
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 04 January 2024
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1288660
 Yang Jia1,†
Yang Jia1,† Xiaoling Liang2,†
Xiaoling Liang2,† Lini Liu1
Lini Liu1 Huixi Ma1
Huixi Ma1 Chenhao Xu3
Chenhao Xu3 Jingyuan Zeng3
Jingyuan Zeng3 Rong Xu1
Rong Xu1 Lu Ye4*‡
Lu Ye4*‡ Linjun Xie1*‡
Linjun Xie1*‡
Background: The development of prenatal diagnosis technology allows prompt detection of severe fetal diseases. To address adverse factors that threaten fetal survival, fetal therapy came into existence, which aims to preserve the function after birth to a higher degree and improve the quality of life.
Objective: To conduct a comprehensive bibliometric analysis of studies on fetal therapy in the past decade and explore the research trends and hotspots in this field.
Methods: We conducted a systematic search on the Web of Science Core Collection to retrieve studies related to fetal therapy published from 2012 to 2022. VOSviewer and CiteSpace were used to analyze the key features of studies, including annual output, countries/regions, institutions, authors, references, research hotspots, and frontiers.
Results: A total of 9,715 articles were included after eliminating duplicates. The annual distribution of the number of articles showed that the number of articles published in fetal therapy had increased in the past decade. Countries and institutions showed that fetal therapy is more mature in the United States. Author analysis showed the core investigators in the field. Keyword analysis showed the clustering and emergence frequency, which helped summarize the research results and frontier hotspots in this field. The cocited references were sorted out to determine the literature with a high ranking of fetal therapy in recent years, and the research trend in recent years was analyzed.
Conclusions: This study reveals that countries, institutions, and researchers should promote wider cooperation and establish multicenter research cooperation in fetal therapy research. Moreover, fetal therapy has been gradually explored from traditional surgical treatment to gene therapy and stem cell therapy. In recent years, fetoscopic laser surgery, guideline, and magnetic resonance imaging have become the research hotspots in the field.
With the rapid development of prenatal ultrasound diagnosis, molecular genetics, and interventional prenatal diagnosis techniques, many fetal diseases can be screened and diagnosed during the prenatal period (1). Neonatal and pediatric subspecialties have rapidly emerged, and most fetal diseases are well diagnosed before delivery, particularly isolated fetal structural abnormalities that can be corrected after birth (2). However, for a small number of severe fetal diseases, due to their rapid progression and poor prognosis, effective postpartum treatment is still lacking, which has greatly promoted the development of fetal therapy (3). Fetal therapy involves interventions administered during pregnancy to stop the progression of fetal diseases (4). Fetal therapy intends to prevent further disease progression and create conditions for postnatal treatment to reduce perinatal infant mortality and improve their quality of life (5). Fetal therapy is performed during pregnancy and involves invasive procedures; thus, some risks are unavoidable. In 1982, the International Society of Fetal Medicine and Surgery (IFMSS) first proposed principles that must be followed in fetal treatment to regulate the indications and procedures for treatment (6). Subsequently, in 2017, the North American Fetal Therapy Network and IFMSS members revised and supplemented the 1982 Fetal Therapy Basic Principles to promote further development in the field (7). They constructively suggested that fetal therapy was no longer limited to improving survival rates but increasingly focused on maternal reproductive health. Researchers should focus on reducing morbidity, improving the long-term prognosis of patients, balancing maternal and fetal risks associated with treatment, and establishing multidisciplinary teams. Fetal treatment is the reversal of pathological changes, restoration of normal anatomy, or preservation of physiological functions under prenatal intervention. Therefore, understanding the current status, main research institutions, and hotspots of fetal therapy will help with further research on the occurrence and development, treatment, and long-term prognosis of fetal diseases.
The gradual emergence of bibliometric analysis in recent years has brought a certain simplicity to summarizing the main content and cutting-edge hotspots in the research field. Bibliometric analysis is a quantitative analysis that combines mathematical and statistical methods, and it can help researchers understand the characteristics of field development over time (8). An in-depth assessment of research trends and focus on a particular topic are possible, and the results of the bibliometric evaluation can provide recommendations for further research and decision making. CiteSpace and VOSviewer were used to analyze fetal therapy, and their combination can obtain an intuitive visual network diagram in this field. To reveal the structure of the research domain, CiteSpace visual analysis generates cocitation networks based on reference citations (9).
Most of the existing reviews of fetal therapy summarize the status quo of treatment methods and fetal diseases; however, a macrosummary and induction of the whole field of fetal therapy remain unknown. In recent years, CiteSpace and VOSviewer have been widely used in many fields. Currently, targeted bibliometric analysis of scientific research on fetal therapy worldwide is lacking (10). Therefore, this study aimed to review the basics, hotspots, and frontiers of fetal therapy through bibliometric analysis.
All published articles were available through January 14, 2023 at the Science Core Collection to eliminate large errors caused by routine database changes. The core collection of the Web of Science (WOS) was used as the data source. The search strategy used was as follows: [TS = (“fetal therapies” OR “fetal therapy” OR “fetal surgery” OR “fetoscopy” OR “blood transfusion, intrauterine” OR “intrauterine transfusion” OR “ex utero intrapartum technique procedures”)] AND [Language = (English)] AND [document type = (“article” OR “review”)], and the retrieval time was limited from January 1, 2012 to December 31, 2022. The following exclusion criteria were used to screen the obtained study: articles not published formally, meeting summaries and meeting minutes, early online publications, repeated articles unrelated to the topic, book chapters, and incomplete articles. Figure 1 provides detailed information on enrollment and selection. Because data were retrieved directly from the database, ethical approval was not needed.
The included studies were analyzed using VOSviewer and CiteSpace (6.1.R6) software. VOSviewer, a bibliometric software with strong graphical capabilities, is suitable for processing large-scale data and can be used to construct relational networks and data visualization (11). Using a visual map created by VOSviewer, nodes represent countries, institutions, authors, or keywords, which can be connected via coauthorship, citation, co-occurrence, and cocitation analysis. The size of the node was determined by the weight of the element, such as the number of publications, citations, or frequency of occurrence. Each node was assigned a color, and the same color represented the same cluster, which was a set of items in the network with similar properties. The link between nodes represented the correlation between elements, and the thickness of the link represented the strength of the link. Total link strength (TLS) was used for the quantitative evaluation of chains. A similar analysis was used to create a visual map of countries or regions, organizations, and authors published in each period to map the evolution of these elements (12). CiteSpace is an important bibliometric analysis software (13) that can realize the understanding of the main fields, research hotspots, and frontiers of fetal therapy.
A total of 9,715 articles related to fetal therapy from 2012 to 2022 were retrieved from the WOS Core Collection. The outputs of fetal therapy published annually are shown in Figure 2. Interest in intrauterine interventions has increased dramatically over the past 10 years. The annual number of publications worldwide increased from 675 in 2012 to 1,171 in 2020. The number of articles published in 2021 and 2022 were slightly lower than those in previous years; however, the number of articles was still increasing. The citation frequency was also an important indicator for academic quantitative evaluation. During the last decade, the citation frequency of the study annually increased and it was the highest in 2021. In the past decade, the number and citation frequency of articles in the field of fetal therapy have been increasing, which indicates that research in this field is in a booming period.
The number of publications in a country reflects the level and effect of research on the relevant field in that country. A total of 9,715 articles were published in the field of fetal therapy from 133 countries/regions. Table 1 lists the top ten countries with the highest number of papers. As shown in Table 1, the top three countries were the United States (3,334), China (987), and the United Kingdom (803), and these countries accounted for >50% of the publications and made important contributions to the research in this field. Regarding the number of citations, the top three countries were the United States, the United Kingdom, and Australia. The United States ranked first in the number of publications and citations in this field. This indicates its important role in fetal therapy and provides many excellent articles for researchers in the same field. Figure 3 illustrates the top 30 countries with >70 published articles generated via VOSviewer. As shown in this figure, research forces are mainly concentrated in the United States, China, United Kingdom, Italy, Canada, and Australia. Moreover, countries maintain a relatively close cooperative relationship, which also greatly promotes the continuous development of fetal therapy.
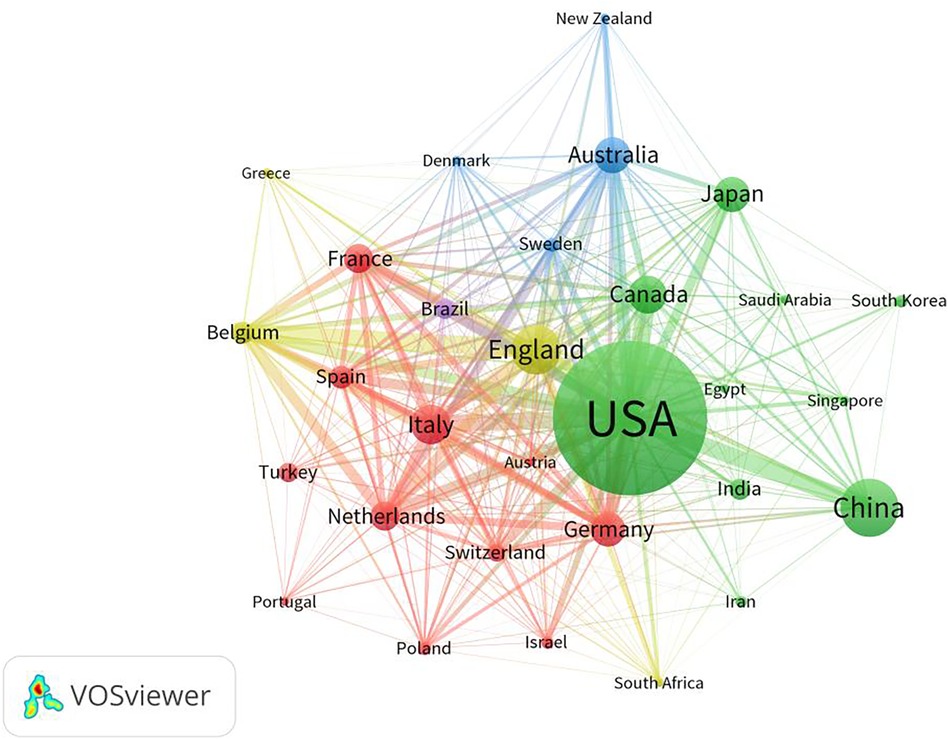
Figure 3. Map network of top 30 countries. Different colors indicate different clusters, and the size of circles indicate the number of publications. The thickness of the lines represents the link strength of the countries.
In total, 345 institutions have published research articles on fetal therapy. Table 2 lists the top ten institutions with the largest number of publications on fetal therapy. The top three institutions were University of Pennsylvania, the University of Toronto, and University College London. Four of the top ten institutions are located in the United States, two in Australia, and the rest in Canada, the United Kingdom, Netherlands, and Belgium, confirming the pre-eminent position of the United States, the United Kingdom, Australia, and Canada in this field. Interestingly, regarding the number of citations, the top four institutions were University of Pennsylvania,University College London, Leiden University, and the University of Toronto, which publish articles that receive more attention from researchers in the same field. In terms of total connection strength, Katholieke University of Leuven, University of Pennsylvania, and the University College London interacted more frequently with other research institutions.
Figure 4 is a visual map generated via VOSviewer for institutions with >74 published articles. As shown, the main research power in this field is concentrated in University of Pennsylvania, University of Toronto, University College London, Baylor College of Medicine, and University of San Francisco.
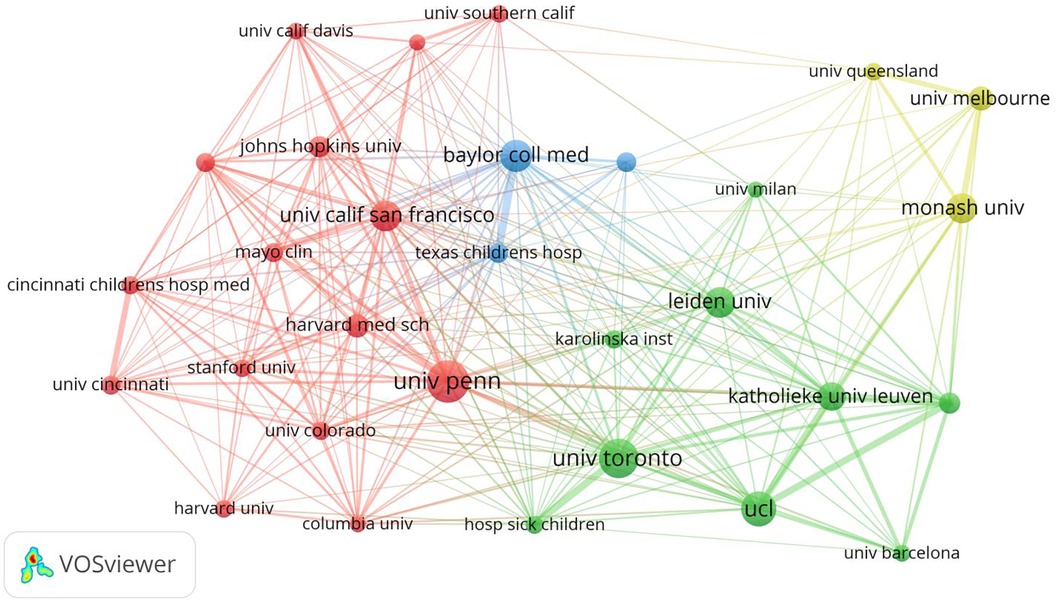
Figure 4. Map network of top 30 institutions. Different colors indicate different clusters, and the size of circles indicate the number of publications. The thickness of the lines represents the link strength of the institutions.
Table 3 lists the most influential experts in fetal therapy in the last decade, ranked by the number of publications. The top three citations were Deprest J, Chmait RH and Oepkes D, Deprest J, Adzick NS and Oepkes D, in this order. Statistically, Deprest J ranked first in both the number of publications and the number of citations, indicating that his outstanding contributions to fetal therapy have been recognized by researchers in the same field.
The analysis of keyword co-occurrence provides a theoretical basis for the in-depth understanding of the distribution and evolution of various research hotspots within a topic. As a highly simplified form of the content of the paper, keywords can directly and simply express the topic to a certain extent. Keywords selected by the author when submitting the manuscript for publication were extracted using VOSviewer. We analyzed keywords extracted from 9,715 publications. Figure 5 shows detailed data on the co-occurrence of the top 30 keywords. Among them, “management,” “prenatal diagnosis,” “fetal surgery,” and related keywords are the most significant.
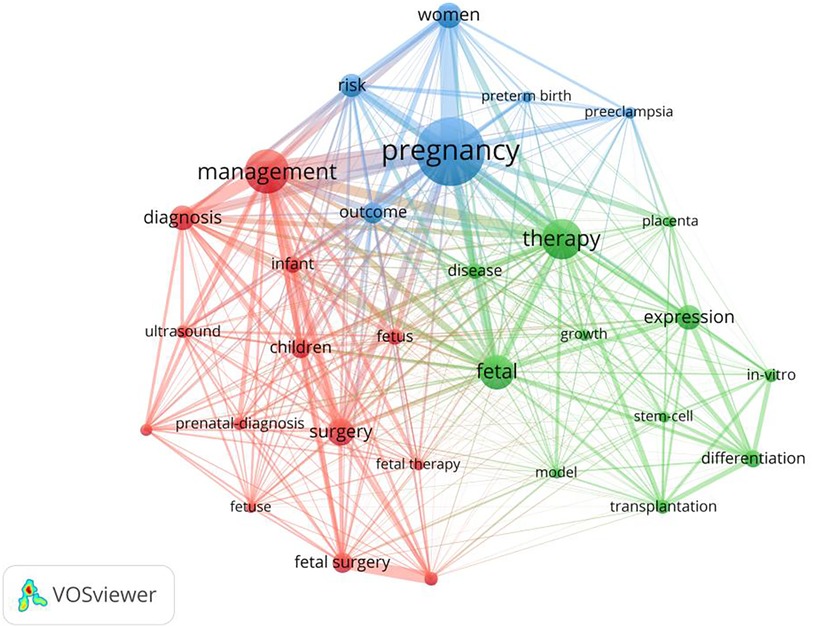
Figure 5. Map network of top 30 keywords. Different colors indicate different clusters, and the size of circles indicate the number of publications.
As shown in Figure 6, in the keyword cluster analysis, keywords were divided into 20 clusters, including #0 cell therapy, #1 congenital diaphragmatic hernia, #2 expression, #3 gene therapy, #4 congenital heart disease, #5 HIV, #6 gestational diabetes mellitus, #7 fetal surgery, #8 gestational age, #9 pregnant women, #10 preterm birth, #11 twin–twin transfusion syndrome, #12 laser coagulation, #13 pulmonary hypoplasia, #14 differentiation, #15 risk factor, #16 mesenchymal stem cells, #17 myelomeningocele, #18 management, #19 fetal fibronectin, and #20 twin pregnancy.
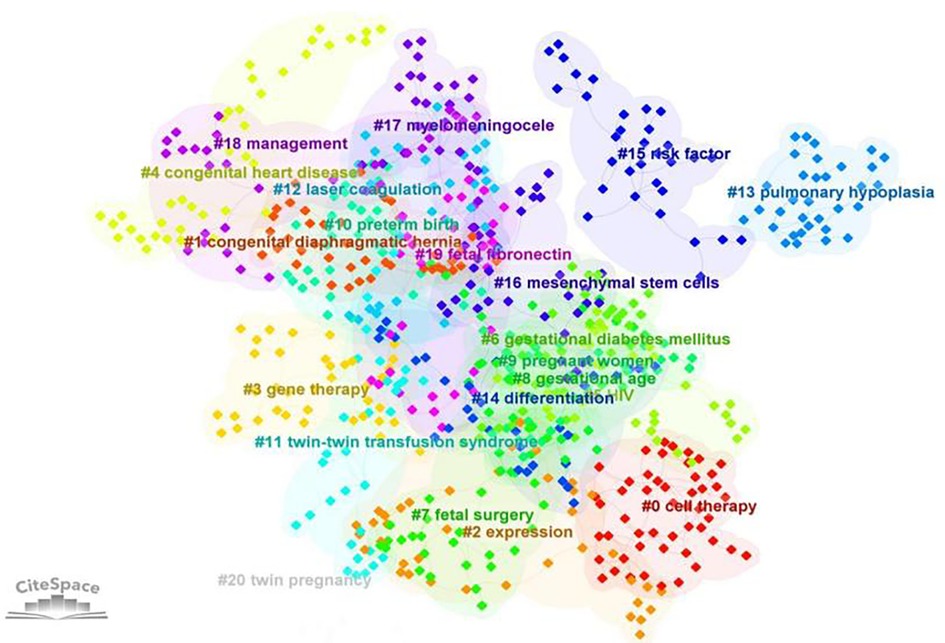
Figure 6. Clustering analysis of keywords (the order is from 0 to 22; the smaller the number, the more keywords the cluster contains, and therefore the more attention it receives).
CiteSpace was used to detect frequently occurring words, known as citation burst keywords, which can reflect cutting-edge topics and research development. Figure 7 shows the top 25 keywords with the strongest citation burst. The keyword burst analysis continued until 2022, indicating the hotspots in fetal therapy research. Of the 25 keywords detected, magnetic resonance imaging (MRI), guideline, and fetoscopic laser surgery are hot topics in recent years.
Cocitation analysis focused on research topics that were closely related to a particular field. To further explore its development trend, Table 4 lists our top ten cited studies during the study period. Overall, articles have low citation rates when newly published; thus, the current citation count may lag behind the actual value of articles. We also identified the top 25 articles with the strongest citation outbreak (Figure 8), which is considered an indicator of research frontiers or emerging trends. Combining these two study types helped identify key studies that influenced developments in fetal therapy.
We selected 9,715 articles on fetal therapy from January 2012 to December 2022 for bibliometric analysis and visualization and used study-retrieval programs VOSviewer and CiteSpace to obtain a more accurate and clear understanding of the latest research trends in this field. During the studied decade, the number of research articles on fetal therapy has steadily increased steadily, and the number of citations has also shown an obvious upward trend. Information on the characteristics of the study areas can be obtained from the country/region, institution, and author analysis. The main body of fetal therapy research is primarily concentrated in the United States, China, Britain, and other countries, and the fetal research centers in these countries are the main force of published studies in this field. In the author analysis, not much difference was observed in the number of published articles, which indicates a large space for exploration. Currently, most fetal studies are single-center studies with less cross-institutional communication. In the future, fetal studies should gradually move toward multicenter research.
The top ten cocited literature mainly included myelomeningocele surgeries, fetal laser treatment of twin transfusion syndrome, and ultrasound examination of twin pregnancy. The fetal surgeries related to myelomeningocele and twin transfusion syndrome are more mature in clinical application, twin ultrasound examination is completely different from that performed in single pregnancies, and prenatal monitoring is needed to avoid adverse complications.
By showing the co-cited references, the relationship between many topics in the discipline is sorted, and the reader is more familiar with it. From the strongest citation burst, the top 25 articles reflect the mature clinical application of diseases in fetal therapy, and the twin–twin transfusion syndrome, myelomeningocele, diaphragmatic hernia, and fetal congenital heart diseases are still the hotspots of research improvement in recent years. To avoid complications following treatment, cell therapy has gradually entered the field of view of researchers and become a hot research topic in recent years. By combining the most frequently cited references with the strongest citation burst, high values that contribute to the field of fetal therapy were identified. It is an essential reference for researchers who want to understand fetal therapy.
Our keyword clusters cover the influence of maternal factors on fetal therapy, indications for fetal therapy, therapeutic means, and complications of fetal therapy, and these are major research topics in this field.
Fetal surgery, gene therapy, and stem cell therapy were the main fetal therapies mentioned in the keyword analysis in the past ten years. Fetal surgery, as a form of correction treatment, is a major topic in fetal therapy (14–26). At present, more mature fetal surgical methods include open hysterotomy, small-access hysteroscopy, and image-guided percutaneous fetal access (27), which have been applied to diseases such as twin–twin transfusion syndrome, thoracic malformations, fetal airway obstructions, myelomeningocele, and aortic valve stenosis (28). Although problems such as premature rupture of membranes and fetal distress may occur after invasive treatment (29), the benefits of fetal treatment against those of congenital diaphragmatic hernia, severe congenital heart disease (30–32), myelomeningocele, and twin transfusion syndrome must be evaluated to determine the fetal treatment that has achieved some success. The benefits of fetal surgical treatment outweigh the risks associated with treatment, resulting in increased survival and improved organ function. Surgical treatment of severe congenital diaphragmatic hernia can significantly improve the survival rate at discharge (33–37). The modified laser surgery for twin–twin transfusion syndrome has reduced complications associated with previous conventional laser treatment (38), and surgical repair of a fetus with myelomeningocele has led to a significant reduction in hindbrain herniation, decrease in shunt-dependent hydrocephalus, and improvement in lower-extremity motor function (39, 40).
Accordingly, gene therapy and cell therapy have become new hot topics (41–43). Gene therapy has yielded satisfactory results in theoretical studies on animal models of hemophilia, muscular dystrophy, central nervous system disease, cystic fibrosis, and other pulmonary diseases (44). Maternal risk from prenatal gene therapy is more dependent on the carrier used and the mode of administration.So far, animal studies have not demonstrated any significant germline or maternal effect of prenatal gene therapy (45). This has considerably helped gene therapy research advancement and exploration.
In cell therapy, hematopoietic stem cell research (46) is in the stage of animal models; however, it has successfully overcome important obstacles such as engraftment and immune barriers (47). It possesses the potential to be used in the clinical treatment of fetal anemia in the future. Prenatal mesenchymal stem cell (MSC) transplantation has been used to treat fetal osteogenesis imperfecta in humans, which is characterized by lower levels of bone implantation and improved linear growth, mobility, and fracture rates after treatment, compared with other patients with osteogenesis imperfecta. These favorable results make fetal MSC transplantation a promising option for clinical applications (48). Furthermore, in an animal model that is prone to preterm birth and premature rupture of membranes after surgery, transamniotic stem cell therapy enhanced postoperative recovery to a greater extent by promoting local tissue regeneration at the surgical site (49). The use of biological cells or collagen replacement drugs can also reduce the incidence of complications of inclusion body cysts and myelomeningocele surgery (50), which has implications for the perfection of myelomeningocele surgery.
The most common complications of fetal surgery are premature delivery, premature rupture of membranes,and placental abruption, etc. In recent years, the artificial uterus (AU) has also introduced brought a new perspective to fetal therapy. AU attempts to mimic the human nature environment of the human uterus and thus, provides an alternative for placental insufficiency or high-risk pregnancy caused by premature membrane rupture. For example, AU supports the lungs to mature to the extent where independent normal breathing is achieved, avoids immature development, and infection, and reduces the incidence of premature brain injury, such as intracranial hemorrhage in preterm infants. Interestingly, AU has social benefits as well. AU enables infertile couples and women who have undergone hysterectomy to achieve pregnancy. Thus, the AU is a useful surrogate alternative. However, AU cannot fully replicate the role of placenta in fetal development, such as gas exchange, metabolic waste product clearance, nutritional support, development of temporary immune system, production of various hormones, enzymes and cytokines to support the growth of the fetus. Thus, AU cannot completely duplicate uterine function, but it can be considered as a type of fetal treatment.
Keywords with strong burst strength are implicated as those that received special attention by the scientific community during a specific period and could therefore represent research hotspots and frontiers of a special field or subject in one period. Therefore, three research hotspots were selected for in-depth analysis, namely, fetoscopic laser surgery, guideline, and MRI, which are the current research frontiers.
At present, fetal laser therapy for twin–twin transfusion syndrome (51) is relatively mature; however, postoperative neurodysplasia may occur (52, 53), which still needs further improvement. Over the past decade, fetal laser therapy for chorionic angioma, sacrococcygeal teratoma, lower urinary tract obstruction, chest mass, and airway obstruction have emerged, and more post-treatment data are needed in the future to determine whether fetal laser therapy is a more beneficial treatment option (54–56).
Moreover, magnetic resonance examination, as an effective supplement to prenatal ultrasound diagnosis, can reflect the complex anatomical structure more directly and play an important role in late fetal imaging (57). It has contributed to the determination of fetal intrauterine intervention indications, intervention program selection, organ function evaluation, and so on. For example, in severe congenital heart disease, MRI can accurately reflect the fetal heart anatomy, tetralogy of Fallot in the absence of a pulmonary valve, severe Ebstein malformations with heart failure, or in some cases severe pulmonary comorbidities due to bronchial or lung compression or lung dysplasia. It can be used for fetal risk assessment and management planning (30), and additional information on fetal circulation distribution and fetal oxygen transport can be obtained, which opens the possibility for minimally invasive fetal cardiac interventional therapy and the development of fetal management strategies with neuroprotective effect (58). The research focuses on T1- and T2-weighted imaging, diffusion tensor imaging, quantitative imaging of T1/T2 relaxation time, myelin water fraction, and magnetization transfer ratio in nervous system diagnosis. Other emerging MRI techniques, such as magnetic sensitive mapping and phase imaging, are expected to help characterize the microstructure of the developing white matter (59, 60). In the digestive system, MRI allows for the visualization of the extent and pattern of dilated bowel loops (61). In the skeletal musculoskeletal system, the length and morphology of the ossified bone can be assessed, and three-dimensional imaging can provide a comprehensive assessment of fetal bone morphology. Dynamic MRI helps describe distal limb abnormalities, fetal dyskinesia, and contraction (62).
The American College of Obstetricians and Gynecologists, American Academy of Pediatrics, and Maternal-Fetal Medical Society regularly issue guidelines on the screening, diagnosis, and treatment of fetal diseases to provide standardized methods for the prenatal diagnosis and management of fetal diseases, achieve standardized and safe diagnosis and treatment, and maximize clinical care. Continuous updating of clinical guidelines in fetal therapy also prompts researchers to pay attention to recent problems and make targeted exploration to achieve qualitative breakthroughs in fetal therapy.
HIV is commonly used as an exclusion criterion in studies on fetal treatment. Elrod J (63) attempted to repair fetal spina bifida in a pregnant woman with HIV infection, and the fetus was confirmed to be free of HIV infection after birth. This case report also brought some inspiration to fetal treatment. While emphasizing the progress of overall medical research, we should also monitor progress in individualized medicine, rather than strictly following outdated standards.
The effective control of diseases during pregnancy is also an important aspect of protecting the fetus. The specificity of prenatal treatment lies in the relationship between the mother and fetus. When treating the fetus, the safety of the pregnant woman should also be ensured, and the negative effect on the fetus should also be ensured in the control of pregnancy diseases. For example, zidovudine as an antiretroviral therapy during pregnancy affects fetal cardiac function (64, 65), glyburide has limited control of gestational diabetes mellitus, and metformin is associated with preterm birth (66–71). Angiotensin-converting enzyme inhibitors should be avoided for hypertension in early pregnancy (72, 73).
The research content and hotspots in the past decade reflect the gradual progress of fetal therapy; thus, more fetal therapy methods will be applied to clinical practice in the future.Based on the frequency of the keywords (Table 5), we listed the current fetal diseases in fetal therapy to provide clarity on the diseases that concern fetal therapy research.
This study still has some limitations. First, data sources were limited to the WOS. Given the limitations of existing software, directly combining search results from multiple databases is not possible. We used WOS instead of other databases, such as Embase and PubMed. It is recognized as the most commonly used and suitable bibliometric database in scientometrics in a format that can be directly recognized by the metrological software used in this study. In addition, the language was limited to English and therefore did not include few multilingual studies. The effect of recently published high-quality articles may be underestimated because they may not have had enough time to accumulate enough citations. A follow-up study is necessary to assess the effect of these articles on the field. This study analyzed the research on fetal therapy in the past decade and summarized the current status and research trends in this field, which is believed to provide some reference for future studies.
To our knowledge, this study provides the first multidimensional analysis of fetal therapy studies from a bibliometric perspective. By reviewing the publications in the past decade, this bibliometrics visually presents fetal therapy research, including publication trends, institutions, authors, and country analyses, summarizes research content, and identifies research hotspots. These results allow the research community to identify emerging themes and frontiers to guide future fetal therapy research.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YJ: Data curation, Formal analysis, Writing – original draft. XL: Data curation, Writing – original draft. LL: Data curation, Methodology, Writing – original draft. HM: Data curation, Writing – original draft. CX: Data curation, Writing – original draft. JZ: Data curation, Writing – original draft. RX: Supervision, Writing – review & editing. LY: Supervision, Writing – review & editing. LX: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by National Natural Science Foundation of China (82120108015, 82102020, 82071874, 81971586), Sichuan Province Key R & D Project (2023YFS0020) and Sichuan Science and Technology Program (2020YJ0029, 2017TD0005, 23ZDYF2519).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bergh E, Buskmiller C, Johnson A. The future of fetal surgery. Obstet Gynecol Clin North Am. (2021) 48(4):745–58. doi: 10.1016/j.ogc.2021.06.004
2. David AL, Spencer RN. Clinical assessment of fetal well-being and fetal safety indicators. J Clin Pharmacol. (2022) 62 Suppl 1(Suppl 1):S67–78. doi: 10.1002/jcph.2126
3. Grandt J, Gottschalk I, Geipel A, Gembruch U, Simonini C, Weber E, et al. Intrauterine thoracoamniotic shunting of fetal hydrothorax with the somatex intrauterine shunt: intrauterine course and postnatal outcome. J Clin Med. (2022) 11(9):2312. doi: 10.3390/jcm11092312
4. Meller CH, Grinenco S, Aiello H, Córdoba A, Sáenz-Tejeira MM, Marantz P, et al. Congenital heart disease, prenatal diagnosis and management. Arch Argent Pediatr. 2020;118(2):e149–61. English, Spanish. doi: 10.5546/aap.2020.eng.e149
5. van Mieghem T, Baud D, Devlieger R, Lewi L, Ryan G, De Catte L, et al. Minimally invasive fetal therapy. Best Pract Res Clin Obstet Gynaecol. (2012) 26(5):711–25. doi: 10.1016/j.bpobgyn.2012.03.005
6. Harrison MR, Filly RA, Golbus MS, Berkowitz RL, Callen PW, Canty TG, et al. Fetal treatment 1982. N Engl J Med. (1982) 307(26):1651–2. doi: 10.1056/NEJM198212233072623
7. Moon-Grady AJ, Baschat A, Cass D, Choolani M, Copel JA, Crombleholme TM, et al. Fetal treatment 2017: the evolution of fetal therapy centers—a joint opinion from the international fetal medicine and surgical society (IFMSS) and the north American fetal therapy network (NAFTNet). Fetal Diagn Ther. (2017) 42(4):241–8. doi: 10.1159/000475929
8. Guler AT, Waaijer CJ, Palmblad M. Scientific workflows for bibliometrics. Scientometrics. (2016) 107:385–98. doi: 10.1007/s11192-016-1885-6
9. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
10. Dhombres F, Bodenreider O. Trends in fetal medicine: a 10-year bibliometric analysis of prenatal diagnosis. Stud Health Technol Inform. (2017) 245:853–7. doi: 10.3233/978-1-61499-830-3-853
11. Arruda H, Silva ER, Lessa M, Proença D Jr, Bartholo R. VOSviewer and bibliometrix. J Med Libr Assoc. (2022) 110(3):392–5. doi: 10.5195/jmla.2022.1434
12. van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
13. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
14. Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. (2011) 364(11):993–1004. doi: 10.1056/NEJMoa1014379
15. Belfort MA, Whitehead WE, Shamshirsaz AA, Bateni ZH, Olutoye OO, Olutoye OA, et al. Fetoscopic open neural tube defect repair: development and refinement of a two-port, carbon dioxide insufflation technique. Obstet Gynecol. (2017) 129(4):734–43. doi: 10.1097/AOG.0000000000001941
16. Pedreira DA, Zanon N, Nishikuni K, Moreira de Sá RA, Acacio GL, Chmait RH, et al. Endoscopic surgery for the antenatal treatment of myelomeningocele: the CECAM trial. Am J Obstet Gynecol. (2016) 214(1):111.e1–111.e11. doi: 10.1016/j.ajog.2015.09.065
17. Moldenhauer JS, Soni S, Rintoul NE, Spinner SS, Khalek N, Martinez-Poyer J, et al. Fetal myelomeningocele repair: the post-MOMS experience at the children’s hospital of Philadelphia. Fetal Diagn Ther. (2015) 37(3):235–40. doi: 10.1159/000365353
18. Farmer DL, Thom EA, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, et al. The management of myelomeningocele study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol. (2018) 218(2):256.e1–256.e13. doi: 10.1016/j.ajog.2017.12.001
19. Kabagambe SK, Jensen GW, Chen YJ, Vanover MA, Farmer DL. Fetal surgery for myelomeningocele: a systematic review and meta-analysis of outcomes in fetoscopic versus open repair. Fetal Diagn Ther. (2018) 43(3):161–74. doi: 10.1159/000479505
20. Johnson MP, Bennett KA, Rand L, Burrows PK, Thom EA, Howell LJ, et al. The management of myelomeningocele study: obstetrical outcomes and risk factors for obstetrical complications following prenatal surgery. Am J Obstet Gynecol. (2016) 215(6):778.e1–e9. doi: 10.1016/j.ajog.2016.07.052
21. Slaghekke F, Lopriore E, Lewi L, Middeldorp JM, van Zwet EW, Weingertner AS, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet. (2014) 383(9935):2144–51. doi: 10.1016/S0140-6736(13)62419-8
22. Tulipan N, Wellons JC 3rd, Thom EA, Gupta N, Sutton LN, Burrows PK, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr. (2015) 16(6):613–20. doi: 10.3171/2015.7.PEDS15336 Erratum in: J Neurosurg Pediatr. 2022 Nov 04;31(1):87-89. PMID: 26369371; PMCID: PMC5206797.26369371
23. Khalil A, Rodgers M, Baschat A, Bhide A, Gratacos E, Hecher K, et al. ISUOG Practice guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. (2016) 47(2):247–63. doi: 10.1002/uog.15821. Erratum in: Ultrasound Obstet Gynecol. 2018 Jul;52(1):140. PMID: 26577371.26577371
24. Lin TY, Wataganara T, Shaw SW. From non-invasive to invasive fetal therapy: a comprehensive review and current update. Taiwan J Obstet Gynecol. (2021) 60(4):595–601. doi: 10.1016/j.tjog.2021.05.004
25. Dick JR, Wimalasundera R, Nandi R. Maternal and fetal anaesthesia for fetal surgery. Anaesthesia. (2021) 76(Suppl 4):63–8. doi: 10.1111/anae.15423
27. Kunisaki SM, Jennings RW. Fetal surgery. J Intensive Care Med. (2008) 23(1):33–51. doi: 10.1177/0885066607310240
28. Sudhakaran N, Sothinathan U, Patel S. Best practice guidelines: fetal surgery. Early Hum Dev. (2012) 88(1):15–9. doi: 10.1016/j.earlhumdev.2011.11.006
29. Teefey CP, Soni S, Khalek N. Maternal fetal surgery: intervention and management. Clin Obstet Gynecol. (2020) 63(2):455–67. doi: 10.1097/GRF.0000000000000534
30. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American heart association. Circulation. (2014) 129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d Erratum in: Circulation. 2014 May 27;129(21):e512.24763516
31. Freud LR, Tworetzky W. Fetal interventions for congenital heart disease. Curr Opin Pediatr. (2016) 28(2):156–62. doi: 10.1097/MOP.0000000000000331
32. Hunter LE, Simpson JM. Prenatal screening for structural congenital heart disease. Nat Rev Cardiol. (2014) 11(6):323–34. doi: 10.1038/nrcardio.2014.34
33. Wang W, Pei L, Zhang Y, Chen W, Liu J, Jiang Y, et al. Neuraxial anesthesia in ex utero intrapartum therapy for parturients with fetal congenital diaphragmatic hernia: a prospective observational study. Int J Obstet Anesth. (2022) 52:103599. doi: 10.1016/j.ijoa.2022.103599
34. Ruano R, Martinovic J, Aubry MC, Dumez Y, Benachi A. Predicting pulmonary hypoplasia using the sonographic fetal lung volume to body weight ratio–how precise and accurate is it? Ultrasound Obstet Gynecol. (2006) 28(7):958–62. doi: 10.1002/uog.3853
35. Cruz-Martínez R, Shazly S, Martínez-Rodríguez M, Gámez-Varela A, Luna-García J, Juárez-Martínez I, et al. Impact of fetal endoscopic tracheal occlusion in fetuses with congenital diaphragmatic hernia and moderate lung hypoplasia. Prenat Diagn. (2022) 42(3):310–7. doi: 10.1002/pd.5988
36. Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. (2021) 385(2):107–18. doi: 10.1056/NEJMoa2027030
37. Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med. (2021) 385(2):119–29. doi: 10.1056/NEJMoa2026983
38. Diehl W, Diemert A, Hecher K. Twin-twin transfusion syndrome: treatment and outcome. Best Pract Res Clin Obstet Gynaecol. (2014) 28(2):227–38. doi: 10.1016/j.bpobgyn.2013.12.001
39. Yamashiro KJ, Galganski LA, Hirose S. Fetal myelomeningocele repair. Semin Pediatr Surg. (2019) 28(4):150823. doi: 10.1053/j.sempedsurg.2019.07.006
40. Clayton DB, Thomas JC, Brock JW 3rd. Fetal repair of myelomeningocele: current status and urologic implications. J Pediatr Urol. (2020) 16(1):3–9. doi: 10.1016/j.jpurol.2019.11.019
41. Wagner AM, Schoeberlein A, Surbek D. Fetal gene therapy: opportunities and risks. Adv Drug Deliv Rev. (2009) 61(10):813–21. doi: 10.1016/j.addr.2009.04.011
42. Palanki R, Peranteau WH, Mitchell MJ. Delivery technologies for in utero gene therapy. Adv Drug Deliv Rev. (2021) 169:51–62. doi: 10.1016/j.addr.2020.11.002
43. Loukogeorgakis SP, Flake AW. In utero stem cell and gene therapy: current status and future perspectives. Eur J Pediatr Surg. (2014) 24(3):237–45. doi: 10.1055/s-0034-1382260
44. Santore MT, Roybal JL, Flake AW. Prenatal stem cell transplantation and gene therapy. Clin Perinatol. (2009) 36(2):451–71, xi. doi: 10.1016/j.clp.2009.03.006
45. Peranteau WH, Flake AW. The future of in utero gene therapy. Mol Diagn Ther. (2020) 24(2):135–42. doi: 10.1007/s40291-020-00445-y
46. Witt R, MacKenzie TC, Peranteau WH. Fetal stem cell and gene therapy. Semin Fetal Neonatal Med. (2017) 22(6):410–4. doi: 10.1016/j.siny.2017.05.003
47. Pearson EG, Flake AW. Stem cell and genetic therapies for the fetus. Semin Pediatr Surg. (2013) 22(1):56–61. doi: 10.1053/j.sempedsurg.2012.10.010
48. Ekblad-Nordberg Å, Walther-Jallow L, Westgren M, Götherström C. Prenatal stem cell therapy for inherited diseases: past, present, and future treatment strategies. Stem Cells Transl Med. (2020) 9(2):148–57. doi: 10.1002/sctm.19-0107
49. Hii LY, Sung CA, Shaw SW. Fetal surgery and stem cell therapy for meningomyelocele. Curr Opin Obstet Gynecol. (2020) 32(2):147–51. doi: 10.1097/GCO.0000000000000614
50. Kunpalin Y, Subramaniam S, Perin S, Gerli MFM, Bosteels J, Ourselin S, et al. Preclinical stem cell therapy in fetuses with myelomeningocele: a systematic review and meta-analysis. Prenat Diagn. (2021) 41(3):283–300. doi: 10.1002/pd.5887
51. Lopriore EA, Slaghekke F, Verweij EJ, Haak MC, Middeldorp AJM, Lopriore E. Neonatal outcome in twin-to-twin transfusion syndrome Not treated with fetoscopic laser surgery. Twin Res Hum Genet. (2022) 25(1):45–9. doi: 10.1017/thg.2022.5
52. Behrendt N, Galan HL. Twin-twin transfusion and laser therapy. Curr Opin Obstet Gynecol. (2016) 28(2):79–85. doi: 10.1097/GCO.0000000000000247
53. Gupta N. Surgical techniques for open fetal repair of myelomeningocele. Childs Nerv Syst. (2017) 33(7):1143–8. doi: 10.1007/s00381-017-3439-5
54. Peiro JL, Nolan HR, Alhajjat A, Diaz R, Gil-Guevara E, Tabbah SM, et al. A technical look at fetoscopic laser ablation for fetal laryngeal surgical recanalization in congenital high airway obstruction syndrome. J Laparoendosc Adv Surg Tech A. (2020) 30(6):695–700. doi: 10.1089/lap.2019.0808
55. Torres Montebruno X, Martinez JM, Eixarch E, Gómez O, García Aparicio L, Castañón M, et al. Fetoscopic laser surgery to decompress distal urethral obstruction caused by prolapsed ureterocele. Ultrasound Obstet Gynecol. (2015) 46(5):623–6. doi: 10.1002/uog.14876
56. Mathis J, Raio L, Baud D. Fetal laser therapy: applications in the management of fetal pathologies. Prenat Diagn. (2015) 35(7):623–36. doi: 10.1002/pd.4587
57. Welsh RC, Nemec U, Thomason ME. Fetal magnetic resonance imaging at 3.0 T. Top Magn Reson Imaging. (2011) 22(3):119–31. doi: 10.1097/RMR.0b013e318267f932
58. Sun L, Macgowan CK, Portnoy S, Sled JG, Yoo SJ, Grosse-Wortmann L, et al. New advances in fetal cardiovascular magnetic resonance imaging for quantifying the distribution of blood flow and oxygen transport: potential applications in fetal cardiovascular disease diagnosis and therapy. Echocardiography. (2017) 34(12):1799–803. doi: 10.1111/echo.13760
59. Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. (2014) 276:48–71. doi: 10.1016/j.neuroscience.2013.12.044
60. Tee LM, Kan EY, Cheung JC, Leung WC. Magnetic resonance imaging of the fetal brain. Hong Kong Med J. (2016) 22(3):270–8. doi: 10.12809/hkmj154678
61. Marine MB, Forbes-Amrhein MM. Magnetic resonance imaging of the fetal gastrointestinal system. Pediatr Radiol. (2020) 50(13):1895–906. doi: 10.1007/s00247-020-04677-2
62. Chauvin NA, Victoria T, Khwaja A, Dahmoush H, Jaramillo D. Magnetic resonance imaging of the fetal musculoskeletal system. Pediatr Radiol. (2020) 50(13):2009–27. doi: 10.1007/s00247-020-04769-z
63. Elrod J, Ochsenbein-Kölble N, Mazzone L, Zimmermann R, Berger C, Speck RF, et al. Fetal-Maternal surgery for spina bifida in a HIV-infected mother. Fetal Diagn Ther. (2022) 49(1-2):25–8. doi: 10.1159/000521788
64. García-Otero L, López M, Goncé A, Fortuny C, Salazar L, Valenzuela-Alcaraz B, et al. Cardiac remodeling and hypertension in HIV-uninfected infants exposed in utero to antiretroviral therapy. Clin Infect Dis. (2021) 73(4):586–93. doi: 10.1093/cid/ciab030
65. García-Otero L, López M, Gómez O, Goncé A, Bennasar M, Martínez JM, et al. Zidovudine treatment in HIV-infected pregnant women is associated with fetal cardiac remodelling. AIDS. (2016) 30(9):1393–401. doi: 10.1097/QAD.0000000000001066
66. Kallem VR, Pandita A, Pillai A. Infant of diabetic mother: what one needs to know? J Matern Fetal Neonatal Med. (2020) 33(3):482–92. doi: 10.1080/14767058.2018.1494710
67. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. (2010) 23(3):199–203. doi: 10.3109/14767050903550659
68. Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes Mellitus. Endocr Rev. (2022) 43(5):763–93. doi: 10.1210/endrev/bnac003
69. Subiabre M, Silva L, Toledo F, Paublo M, López MA, Boric MP, et al. Insulin therapy and its consequences for the mother, foetus, and newborn in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. (2018) 1864(9 Pt B):2949–56. doi: 10.1016/j.bbadis.2018.06.005
70. Young BC, Ecker JL. Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment? Curr Diab Rep. (2013) 13(1):12–8. doi: 10.1007/s11892-012-0338-8
71. Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. Br Med J. (2015) 350:h102. doi: 10.1136/bmj.h102
72. Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones (Athens). (2015) 14(2):211–23. doi: 10.14310/horm.2002.1582
Keywords: fetal therapy, bibliometric analysis, trend, CiteSpace, VOSviewer
Citation: Jia Y, Liang X, Liu L, Ma H, Xu C, Zeng J, Xu R, Ye L and Xie L (2024) Trends in research related to fetal therapy from 2012 to 2022: a bibliometric analysis. Front. Pediatr. 11:1288660. doi: 10.3389/fped.2023.1288660
Received: 11 September 2023; Accepted: 13 December 2023;
Published: 4 January 2024.
Edited by:
Chiara Grimaldi, Meyer Children’s Hospital, ItalyReviewed by:
Giulia Fusi, Meyer Children’s Hospital, Italy© 2024 Jia, Liang, Liu, Ma, Xu, Zeng, Xu, Ye and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Ye Y2x0d29AMTYzLmNvbQ== Linjun Xie eGllbGluanVuMjAxNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Abbreviations TLS, total chain strength; MSC, mesenchymal stem cell; MRI, magnetic resonance imaging.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.