- 1Department of Dermatology, Weill Cornell Medicine, New York, NY, United States
- 2Mycotic Diseases Branch, Division of Foodborne, Waterborne and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, United States
- 3Departments of Dermatology and Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
We retrospectively reviewed physician diagnostic and treatment practices for pediatric tinea capitis at an academic institution over 16 years, in assessing adherence with published guidelines. We demonstrate the need to increase utilization of confirmatory testing and systemic therapy, and call for directed pediatrician education towards these goals.
Introduction
Tinea capitis (TC), a fungal scalp infection, is the most common childhood dermatophytosis worldwide (1). TC primarily affects children aged 3–14 years, particularly Black males, and is most often caused by Microsporum and Trichophyton species. In the United States, T. tonsurans is the most common causative species (2). American Academy of Dermatology guidelines (1996, most recent year) and the American Academy of Pediatrics Committee on Infectious Disease (2021 Red Book, most recent year) emphasize confirmatory testing of suspected TC and treatment with systemic antifungals (3, 4). Confirmatory testing is important given possibility of misdiagnosis, ineffectiveness of topicals against TC, and need for antifungal stewardship in an era of emerging antifungal-resistant dermatophytes (5). Because data on guideline adherence are lacking, we aimed to capture TC diagnostic and treatment practices.
Methods
After Weill Cornell Medicine Institutional Review Board approval (22-09025241), Weill Cornell Medicine EPIC database was queried for patients 0–18 years diagnosed with TC (International Classification of Diseases-9 code 110.0, International Classification of Diseases-10 code B35.0) 10/1/2005-9/30/2021. Demographics, diagnosing physician specialty, diagnostic test(s) performed, and treatment(s) prescribed were described. Chi-squared tests compared diagnostic and treatment practices for dermatologists vs. pediatricians (α < 0.05).
Results
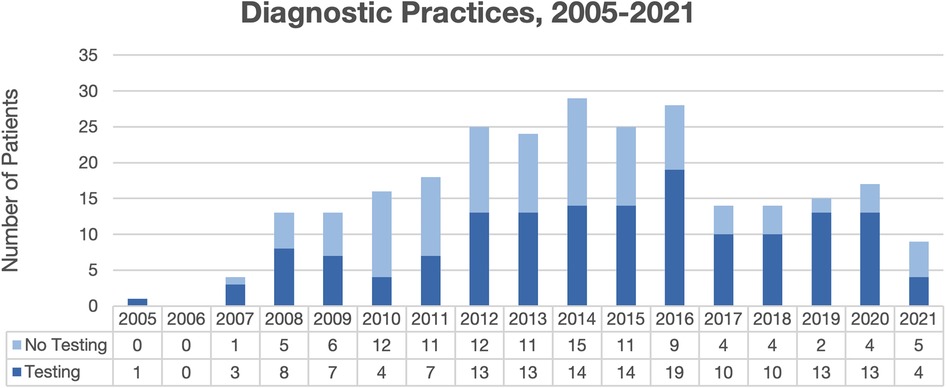
Overall, 265 total patient visits were included, comprising 239 patients, with average age 4.4 years, 56.5% male, and 70.8% Black (Table 1). Most were diagnosed by dermatologists (49.8%) or pediatricians (49.1%). Diagnostic testing was performed in 57.7% of encounters, most commonly fungal cultures (94.8%) and potassium hydroxide (KOH) preparation (54.9%) (Table 2). Testing was performed more often by dermatologists than pediatricians (96.2% vs. 20.0%, p < 0.00001), with testing practices by specialty relatively stable over the study period (Figure 1). Identified species included 77.1% T. tonsurans, 4.2% T. rubrum, and 2.1% M. canis. Systemic therapy was prescribed most often (92.6%), and more commonly by dermatologists than pediatricians (96.6% vs. 88.6%, p = 0.02). Common systemics were griseofulvin (86.7%) and terbinafine (16.0%), with terbinafine more often utilized by dermatologists (30.0%) than pediatricians (1.8%). Systemics besides griseofulvin were utilized more often 2014–2021 vs. 2005–2013 (24.4% vs. 11.2%, p = 0.01). Common topicals were ketoconazole (69.5%) and clotrimazole (20.7%), with clotrimazole only prescribed by pediatricians.
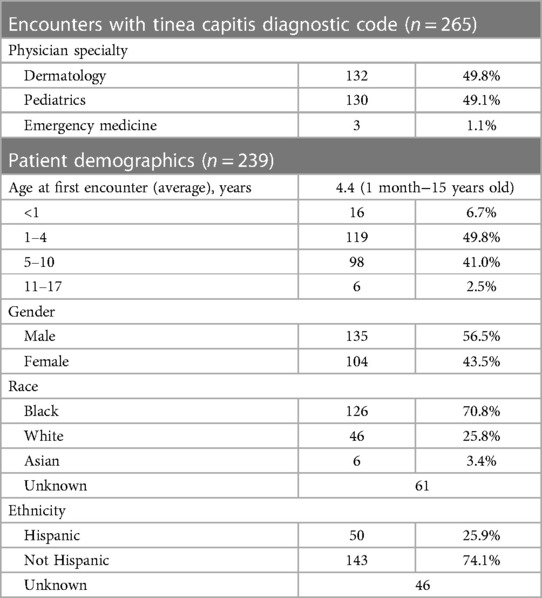
Table 1. Diagnosing physician specialty and demographics of patients with tinea capitis diagnoses from 2005 to 2021.
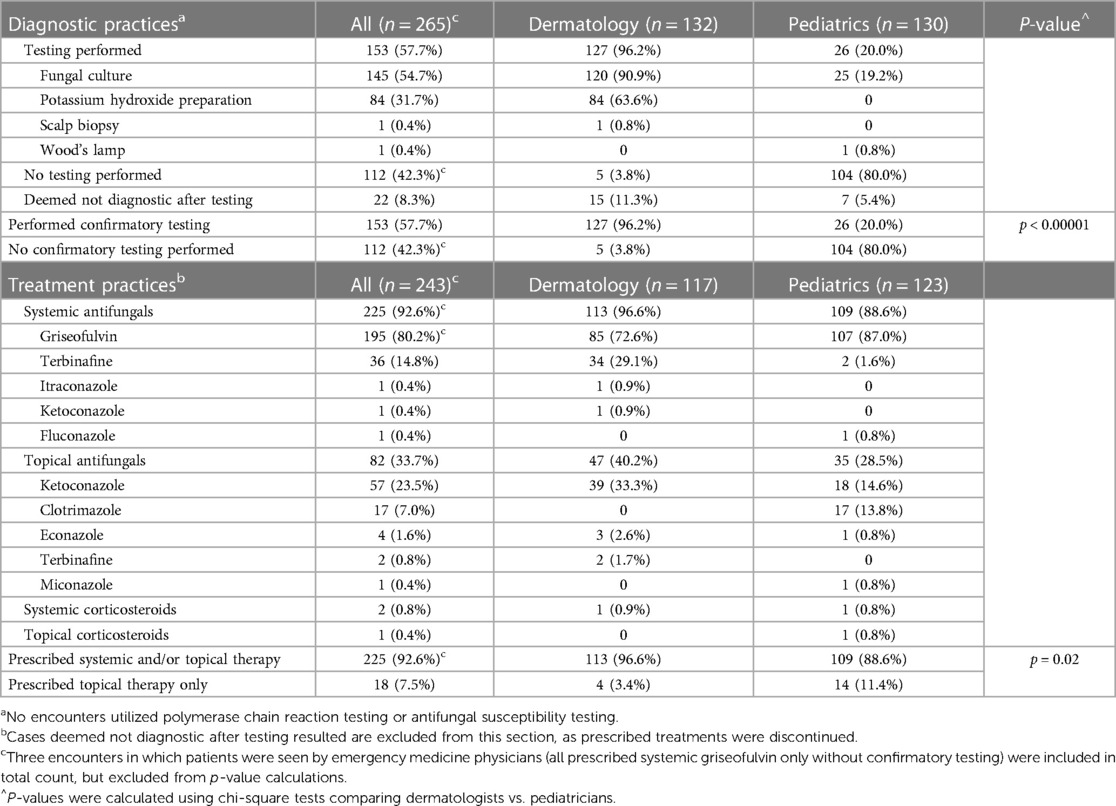
Table 2. Diagnostic and treatment practices for patients with tinea capitis diagnoses from 2005 to 2021.
Discussion
In this 16-year retrospective review of TC patients at Weill Cornell Medicine, virtually all dermatologists, but only one-fifth of pediatricians, performed confirmatory testing. In a national commercial database study of 3.9 million pediatric TC encounters, confirmatory testing was infrequent (21.9%), with dermatologists testing more often than pediatricians (51.0% vs. 16.4%, p < 0.01), suggesting similar testing practices between academic and community pediatricians (6). KOH preparations were documented in approximately two-thirds of dermatologist encounters and in no pediatric encounters. Low testing rates by pediatricians may be due to lack of recognition of importance of diagnostic confirmation emphasized by American Academy of Dermatology guidelines and American Academy of Pediatrics Red Book recommendations, long fungal culture turnaround times competing with prompt treatment initiation, and lack of training or required Clinical Laboratory Improvement Amendments certification for KOH examinations (3, 4, 6).
Consistent with TC treatment guidelines, most patients were prescribed systemic antifungals (92.6%). Using topical antifungals (creams, shampoos) alone is discouraged, due to lack of hair follicle root penetration. However, 11.4% of pediatricians prescribed topical therapy only, similar to the aforementioned commercial database study reporting a 10.1% rate for pediatricians, emphasizing the need for directed education (6). For systemic therapies, a systematic review of 38 TC clinical trials reported overall 92% and 72% complete cure rates for terbinafine and griseofulvin, respectively, terbinafine being more effective for Trichophyton and griseofulvin more effective for Microsporum species (7). Terbinafine is regarded as first-line treatment in the US, given vast predominance of Trichophyton TC and shorter treatment course (6–8 weeks) (7). However, since terbinafine was infrequently prescribed by pediatricians (1.6%), directed education is necessary.
Limitations include single-center design and small sample size. However, our study may accurately reflect US practices, given congruence with the commercial database study (5).
We highlight opportunities to increase utilization of confirmatory testing and systemic therapy, and call for directed pediatrician education towards these goals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of BRANY, Institutional Review Board of Weill Cornell Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective review nature of study.
Author contributions
JH: Writing – original draft, Writing – review & editing. JG: Writing – review & editing. AP: Writing – review & editing. SL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
AP is an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, and UCB; a consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer-Ingelheim, Bristol Myers Squibb, Castle Creek, Eli Lilly, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi/Genzyme, Seanergy, TWI Biotechnology, and UCB; and on the Data and Safety Monitoring Board (DSMB) for AbbVie, Abeona, Catawba, Galderma, and InMed. SL is a consultant for Eli Lilly, Ortho-Dermatologics, Belle Torus Corporation, and Moberg Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
1. Leung AKC, Hon KL, Leong KF, Barankin B, Lam JM. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. (2020) 14(1):58–68. doi: 10.2174/1872213X14666200106145624
2. Gold JAW, Benedict K, Lockhart SR, Lipner SR. Epidemiology of tinea capitis causative species: an analysis of fungal culture results from a major United States national commercial laboratory. J Am Acad Dermatol. (2023) 89(2):382–4. doi: 10.1016/j.jaad.2023.03.03
3. Drake LA, Dinehart SM, Farmer ER, Goltz RW, Graham GF, Hardinsky MK, et al. Guidelines of care for superficial mycotic infections of the skin: tinea capitis and tinea barbae. Guidelines/outcomes committee. American academy of dermatology. J Am Acad Dermatol. (1996) 34(2 Pt 1):290–4. doi: 10.1016/s0190-9622(96)80137-x
4. Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH. Tinea capitis (ringworm of the scalp). In: Kimberlin DW, editor. Red book: 2021−2024 report of the committee on infectious diseases. 32nd ed. Itasca, IL: American Academy of Pediatrics (2021):755–9.
5. Zeeshan F, Sabir M, Uddin F. The rising menace of antifungal resistance in dermatophytes among the patients of tinea capitis. J Pak Med Assoc. (2023) 73(1):43–8. doi: 10.47391/JPMA.5307
6. Gold JAW, Benedict K, Dulski TM, Lipner SR. Inadequate diagnostic testing and systemic antifungal prescribing for tinea capitis in an observational cohort study of 3.9 million children, United States. J Am Acad Dermatol. (2023) 89(1):133–5. doi: 10.1016/j.jaad.2023.02.009
Keywords: tinea, tinea capitis, fungal infections, diagnosis, treatment
Citation: Hwang JK, Gold JAW, Paller AS and Lipner SR (2023) Low utilization of confirmatory testing for tinea capitis by pediatricians at an academic center in New York, United States, 2005–2021. Front. Pediatr. 11:1297339. doi: 10.3389/fped.2023.1297339
Received: 19 September 2023; Accepted: 7 November 2023;
Published: 17 November 2023.
Edited by:
Despina Contopoulos-Ioannidis, Stanford University, United StatesReviewed by:
Maud Gits-Muselli, Assistance Publique Hopitaux De Paris, FranceZachary McPherson, Children’s Hospital at Westmead, Australia
© 2023 Hwang, Gold, Paller and Lipner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shari R. Lipner shl9032@med.cornell.edu
 Jonathan K. Hwang
Jonathan K. Hwang