- 1Neonatal Intensive Care Unit, Department of Public Health and Pediatrics, University of Turin, Turin, Italy
- 2Laboratorio Della Conoscenza Carlo Corchia—APS, Florence, Italy
Introduction: Acute intestinal diseases (AID), including necrotizing enterocolitis and spontaneous intestinal perforation, are a group of conditions that typically present in preterm infants, and are associated with an elevated mortality and morbidity rate. The risk factors for these diseases remain largely unknown. The aim of the study is to identify the correlation between twinning and the development of AID.
Methods: A single-center retrospective case–control study was conducted. We recruited all infants with a diagnosis of AID, confirmed by anatomopathology, recovered in NICU between 2010 and 2020. Considering the rarity of the outcome, 4 matched controls for each subject were randomly chosen from the overall population of newborns. Odds Ratio (OR) and 95% Confidence Interval (CI) were calculated using a conditional logistic regression model and a multivariate model by the creation of a Directed Acyclic Graph (www.dagitty.net).
Results: The study population resulted in 65 cases and 260 controls. The two groups present similar median gestational age and mean birthweight in grams. The cases have a higher frequency of neonatal pathology (defined as at least one of patent ductus arteriosus, early or late sepsis, severe respiratory distress) (84.6% vs. 51.9%), medically assisted procreation (33.8% vs. 18.8%) and periventricular leukomalacia (10.8% vs. 2.7%), and a lower frequency of steroids prophylaxis (67.7% vs. 86.9%). About 50% of cases needed surgery. The OR for the direct effect were difference from one using logistic regression booth without and with repeated measures statements: from 1.14 to 4.21 (p = .019) and from 1.16 to 4.29 (p = .016), respectively.
Conclusions: Our study suggests that twinning may be a risk factor for the development of AID. Due to the small number of cases observed, further studies on larger populations are needed.
1. Introduction
Neonatal acute intestinal diseases (AID) are surgical intestinal disorders without mechanical obstruction typical of preterm newborns, including necrotizing enterocolitis (NEC) and spontaneous intestinal perforation (SIP). Despite recent advancements in neonatal care, these surgical intestinal disorders are still associated with a high mortality rate and a high prevalence of long-term morbidity in affected preterm infants.
NEC is the most frequent disease of the gastrointestinal tract of preterm infants and represents the most common cause of mortality and morbidity in the Neonatal Intensive Care Unit (NICU) (1, 2). Multiple population-based studies have reported the incidence of NEC to vary from 2 to 13% in NICU population, although there is substantial variability in incidence reported from different parts of the world (3, 4). Prognosis of NEC is related to gestational age (GA), with an estimated overall mortality from confirmed NEC of 25%, rising to 50% in Extremely Low Birth Weight (ELBW) infants (5). Pathogenesis of NEC is still partially unknown, but certainly multifactorial. The clinical presentation can be insidious or fulminant. Treatments involve supportive clinical management and consist of stopping enteral feedings and providing parenteral nutrition, intestinal decompression by nasogastric suctioning, and empiric administration of broad-spectrum antibiotics. In severe cases, surgical management often consists of peritoneal drain placement or exploratory laparotomy with possible bowel resection and percutaneous enterostomy placement (1).
SIP, characterized by the presence of focal intestinal perforation with no or minimal adjacent bowel inflammation, has confirmed to be a separate disease entity from NEC, because necrosis, inflammation of the intestinal mucosa or alterations in blood flow are generally not observed (6). Mortality rate is lower in infants with SIP in comparison with those with NEC, however these patients are particularly fragile and susceptible to short- and long-term complications (7, 8). SIP is a surgical disease, and the treatment is based on two main options currently used: exploratory laparotomy with bowel resection, or peritoneal drainage, which can be used either as a stabilizing procedure or a definitive treatment. Another minimally invasive and less used option is peritoneal needle aspiration (9).
It is therefore important to identify as many AID risk factors as possible in order to prevent them or, where the risk is unavoidable, perform early intervention. Regarding SIP risk factor, few studies have been conducted on this topic and there are not many certainties. On the other hand, for NEC risk factors, several associations were investigated and classified in antenatal, perinatal and postnatal. Established risks include prematurity and low birth weight (5).
Other certain risk factors reported currently in Literature are: prolonged rupture of membranes and maternal chorioamnionitis; compromised fetal blood flow before or at the time of delivery that may result in fetal ischemia; Intrauterine growth restriction (IUGR) especially if associated with abnormal Doppler studies; many typical complications associated with prematurity and some medication routinely administered to NICU's patients (sepsis, anemia, Patent ductus arteriosus); bacterial colonization of the gut and formula-feeding (5, 10–23).
In addition to the already known risk factors, clinical observations, supported by a plausible biological explanation, suggest that twinning may be associated with the risk of AID. In fact, many studies outlined that twin pregnancies present alterations not only in placental but also in fetal microcirculation with a greater frequency than that observed in single pregnancies. Those alterations of the microcirculatory perfusion of gastrointestinal tract, before or at the time of delivery, may lead to intestinal diseases development (24, 25).
The aim of this study is to identify a possible association between twinning and the development of NEC and/or SIP.
2. Methods
The study population was extracted from the St. Anne's Hospital dataset and selected from infants born between 2010 and 2020 admitted to the neonatal intensive care unit. All cases -defined as infants who developed NEC or SIP from birth to discharge- were collected. For each case, 4 controls -defined as infants who did not develop NEC or SIP from birth to discharge- were randomly extracted, with a final case:controls ratio of 1:4.
In the description of the sample, the categorical variables were presented as frequencies (percent), while the continuous variables were presented as mean (standard deviation) or median (interquartile range) according to their distribution.
The effect of twinning was analyzed using a logistic regression. The use of DAG allowed to identify the covariate for the estimate of direct and total effect; where the direct effect is the effect of twinning vs. singleton all the other covariate been equal, i.e., the effect added by twinning alone, and total effect is the effect of twinning vs. singleton, i.e., direct plus mediate by other variables (as gestational age) effect (Figure 1). The adjustment variables for direct effect were sex, gestational age (weeks), birth weight (z-score using INeS charts as reference) (26), intra uterine growth restriction, chorioamnionitis, premature rupture of membranes, neonatal pathology (defined as at least one of patent ductus arteriosus, early or late sepsis, severe respiratory distress), perinatal asphyxia, steroid prophylaxis (at least one cycles). The adjustment variables for total effect were maternal obesity (BMI ≥ 35 kg/m2), medically assisted procreation, maternal age.
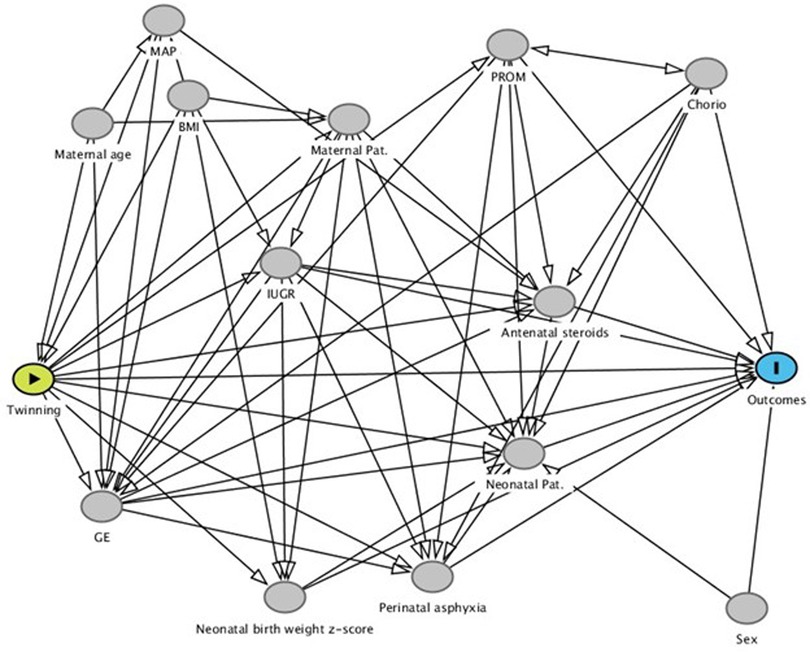
Figure 1. Directed acyclic graph. This graph helps in visualizing the relationships between the variables and in allows to identify the covariate for the estimate of direct and total effect.
A further analysis was performed including the repeated statement in logistic regression to model the covariance between the twin siblings, considering the couples of twins' outcomes repeated measures.
Only records with missing values of one or more variables included in the models were excluded.
The reference group was control group.
3. Results
From 2010 to 2020, 72 neonates admitted at St. Anne's Hospital NICU were affected by NEC or SIP. From these 7 (8.9%) neonates were excluded due to missing maternal values (BMI and age). Their gestational age ranged from 24 to 34 weeks, 1 mother had medically assisted procreation, 3 (43%) were boys. The study population resulted in 325 newborns (65 cases and 260 controls) and the description of the two groups is reported in Table 1.
The median gestational age is similar. In cases the frequency of boys is slightly higher while the frequency of twins is higher of 10% (46.2% cases vs. 35.0% controls). The two groups present similar mean birthweight in grams, but a mean z-score lower in case than in control group. The cases have a higher frequency of neonatal pathology (defined as at least one of patent ductus arteriosus, early or late sepsis, severe respiratory distress) (84.6% cases vs. 51.9% controls), medically assisted procreation (33.8% cases vs. 18.8% controls) and periventricular leukomalacia (10.8% cases vs. 2.7% controls), and a lower frequency of intrauterine growth restriction (18.5% cases vs. 23.1%controls) and steroids prophylaxis (67.7% cases vs. 86.9% controls). While early sepsis is comparable between the two groups (3.1% cases vs. 4.2% controls), the frequency of late sepsis is more than 5 times in cases than in controls (43.1% cases vs. 8.9% controls). About 30% of cases presented perinatal distress vs. 13% of controls.
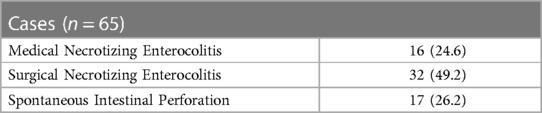
Table 2 reports the frequencies of NEC, SIP and need for surgery for these conditions in cases. About 50% of cases needed surgery.
The Odds Ratio estimates are reported in Figure 2. While the total effect does not show significant difference, the ORs for the direct effect were difference from one using logistic regression booth without and with repeated measures statement: from 1.14 to 4.21 (p = .019) and from 1.16 to 4.29 (p = .016), respectively.
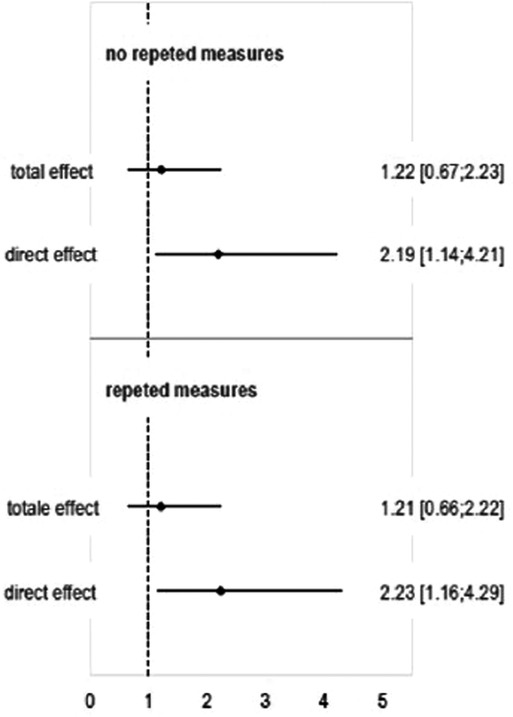
Figure 2. Direct and total effect ORs [95% CI] estimated using logistic regression and logistic regression with repeated measures (i.e. the 21 pairs of twins were considered as repeat measures in order to account for covariance between twin siblings).
4. Discussion
In last decades, the improvement of neonatal techniques has allowed a greater survival of premature infants, with increasingly satisfying long-term outcomes. Despite this recent advancement, incidence of AID has remained relatively stable in infant populations with a high risk of mortality and significant short- and long-term morbidity (27, 28).
The management of AID has remained almost stable over the years, so prevention and identification of the risk factors are mandatory.
In Literature, many risk factors have been analyzed and confirmed by recent studies, while others are still controversial or not fully considered (5, 10–23). Few studies considered the possibility that twinning was a yet unknown risk factor, despite its clinical and biological plausibility. Literature data are mainly focused on the NEC and twinning has been used as a paradigm in the understanding of etiopathogenesis (29–31). Conversely, in studies focused on specific types of twin pregnancy, NEC is considered as one of the neonatal morbidities under analysis and in these cases an association was observed (32, 33).
Regarding the characteristics of our two populations of cases and controls, the main data observed were in line with the literature. Among population of cases, we observed a higher frequency of some neonatal risk factors associated with AID:
- perinatal asphyxia: poor neonatal transition
- PDA: an association between the presence of PDA and the development of intestinal surgical pathologies seems to emerge from numerous studies in recent years (15, 34).
- Sepsis: the same can be said of the association between septic pathology of the newborn and the risk of developing NEC (10, 20)
- Male sex (33)
In recent years, a possible protective role of prenatal administration of corticosteroids towards the development of NEC has emerged, and also in our population we observed a lower frequency of steroids prophylaxis in cases compared to controls.
It is also interesting to note the higher number of assisted fertilization pregnancies among cases. This observation can be explained considering that the pregnancies obtained with medically assisted procreation are often at risk for preterm labor and pre or postnatal complications, and they generate twin pregnancies more frequently than spontaneous.
The core of our study is the relationship between twinning and the development of AID. The construction of a DAG allowed us to analyze this association without considering the confounding factors that could modify the association between the risk factor and the outcome.
This data is extremely interesting because it seems to indicate that twinning is not only a risk factor of premature birth, per se known as a major risk factor for the onset of AID in the newborn, but that it constitutes in itself a direct risk factor of AID.
The ORs for direct effect resulted higher respect to ORs for total effect. This result can be explained from the different distribution of variables included in multivariable analysis: lower z-score and steroids prophylaxis, intrauterine growth restriction, premature rupture of membranes, and higher perinatal distress.
The case-control study has the advantage of allowing us to have information on the association between two variables in a short time and is useful for exploratory purposes, especially when the risk is rare. Like all studies with retrospective data collection, whether prospective or case-control, the major disadvantage is related to the possible bias due to collected variables not specifically for the purpose. However, it must be considered that this affordability is the same for clinical purposes only. The main limitation of the study lies in the sample size that is about 30%. Despite this, the direct effect results significant. Future studies, possibly multicentric, are needed to expand the sample size and confirm our results. In future studies, it would also be interesting to collect more data related to both twins, regardless of whether they are both cases and/or controls to have a complete picture of the twin population considered. Finally, it would also be important to collect detailed data related to nutrition (i.e., type of nutrition, method of nutrition administration, the rhythm of increasing the amount of meal, day of life for beginning the nutrition) because in a multicenter study the nutritional protocols may differ substantially between centers, and then these variables will also be included in the multivariate analysis.
5. Conclusions
AID still remains one of the leading causes of death in preterm infants, with still largely unclear characteristics. The ever more in-depth knowledge of the risk factors that favor the onset of these diseases has made it possible to prevent and early recognize them. Twinning was not yet included in the group of the recognized risk factor, and few studies considered it before. This study allowed to identify an association between twinning and the development of NEC and SIP, providing a deeper knowledge of these diseases and a possibility of prevention for the future. Given the still small number of the sample analyzed, it is desirable that studies conducted on larger populations allow to confirm the observed data and to deepen it in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
CP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ES: Conceptualization, Formal Analysis, Methodology, Writing – original draft. LR: Methodology, Writing – original draft, Writing – review & editing. MC: Data curation, Writing – original draft. FP: Data curation, Writing – original draft. AC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364(3):255–64. doi: 10.1056/NEJMra1005408
2. Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. (2012) 129:1019–26. doi: 10.1542/peds.2011-3028
3. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20:344. doi: 10.1186/s12887-020-02231-5
4. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F182–9. doi: 10.1136/archdischild-2017-313880
5. Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. (2012) 129:e298–304. doi: 10.1542/peds.2011-2022
6. Vongbhavit K, Underwood MA. Intestinal perforation in the premature infant. J Neonatal Perinatal Med. (2017) 10(3):281–9. doi: 10.3233/NPM-16148
7. Fisher JG, Jones BA, Gutierrez IM, Hull MA, Kang KH, Kenny M, et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg. (2014) 49:1215–9. doi: 10.1016/j.jpedsurg.2013.11.051
8. Shah J, Singhal N, da Silva O, Rouvinez-Bouali N, Seshia M, Lee SK, et al. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol. (2015) 35(8):595–600. doi: 10.1038/jp.2015.41
9. Gébus M, Michel J-L, Samperiz S, Harper L, Alessandri JL, Ramful D. Management of neonatal spontaneous intestinal perforation by peritoneal needle aspiration. J Perinatol. (2018) 38(2):159–63. doi: 10.1038/jp.2017.170
10. Drenckpohl D, Knaub L, Schneider C, McConnell C, Wang H, Macwan K. Risk factors that may predispose premature infants to increased incidence of necrotizing enterocolitis. ICAN Infant Child Adolesc Nutr. (2010) 2(1):37–44. doi: 10.1177/1941406409359195
11. Rose AT, Patel RM. A critical analysis of risk factors for NEC. Semin Fetal Neonatal Med. (2018) 23(6):374–9. doi: 10.1016/j.siny.2018.07.005
12. Luig M, Lui K. Epidemiology of necrotizing enterocolitis–part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. (2005) 41(4):174–9. doi: 10.1111/j.1440-1754.2005.00583.x
13. Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal doppler results. Arch Dis Child Fetal Neonatal Ed. (2005) 90(5):F359–63. doi: 10.1136/adc.2004.060350
14. Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. (2016) 315(9):889–97. doi: 10.1001/jama.2016.1204
15. Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. (2005) 40(2):184–8. doi: 10.1097/00005176-200502000-00019
16. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. (2017) 5(1):3. doi: 10.1186/s40168-017-0248-8
17. Vongbhavit K, Underwood MA. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther. (2016) 38:716–32. doi: 10.1016/j.clinthera.2016.01.006
18. Nino DF, Sodhi CP, Hackman DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13(10):590–600. doi: 10.1038/nrgastro.2016.119
19. Good M, Sodhi CP, Hackam DJ. Evidence based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol. (2014) 10(7):875–84. doi: 10.1586/1744666X.2014.913481
20. Su Y, Xu RH, Guo LY, Chen XQ, Han WX, Ma JJ, et al. Risk factors for necrotizing enterocolitis in neonates: a meta-analysis. Front Pediatr. (2023) 10:1079894. doi: 10.3389/fped.2022.1079894
21. Alganabi M, Lee C, Bindi E, Li B, Pierro A. Recent advances in understanding necrotizing enterocolitis. F1000Res. (2019) 8:F1000 Faculty Rev-107. doi: 10.12688/f1000research.17228.1
22. Campos-Martinez AM, Expósito-Herrera J, Gonzalez-Bolívar M, Fernández-Marin E, Uberos J. Evaluation of risk and preventive factors for necrotizing enterocolitis in premature newborns. A systematic review of the literature. Front Pediatr. (2022) 10:874976. doi: 10.3389/fped.2022.874976
23. Ahle M, Drott P, Elfvin A, Andersson RE. Maternal, fetal and perinatal factors associated with necrotizing enterocolitis in Sweden. A national case-control study. PLoS One. (2018) 13:e0194352. doi: 10.1371/journal.pone.0194352
24. Altorjay ÁT, Nyári T, Gyurkovits Z, Németh G, Surányi A. Evaluation of placental vascularization indices in monochorionic diamniotic and dichorionic diamniotic twin pregnancies. Eur J Obstet Gynecol Reprod Biol. (2018) 228:225–31. doi: 10.1016/j.ejogrb.2018.05.040
25. Hubinont C, Lewi L, Bernard P, Marbaix E, Debiève F, Jauniaux E. Anomalies of the placenta and umbilical cord in twin gestations. Am J Obstet Gynecol. (2015) 213(4 Suppl):S91–S102. doi: 10.1016/j.ajog.2015.06.054
26. Lewi L, Deprest J, Hecher K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am J Obstet Gynecol. (2013) 208(1):19–30. doi: 10.1016/j.ajog.2012.09.025
27. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. JPGN. (2010) 51:353–61. doi: 10.1097/MPG.0b013e3181da213e
28. Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol. (2012) 32:552–8. doi: 10.1038/jp.2011.176
29. Dilli D, Eras Z, Ozkan Ulu H, Dilmen U, Durgut Şakrucu E. Does necrotizing enterocolitis affect growth and neurodevelopmental outcome in very low birth weight infants? Pediatr Surg Int. (2012) 28:471–6. doi: 10.1007/s00383-012-3051-4
30. Samm M, Curtis-Cohen M, Keller M, Chawla H. Necrotizing enterocolitis in infants of multiple gestation. Am J Dis Child. (1986) 140:937–9. doi: 10.1001/archpedi.1986.02140230107045
31. Powell RW, Dyess DL, Luterman A, Simon NP, Ramenofsky ML. Necrotizing enterocolitis in multiple-birth infants. J Pediatr Surg. (1990) 25:319–21. doi: 10.1016/0022-3468(90)90076-L
32. Burjonrappa SC, Shea B, Goorah DJ. NEC in twin pregnancies: incidence and outcomes. Neonatal Surg. (2014) 3(4):45. 26023516; PMCID: PMC4420336.
33. Ward C, Caughey AB. Late preterm births: neonatal mortality and morbidity in twins vs. singletons. J Matern Fetal Neonatal Med. (2022) 35(25):7962–7. doi: 10.1080/14767058.2021.1939303
34. Okuyama H, Ohfuji S, Hayakawa M, Urushihara N, Yokoi A, Take H, et al. Risk factors for surgical intestinal disorders in VLBW infants: case-control study. Pediatr Int. (2016) 58(1):34–9. doi: 10.1111/ped.12815
35. Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. (2018) 319(12):1221–38. doi: 10.1001/jama.2018.1896
Keywords: acute intestinal diseases, necrotizing enterocolitis, spontaneous intestinal perforation, twin, twinning, preterm newborn, newborn
Citation: Peila C, Spada E, Riboldi L, Capitanio M, Pellegrino F and Coscia A (2023) Twinning as a risk factor for neonatal acute intestinal diseases: a case-control study. Front. Pediatr. 11:1308538. doi: 10.3389/fped.2023.1308538
Received: 6 October 2023; Accepted: 1 December 2023;
Published: 14 December 2023.
Edited by:
Eugene S. Kim, Cedars Sinai Medical Center, United StatesReviewed by:
Eveline Shue, Cedars Sinai Medical Center, United StatesGabriela Corina Zaharie, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
© 2023 Peila, Spada, Riboldi, Capitanio, Pellegrino and Coscia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: L. Riboldi bG9yZW56by5yaWJvbGRpQHVuaXRvLml0
 C. Peila
C. Peila E. Spada
E. Spada L. Riboldi1*
L. Riboldi1* F. Pellegrino
F. Pellegrino A. Coscia
A. Coscia