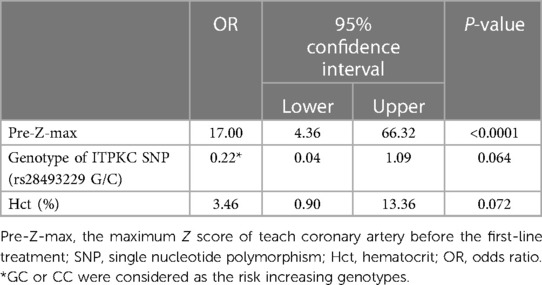
Table 2. Top three factors in a backward-forward stepwise logistic regression analysis for CAA risk in IVIG plus CsA group.
3.2. Risk factors for resistance to initial treatment
Baseline data of the patients by treatment group and by patients' response are summarized in Table 3. In the IVIG plus CsA group, 48 patients (55.8%) responded to the first-line treatment and 38 (44.2%) were resistant. The treatment-resistant patients included 15 (17.4%) with persistent fever and 23 with relapsed fever. In the IVIG group, 48 patients (55.2%) responded to the first-line treatment and 39 (44.8%) were resistant. Those resistant to IVIG included 32 with persistent fever and 7 with relapsed fever.

Table 3. Difference in baseline data of KD patients who responded or not responded to the initial treatment in IVIG plus CsA and IVIG groups.
In the IVIG plus CsA group, treatment-resistant patients had a higher ALT, higher TB, and lower Na. Meanwhile, in the IVIG group, treatment-resistant patients had a higher Neutrophil % than treatment responders (Table 3). In backward-forward stepwise multivariate logistic regression, treatment-resistant patients had a lower lymphocyte % (Ly%) and higher body temperature in both treatment groups. High serum TB levels were associated with a resistance to IVIG plus CsA, which were not associated with a response to IVIG alone (Table 4).
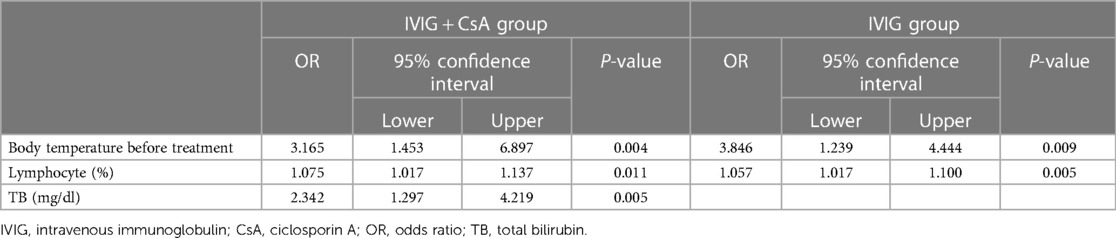
Table 4. Result of a backward-forward stepwise logistic regression analysis for resistance to the initial treatment.
4. Discussion
In this study, we investigated the baseline clinical characteristics associated with CAAs in KD patients treated with IVIG plus CsA. The risk factor for CAA complications was the pre-Z-max, and this factor was a strong predictor of CAAs both in the IVIG plus CsA group and in the IVIG alone group. Several reports have linked pre-Z-max to the development of CAAs (16–19). In the Post-RAISE study, which was done to evaluate the effectiveness of a combination treatment consistent of IVIG and predonisolone on reduction of CAAs, the baseline risk factors for CAA were examined in patients with a Gunma score of either ≥5 or <5. The authors determined that the risk factors for CAAs in both groups were a pre-Z-max ≥2.5, age younger than 12 months, and non-responsiveness to initial treatment, although the rankings of these three risk factors differed between previous studies (20, 21). The coronary artery findings in the early phase of KD should provide vital information for predicting coronary artery outcomes and our data re-confirm this issue.
Our univariate analysis suggested that anemia might be one of the highly important risk factors for CAAs. Baseline Hct, Hgb, and number of RBCs in the IVIG plus CsA group were shown as risk factors. Anemia has been linked to the development of CAAs (22–24). In addition, Kim et al. reported that ferritin was strongly associated with CAAs (25). Another cause of anemia might be inflammation in KD. Ferritin and haptoglobin are acute phase reactant proteins and their elevation is related to the acute phase response.
The incidence of CAAs in the IVIG plus CsA group showed a nonsignificant tendency to be lower in patients with the risk allele (C) of ITPKC than in those without this risk allele (odds ratio 0.22, 95% confidence interval 0.04–1.09, p = 0.064) (Table 2). Onouchi et al. has reported that patients with KD susceptibility alleles of ITPKC and/or CASP3 were more prone to be IVIG resistance and to develop CAAs (9). While genetic studies affirmed the C allele as a risk variant for CAA, the findings in the current study didn't make conclusion about using the C allele to decide who should receive CsA. This study has inadequet power for this purpose and we need further study to evaluate this issue.
Treatment resistance in the IVIG plus CsA group is 44.2%, which was similar as that in the IVIG alone group (44.8%). This fact is unlikely to motivate physicians to select IVIG plus CsA treatment for high-risk KD. In this study, we could not find positive factors for choosing the IVIG plus CsA treatment compared to the IVIG alone. Resistance to first-line treatment, both in IVIG plus CsA group and IVIG alone group, revealed that body temperature before treatment and percent of lymphocytes were significantly related to resistance. Body temperature before treatment is a simple but important parameter that can be readily obtained worldwide. In a previous study, body temperature before treatment has been reported to be a good predictor of IVIG resistance (26).
Serum TB level was an independent predictor of first-line therapy resistance in the IVIG plus CsA group but not in the IVIG alone group. Because CsA is a fat-soluble drug, the absorption of CsA from the gastrointestinal tract may decrease in patients with cholestasis. However, the trough levels of CsA showed no significant differences between patients with and without a response to IVIG plus CsA (data not shown). Baseline TB levels have also been reported to be a risk factor for resistance to intensified IVIG plus prednisolone treatment (20). Although not significant, patients who were resistant to the standard IVIG treatment tended to have higher TB than those responded to the treatment (Table 3). Thus, we speculate that high TB may reflect the predominance of factors that are difficult to resolve with the addition of CsA or prednisolone in the complex and heterogeneous pathogenesis of refractory KD, and that the relative contribution of these factors may have been accentuated by the administration of CsA or prednisolone.
It is vital to select the most appropriate first-line treatment for KD patients to prevent CAAs. However, we could not confirm any significant independent factor for selecting between IVIG plus CsA and IVIG alone in this study. Since this study is a sub-analysis, we know that there is a problem with the statistical power, which is a limitation of this study. In addition, the research was conducted only on Japanese subjects, and the data cannot be used universally.
In conclusion, Pre-Z-max of coronary arteries was a risk factor significantly associated with CAAs in patients who are high risk for IVIG resistance and received IVIG plus CsA treatment. Baseline serum total bilirubin level was a risk factor associated with resistance to IVIG plus CsA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Chiba University Ethical committee review board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YM: Investigation, Writing – original draft. HH: Conceptualization, Funding acquisition, Supervision, Writing – review & editing, Writing – original draft. YS: Formal analysis, Investigation, Writing – review & editing. YO: Conceptualization, Supervision, Writing – review & editing, Writing – original draft. NK: Investigation, Project administration, Writing – review & editing, Writing – original draft. YO: Formal analysis, Investigation, Writing – review & editing. HH: Formal analysis, Supervision, Writing – review & editing. AH: Conceptualization, Supervision, Writing – review & editing. HS: Conceptualization, Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study is funded by Future Medicine Funds at Chiba University, and Japan Agency for Medical Research and Development (23808515).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. (1967) 16:178–222.6062087
2. Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial complications in adulthood. J Am Coll Cardiol. (2009) 54:1911–20. doi: 10.1016/j.jacc.2009.04.102
3. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
4. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. (1991) 324:1633–9. doi: 10.1056/NEJM199106063242305
5. Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. (2019) 393(10176):1128–37. doi: 10.1016/S0140-6736(18)32003-8
6. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. (2012) 379(9826):1613–20. doi: 10.1016/S0140-6736(11)61930-2
7. Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. (2014) 383(9930):1731–8. doi: 10.1016/S0140-6736(13)62298-9
8. Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. (2008) 40:35–42. doi: 10.1038/ng.2007.59
9. Onouchi Y, Suzuki Y, Suzuki H, Terai M, Yasukawa K, Hamada H, et al. ITPKC and CASP3 polymorphisms and risks for IVIG unresponsiveness and coronary artery lesion formation in Kawasaki disease. Pharmacogenomics J. (2013) 13:52–9. doi: 10.1038/tpj.2011.45
10. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
11. Miura M, Ayusawa M, Fukazawa R, Hamada H, Ikeda S, Ito S, et al. The guidelines on acute stage Kawasaki disease treatment. Pediatr Cardiol Cardiac Surg. (2020) 36(S1):S1.1–.29.
12. Yang JC, Jain S, Capparelli EV, Best BM, Son MB, Baker A, et al. Anakinra treatment in patients with acute Kawasaki disease with coronary artery aneurysms: a phase I/IIa trial. J Pediatr. (2022) 243:173–80. doi: 10.1016/j.jpeds.2021.12.035
13. Kone-Paut I, Tellier S, Belot A, Brochard K, Guitton C, Marie I, et al. Phase II open-label study of anakinra in intravenous immunoglobulin-resistant Kawasaki disease. Arthritis Rheumatol. (2021) 73:151–61. doi: 10.1002/art.41481
14. Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. (2005) 47:232–4. doi: 10.1111/j.1442-200x.2005.02033.x
15. Kobayashi T, Fuse S, Sakamoto N, Mikami M, Ogawa S, Hamaoka K, et al. A new Z score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr. (2016) 29:794–801. doi: 10.1016/j.echo.2016.03.017
16. Fuse S, Mori T, Kuroiwa Y, Hirakawa S. On what day of, illness does the dilatation of coronary arteries in patients with, Kawasaki disease begin? Circ J. (2017) 82:247–50. doi: 10.1253/circj.CJ-17-0046
17. Suzuki T, Kakimoto N, Tsuchihashi T, Suenaga T, Takeuchi T, Shibuta S, et al. Z-score is a possible predictor of the risk of coronary artery lesion development in patients with Kawasaki disease in Japan. Eur J Pediatr. (2021) 180:2797–805. doi: 10.1007/s00431-021-04006-1
18. Son MBF, Gauvreau K, Tremoulet AH, Lo M, Baker AL, de Ferranti S, et al. Risk model development and validation for prediction of coronary artery aneurysms in Kawasaki disease in a north American population. J Am Heart Assoc. (2019) 8:e011319. doi: 10.1161/JAHA.118.011319
19. Crystal MA, Manlholt C, Yeung RSM, Smallhorn JF, McCrindle BW. Coronary artery dilation after Kawasaki disease for children within the normal range. Int J Cardiol. (2009) 136:27–31. doi: 10.1016/j.ijcard.2008.04.019
20. Miyata K, Miura M, Kaneko T, Morikawa Y, Sakakibara H, Matsushima T, et al. Risk factors of coronary artery abnormalities and resistance to intravenous immunoglobulin plus corticosteroid therapy in severe Kawasaki disease: an analysis of post RAISE. Circ Cardiovasc Qual Outcomes. (2021) 14:e007191. doi: 10.1161/CIRCOUTCOMES.120.007191
21. Iio K, Morikawa Y, Miyata K, Kaneko T, Misawa M, Yamagishi H, et al. Risk factors of coronary artery aneurysms in Kawasaki disease with a low risk of intravenous immunoglobulin resistance: an analysis of post RAISE. J Pediatr. (2022) 240:158–63. doi: 10.1016/j.jpeds.2021.08.065
22. Kuo HC, Yang YL, Chuang JH, Tiao MM, Yu HR, Huang LT, et al. Inflammation-induced hepcidin is associated with the development of anemia and coronary artery lesions in Kawasaki disease. J Clin Immunol. (2012) 32:746–52. doi: 10.1007/s10875-012-9668-1
23. Huang YH, Kuo HC, Huang FC, Yu HR, Hsieh KS, Yang YL, et al. Hepcidin-induced iron deficiency is related to transient anemia and hypoferremia in Kawasaki disease patients. Int J Mol Sci. (2016) 17:715. doi: 10.3390/ijms17050715
24. Huang YH, Kuo HC. Anemia in Kawasaki disease: hepcidin as a potential biomarker. Int J Mol Sci. (2017) 18:820. doi: 10.3390/ijms18040820
25. Kim S, Eun LY. Iron deficiency anemia as a predictor of coronary artery abnormalities in Kawasaki disease. Korean J Pediatr. (2019) 62:301–6. doi: 10.3345/kjp.2018.06905
Keywords: Kawasaki disease, coronary artery disease, immunoglobulin, ciclosporin A, subanalysis
Citation: Murayama Y, Hamada H, Shiko Y, Onouchi Y, Kakimoto N, Ozawa Y, Hanaoka H, Hata A and Suzuki H (2023) Risk factors for coronary artery abnormalities and resistance to immunoglobulin plus ciclosporin A therapy in severe Kawasaki disease: subanalysis of the KAICA trial, randomized trial for cicrosporin A as the first-line treatment. Front. Pediatr. 11:1321533. doi: 10.3389/fped.2023.1321533
Received: 14 October 2023; Accepted: 4 December 2023;
Published: 15 December 2023.
Edited by:
Giovanni Filocamo, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, ItalyReviewed by:
Keiichi Hirono, University of Toyama, JapanXiaohui Li, Children's Hospital of Capital Institute of Pediatrics, China
Martina Rossano, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
© 2023 Murayama, Hamada, Shiko, Onouchi, Kakimoto, Ozawa, Hanaoka, Hata and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiromichi Hamada aGlyb21pY2hpLmhhbWFkYUBnbWFpbC5jb20= Hiroyuki Suzuki aHN1enVraUB3YWtheWFtYS1tZWQuYWMuanA=
†ORCID Hiromichi Hamada orcid.org/0000-0002-8990-5265
 Yuri Murayama1
Yuri Murayama1 Hiromichi Hamada
Hiromichi Hamada Yuki Shiko
Yuki Shiko Yoshihiro Onouchi
Yoshihiro Onouchi Hiroyuki Suzuki
Hiroyuki Suzuki