- 1National Pediatrics Center for Familial Mediterranean Fever, “Arabkir” Medical Complex-Institute of Child and Adolescent Health, Yerevan, Armenia
- 2Department of Pediatrics, Yerevan State Medical University, Yerevan, Armenia
- 3Center of Medical Genetics and Primary Health Care, Yerevan, Armenia
- 4Department of Medical Genetics, Yerevan State Medical University, Yerevan, Armenia
- 5Department of Public Health and Health Care Organization, Yerevan State Medical University, Yerevan, Armenia
- 6Nutrition Research Unit, Children’s University Hospital in Zürich, Zürich, Switzerland
Inflammatory bowel disease (IBD) and familial Mediterranean fever (FMF) are inflammatory diseases with complex interactions among genetic, immune, and environmental factors. FMF is a monogenic autoinflammatory disease, characterized by recurrent febrile attacks and polyserositis, and is manifested mainly in childhood. FMF is widespread in Armenia. There are reports on the concurrent occurrence of FMF and IBD. MEFV gene mutations may have a disease-modifying effect on IBD. We have investigated the frequency of MEFV mutations and FMF in Armenian children with IBD and their influence on the clinical course. A total of 69 untreated IBD patients under 18 years of age were enrolled: 52.1% (36) had ulcerative colitis (UC), 21.7% (15) had Crohn's disease (CD), and 26.0% (18) had unclassified colitis (IBD-U). The frequency of FMF among them was 36.2% (25/69), and MEFV mutations were identified in 53.6% (37/69). The highest rate of MEFV mutations and FMF was in UC patients (61.1% and 41.6% respectively). In all, 56.7% (21/37) of IBD patients with MEFV mutations had M694V mutated alleles, mainly in compound heterozygous and heterozygous states. There were no associations in the group of IBD patients with coexisting FMF (25), either between any MEFV mutation and type of IBD or coexistence of FMF. Overall, 36.0% (9/25) of them developed VEO IBD and carried mainly the M694V mutation. We concluded that the carrier frequency of MEFV mutations among Armenian pediatric IBD patients was rather high (53.6%), especially for UC. It was suggested that the MEFV gene is not necessarily a susceptibility gene but most likely modifies the course of IBD. MEFV genetic testing was recommended for Armenian pediatric IBD patients, especially for VEO UC and IBD-U, atypical IBD course, or resistance to the conventional treatment. They should also be asked for isolated febrile attacks, recurrent arthritis, and family history, even in the absence of FMF typical symptoms, to rule out FMF and its complications.
1 Introduction
Inflammatory bowel disease (IBD)—comprising ulcerative colitis (UC), Crohn's Disease (CD), and unclassified colitis (IBD-U)—is the most common form of chronic intestinal inflammation, which has a multifactorial etiology with complex interactions among genetic, immune, and environmental factors (1). Familial Mediterranean fever (FMF; ОMIM 249100) is another inflammatory disease as well. It is the most common autosomal recessively inherited monogenic autoinflammatory disease in the group of hereditary periodic fever syndromes (HPFS), mostly found in Sephardic Jews, Armenians, Turks, and Arabs (2, 3). It is characterized by recurrent attacks of fever and painful aseptic peritonitis, pleuritis, synovitis, elevated acute phase of reactants, and its most severe complication—secondary amyloidosis (4–7). Being an ethnic disease, FMF is widespread in Armenia, with a high rate of MEFV mutation carriers of 1:3–4 (0.21) and a marked FMF prevalence of 54.7 per 10,000 of the total population (8–15). FMF manifests mainly in childhood and often has an early or atypical onset.
The concomitant presence of MEFV mutations in diseases other than FMF may modify their presentation and severity. IBD and FMF are inflammatory disorders sharing some common clinical features such as recurrent abdominal pain, diarrhea, fever, and arthralgia/arthritis, etc. UC and FMF are both characterized by a recurrent pattern of presentation with periods of remission and flares associated with neutrophilic infiltration at the site of injury and dysregulation of apoptosis (16, 17). The MEFV gene, responsible for FMF, is involved in inflammatory reactions through altered leukocyte apoptosis, secretion of interleukin-1beta (IL-1b), activation of the NF-kappa B pathway, and, thereby, the degree of inflammation. IBD appears to be more frequent in FMF-matched ethnic populations. There are reports on the concurrent occurrence of FMF and IBD. As known, the level of awareness of FMF is far from being sufficient, and it is assumed that there may be many patients with FMF who are under observation without an accurate diagnosis (17–20). The possibility of developing intestinal vasculitis in FMF is also suggested (21, 22).
Despite the presence of sufficient data on the possible link between the pathogenesis of IBD and FMF, the question of whether UC and CD co-exist or are associated with FMF remains open. It is known that susceptible CD loci localize to chromosome 16q and contain the NOD2-CARD15 CD susceptibility gene. On the other hand, the gene responsible for FMF, MEFV, is in chromosome 16p13 encoding for pyrin, which has been linked to the apoptotic cascade through the caspase recruitment domain (CARD). It is suggested that the NOD2/CARD15 gene product belongs to the same superfamily of death domain protein. In practice, the concurrent occurrence of FMF and CD remains to be demonstrated. MEFV mutations are not associated with Crohn's disease susceptibility, yet the presence of these mutations appears to be associated with a structuring disease pattern, and extraintestinal disease MEFV mutations may have a disease-modifying effect on IBD (18, 19, 23, 24).
On the other hand, studies on the concordance of FMF and IBD, especially with VEO, have suggested that there is a relationship between IBD and FMF (24–27). Several studies have shown that patients with a diverse spectrum of rare genetic disorders can present with inflammatory bowel disease (monogenic IBD). Patients with these disorders often develop symptoms during infancy or early childhood, along with endoscopic or histological features of Crohn's disease, ulcerative colitis, or IBD unclassified. Defects in interleukin-10 signaling have a Mendelian inheritance pattern with complete penetrance of intestinal inflammation. Several genetic defects that disturb intestinal epithelial barrier function or affect innate and adaptive immune function have incomplete penetrance of the IBD-like phenotype. Due to the broad spectrum of these extremely rare diseases, a correct diagnosis is frequently a challenge and often delayed. In many cases, these diseases cannot be categorized based on standard histological and immunologic features of IBD. Genetic analysis is required to identify the cause of the disorder and offer the patient appropriate treatment options (26, 28–30)
2 Material and methods
2.1 Study setting and data collection methods
The aim of this study was to investigate the frequency of MEFV gene mutations and FMF disease in Armenian children with IBD and their influence on the IBD clinical course.
The objectives were the following: (1) to evaluate the spectrum of MEFV gene mutations and genotypes in Armenian children with IBD; (2) to find out the possible association between MEFV genotypes and the type of IBD and their influence on the IBD clinical course.
The cohort of patients with confirmed IBD patients were hospitalized in the General Pediatrics Department of Arabkir MC- ICAH in 2014–2016 and followed up in the outpatient clinic of the pediatric gastroenterology service of the same hospital up to 2019. IBD diagnosis was determined according to the ESPGHAN Porto criteria (endoscopic, radiologic, and histologic) (31). The inclusion criteria were the following: (1) age of IBD patients under 18 years;(2) the informed consent of parents to participate in the study. The exclusion criterion was refusal to participate in the study.
FMF diagnosis was confirmed in 25 (36.2%) of the 69 enrolled IBD patients based on the Tel Hashomer international criteria and genetic testing (32, 33). The molecular genetic analysis for 12 MEFV gene mutations, most common for Armenians, was performed for all the IBD patients at the Center of Medical Genetics and Primary Health Care of Armenia (M694V, V726A, M680I (G/C), M680I (G/A), R761H, E148Q, F479l, M694I, K695R, P369S, K695R, I692del) by reverse hybridization, restriction analysis, PCR, and sequencing (8–10). IBD patients with concomitant FMF were also followed up in the FMF outpatient clinic of NPC FMF of Arabkir MC—ICAH during the same period.
The patients and their medical charts were examined and reviewed to determine the demographic (age of IBD and/or FMF onset; age at IBD and/or FMF diagnosis; sex; consanguinity) and clinical characteristics of the IBD and FMF patients (symptoms and their duration, IBD type and location, extraintestinal manifestations, therapy approaches, duration of follow-up). The laboratory evaluations of IBD were based on the ESPGHAN conventional IBD diagnostic criteria: hemoglobin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), thrombocyte count, ferritin, albumin level, and fecal calprotectin at diagnosis. The Pediatric UC Activity Index (PUCAI) and the Pediatric CD Activity Index (PCDAI) were used for evaluating the disease severity (34–36). Proteinuria was also checked during the follow-up (proteinuria of more than 1 g/day needs to exclude the presence of amyloidosis in renal biopsies).
The IBD patients were divided into two groups (with and without FMF) depending on the coexisting FMF disease. They were also distributed into the next two groups (with and without MEFV mutations) depending on the presence of the mutations. All the above-mentioned demographic, clinical, and laboratory parameters were compared between these groups of IBD patients.
2.2 Statistical analysis
After checking the normality of the distribution of variables by the Kolmogorov–Smirnov test, non-parametric statistical tests were applied. All the statistical analyses were performed using the software Biostat for Windows (Version 4.03) and SPSS 16.0. Fisher's exact test or chi-square test was used to compare the frequency of MEFV mutations among the UC, CD, and IBD-U patients and their clinical symptoms. The Mann–Whitney U-test was used for the analysis of some demographic and laboratory data of the IBD patients in the groups with/without MEFV gene mutations and with/without FMF. To compare the two medians, the Kruskal–Wallis test was used. P value <0.05 was always considered statistically significant. To describe the demographic and laboratory numerical data, routine descriptive statistics were used.
3 Results
3.1 Patients’ socio-demographic, clinical, and laboratory characteristics
The cohort of 69 patients with an IBD diagnosis confirmed by the international Porto criteria were hospitalized in the General Pediatrics Department of Arabkir MC- ICAH in 2014–2016 and followed up in the outpatient clinic of the pediatric gastroenterology service of the same hospital up to 2019. There were 45 male and 24 female patients with aged between 3 months and 18 years (mean age: 9.36 ± 0.62) at IBD diagnosis. UC was diagnosed in 36 of 69 (52.1%) IBD patients, CD in 15 (21.7%), and IBD-U in 18 children (26.0%).
All the patients were of Armenian nationality, representing all the regions of the country, and most of them lived in urban settlements. The median age of IBD patients was 10 years (range, 0.3–18) at IBD diagnosis. The male/female ratio was 1.87:1 (45 male and 24 female). The mean age of IBD onset was 9.13 ± 0.65 years (range, 0.3–18).
The diagnosis of both IBD and FMF diseases was performed relatively late (at 9 years and 7 years of age, respectively). In the majority of IBD patients with coexisting FMF, the diagnosis of FMF was confirmed after the IBD (19 of 25), which coincides with the data of other authors (36). The average duration of follow-up after diagnosis of IBD was 58.56 ± 7.44 months.
The IBD location was as follows: ileocolitis 12 (17.4%), colitis 22 (33.9%), and pancolitis 35 (50.7%). The UC patients (36) were evaluated with the PUCAI, and most of them (22) had moderate disease activity, 2 had severe, and 9 had mild disease (PUCAI median 45; range 10–65). All the CD patients (15) had severe disease activity (PCDAI median 50, range 30–80).
FMF was confirmed in 25 of 69 (36.2%) IBD patients. Depending on the IBD type, the frequency of FMF disease was as follows: in 15 of 36 (41.6%) UC patients, in 7 of 18 (38.8%) IBD-U, and in 3 of 15 (20.0%) CD. In 6 (8.6%) IBD patients, FMF was confirmed before IBD diagnosis due to the prior testing for MEFV mutations. Genetic analysis was conducted based on an identified history of recurrent episodes of fever with abdominal pain and/or chest pain and/or arthropathy and/or family history of FMF. Following the diagnosis of FMF, treatment with colchicine was started. The mean age of FMF diagnosis was 7.94 ± 1.13 years. The average duration of FMF follow-up was 74.28 ± 10.32 months.
In IBD patients with FMF, the localization site at IBD was as follows: most UC patients (12 of 15; 80%) had pancolitis, and 3 had colitis; 2 of 3 CD patients developed ileocolonic disease, and 1 had pancolitis; 5 of 7 IBD-U children had left-sided colitis, and 2 had pancolitis.
The UC patients with and without FMF had a moderate disease activity on the PUCAI (median 50 and 37.5, range 20–65 and 10–55, respectively) with no significant difference between the groups (p = 0.23; by Kruskal–Wallis). A total of 12 CD patients without FMF had severe activity on the PCDAI (median 50, range 30–80), while 3 CD patients with FMF also had severe activity on the PCDAI (40, 50 and 60 values).
The most frequent symptoms in IBD patients were hematochezia in 56 (81%) patients, abdominal pain or tenesmus in 48 (69.6%), and diarrhea in 45 (65.2%).
Extraintestinal manifestations were noticed in 12 (17.3%) IBD patients, mainly mono-oligoarthritis in 9 patients (13.0%). Perianal disease developed in 4 (5.8%) CD patients (two fistulae and two abscesses). There was no consanguinity. There were no patients with proteinuria either.
All IBD patients were untreated prior to hospitalization. After confirmation of IBD diagnosis, the appropriate treatment was started in the hospital. A total of 54 patients (78%) were treated with Salofalk, 35 (50.7%) with methylprednisolone, and 49 (71%) were receiving immune suppressive treatment with azathioprine.
Conventional colchicine therapy was used in all 25 IBD patients with coexisting FMF and FMF remission, and a positive response was achieved in 22 (88%) of them. The colchicine dosage was increased up to the maximum level in 2 UC patients and in 1 CD patient with a severe M694V homozygous and compound heterozygous genotypes because of a concomitant flare of FMF.
There was no statistical difference in IBD patients with or without FMF when comparing most of the demographic, clinical, and laboratory characteristics, including extraintestinal symptoms, exacerbation rates (p = 0.32), the number of IBD relapses (p = 0.2), and immunosuppressive therapy (p = 0.5) (Table 1). However, lower ferritin blood level (p = 0.05) and longer hematochezia (p = 0.026) were significantly more often observed in IBD patients with concomitant FMF, possibly due to the chronic immune inflammation. The possibility of developing intestinal vasculitis as an integral part of FMF is suggested by some authors due to the high frequency of occult blood detection in stool during attacks of FMF (37–39).
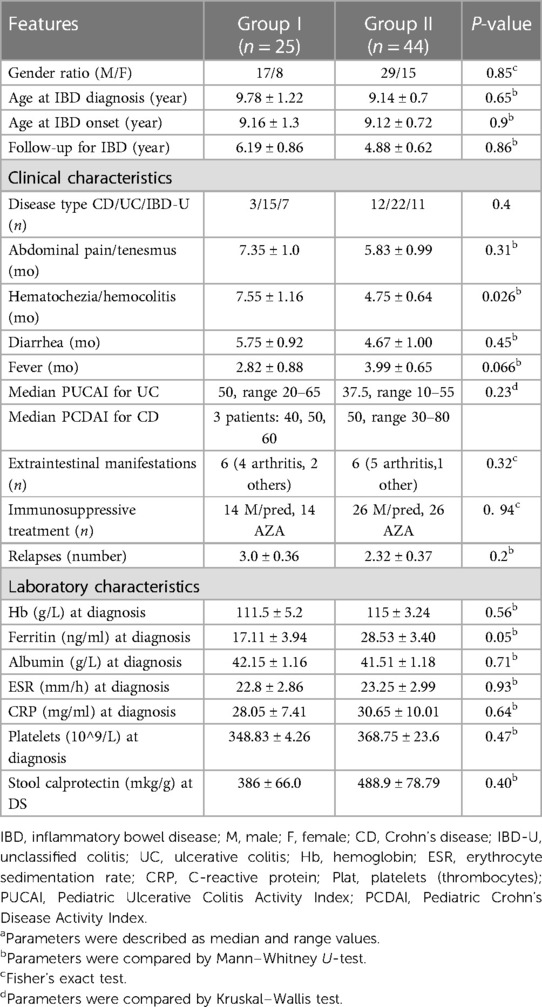
Table 1. Demographic, clinical, and laboratory characteristics of IBD patients with FMF (group I) and without FMF (group II).
All the above-mentioned data were also comparable in most of the features in terms of IBD patients with and without MEFV mutations, and again, no statistically significant difference was found. Particularly, the relationship between the frequency of MEFV mutations and IBD relapses in UC patients (22, 59.4%), IBD-U (11; 29.7%), and CD (4; 10.9%) patients, as well as between the presence of severe M694V mutation and IBD relapses in patients with coexisting FMF (2.67 ± 0.40) and without it (2.54 ± 0.36), was not significant (p = 0.59) (by Mann–Whitney). There was no difference found between the presence of MEFV mutations and the requirement of immunosuppressants, p = 0.5, either. IBD patients of these two groups also had almost the same frequency of extraintestinal symptoms.
3.2 Genetic characteristics of IBD patients with MEFV gene mutations
3.2.1 Distribution of MEFV gene mutations depending on IBD type
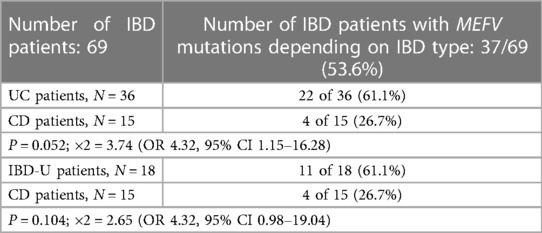
MEFV gene mutations were identified in 37 of 69 (53.6%) IBD patients, and in 33 (47.8%) of them, no MEFV mutations were found. Depending on the IBD type, the frequency of MEFV mutations was as follows: 22 of 36 (61.1%) UC patients, 11 of 18 (61.1%) IBD-U patients, and 4 of 15 (26.7%) CD patients.
As shown in Table 2, the rate of MEFV mutations in UC patients was found to be two times higher than in CD patients (P = 0.052; ×2 = 3.74). The MEFV mutation carriers are four times more likely to have UC than non-carriers (OR 4.32, 95% CI 1.15–16.28); i.e., the presence of MEFV mutation might be considered a risk factor for UC.
Meanwhile, despite the same rate of MEFV mutations (and OR value) in the groups of UC (61.1%; 22 of 36) and IBD-U patients (61.1%; 11 of 18), the comparison of the increased frequency of mutations in the IBD-U group with CD patients was statistically not significant (P = 0.104; ×2 = 2.65) (OR 4.32, 95% CI 0.98–19.04), probably due to the small samples sizes.
3.2.2 Distribution of MEFV gene mutations and genotypes among IBD patients
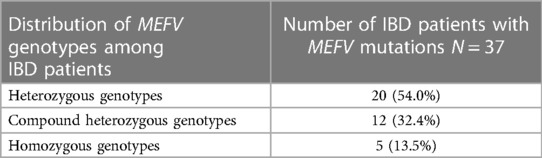
More than half of the IBD patients with MEFV mutations (20 of 37; 54.0%) had heterozygous genotypes, 12 (32.4%) had compound heterozygous genotypes, and 5 (13.5%) had homozygotes (Table 3).
The M694V mutation was the most common and present in 21 of 37 (56.7%) IBD patients with MEFV mutations, mainly in M694V compound heterozygous (10/21; 27%) and heterozygous states (8/21; 21.6%). IBD patients with the MEFV heterozygous genotype had mostly M694V mutation (8 of 17; 47%), less often V726A (6 of 17; 35.2%), and rarely E148Q (2 of 17) and M680I (1 of 17) (Table 4).
3.3 Genetic characteristics of UC patients with MEFV mutations and concomitant FMF
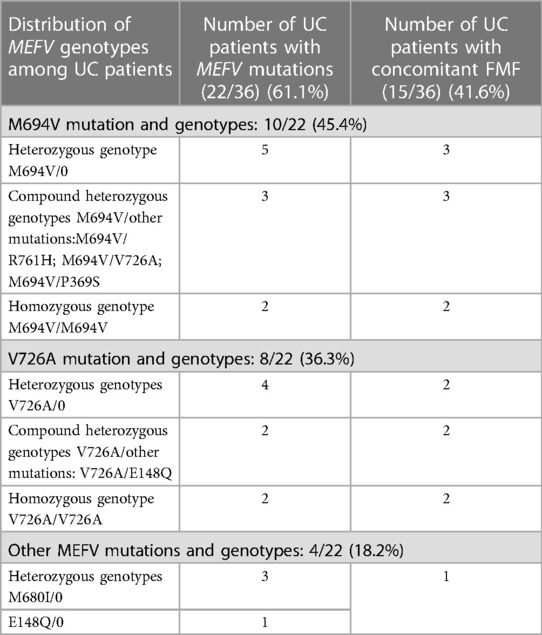
MEFV gene mutations were detected in 22 of 36 (61.1%) UC patients, and most of them had the heterozygous (59.0%; 13 of 22) genotype.
A total of 10 of 22 (45.4%) UC patients with MEFV mutations had severe M694V mutation, mostly (5/10) in heterozygous state (M694V/0), 3/10 patients were had M694V compound heterozygotes (M694V/other mutation), and 2/10 had the M694V homozygous genotype (M694V/M694V). Moreover, 8 of 22 (36.3%) UC patients with MEFV mutations had the V726A mutation, 4 of them were in the heterozygous state (V726A/0), 2 had compound heterozygotes (V726A / E148Q), and 2 had V726A homozygotes (V726A /V726A).
The remaining 4 of the 22 (18.2%) UC patients with MEFV mutations were heterozygotes for other mutations: 3 patients for M680I/0 and 1 for E148Q/0. Most of the UC patients with MEFV mutations had the heterozygous (13 of 22, 59.0%) genotype, mostly for M694V (10 of 22, 45.4%) and V726A (8 of 22, 36.3%) mutated alleles and rarely for M680I (3 of 22, 13.6%).
FMF disease was confirmed in 15 of 36 (41.6%) UC patients, more with compound heterozygous and homozygous genotypes (9/15, 60%) and, again, mainly for the M694V mutation (Table 5). Thus, the M694V mutation and other MEFV mutations were found to be more frequent in UC and IBD-U patients than in CD, but the difference was not significant, p = 0.28.
3.4 Genetic characteristics of CD patients with MEFV mutations and concomitant FMF
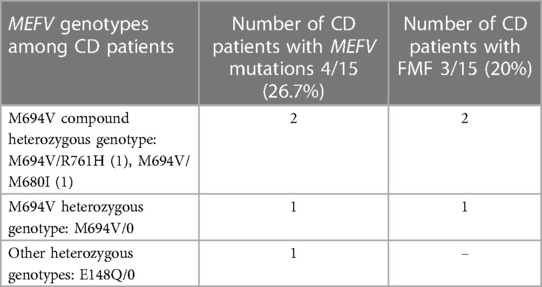
MEFV gene mutations were detected in 4 of 15 (26.7%) CD patients, and 3 (20%) of them had the М694V mutation and confirmed FMF. Just 1 CD patient was the heterozygous carrier for E148Q mutation (without FMF). Thus, FMF disease was confirmed in 3 of 15 (20%) CD patients, with M694V mutation in different states (Table 6).
3.5 Genetic characteristics of IBD-U patients with MEFV mutations and concomitant FMF
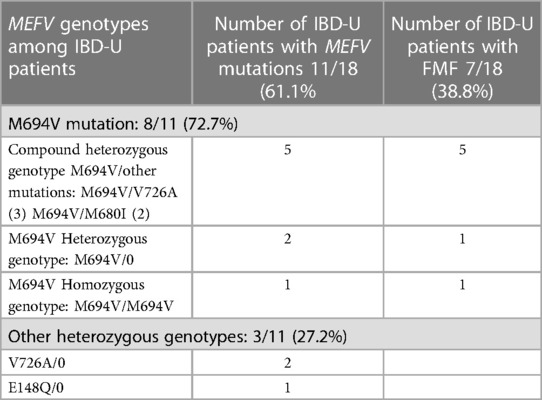
MEFV gene mutations were revealed in 11 of 18 (61.1%) IBD-U patients. In all, 8 (72.7%) had the M694V mutation, mostly (5/8) in the compound heterozygous state (M694V/V726A in 3 patients; M694V/M680I in 2), 2 (18.1%) were heterozygotes (M694V/0), and 1 had the homozygous genotype (M694V/M694V). Furthermore, 3 IBD-U patients with MEFV (27.2%) were heterozygous carriers for other mutations (2 for V726A/0 and 1 for E148Q/0).
Moreover, 7 of 18 (38.8%) IBD-U patients had concomitant FMF disease, and all of them carried the M694V mutation mostly for the M694V compound heterozygous genotypes (5 of 7, 71%). The V726A mutation was the second most frequent after the M694V mutation (Table 7).
Thus, almost half of IBD-U patients with MEFV gene mutations (5/11, 45.4%) had heterozygous genotypes and carried mainly M694V and V726A mutations. FMF disease was confirmed in 7 of 18 (38.8%) IBD-U patients, and all of them had the M694V or V726A mutation, mostly for compound heterozygous and heterozygous genotypes. The majority of UC and IBD-U patients with MEFV mutations had heterozygous genotypes (59.0% and 45.4%, respectively), mostly for M694V and V726A mutated alleles.
3.6 Comparison of the distribution of MEFV gene mutations and genotypes among IBD patients with and without FMF
FMF was confirmed in 25 (36.2%) of 69 IBD patients. Depending on the IBD type the frequency of FMF disease was as follows: 15 of 36 UC patients (41.6%), 7 of 18 IBD-U patients (38.8%), and 3 of 15 CD patients (20.0%).
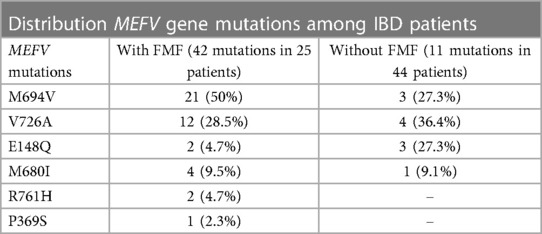
Analysis of the detected MEFV mutations in the group of IBD patients with concomitant FMF (42 mutations in 25 patients) showed that the M694 mutation was the most frequent at 21 of 42 (50%), followed by the V726A mutation in 12 (28.5%), more rarely M680I in 4 (9.5%,), then E148Q in 2 (4.7%), R761H in 2 (4.7%), and, seldom, P369S in 1 (2.3%) (Table 8).
The detection rate of MEFV mutations in IBD patients without FMF (11 mutations in 44 patients) was as follows: 4 (36.4%) V726A, 3 (27.3%) M694V, 3 (27.3%) E148Q, and 1 (9.1%) M680I.
Thus, in IBD patients with FMF (25 patients), the M694V mutation was found in 21 of 42 (50%) of detected mutations. In the group of IBD patients without FMF (44 patients), the M694V mutation was revealed in 3 of 11 (27.3%) of MEFV mutations.
The highest rate of MEFV mutations and FMF disease was recorded in UC patients (61.1% and 41.6%, respectively). However, when comparing the groups of IBD patients with (25 patients) and without FMF (11 patients), we did not find associations either between any MEFV mutation and types of IBD or between MEFV mutations and concomitant FMF (P = 0.28; ×2 = 2.59). In IBD patients with FMF, the M694V mutation was not detected more often than other MEFV mutations. That is, in our cohort of IBD patients, the presence of М694V or other MEFV gene mutations did not depend on concomitant FMF.
3.7 Genetic characteristics of patients with very early-onset IBD (VEO IBD)
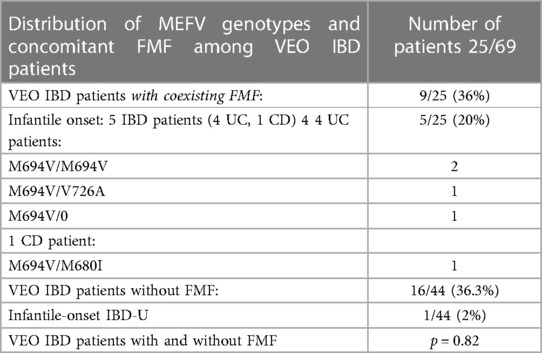
A total of 9 of 25 (36%) IBD patients with coexisting FMF developed very early-onset IBD (VEO IBD) during the first 6 years of life. In all, 5 of them (20%) had infantile-onset IBD within the first year of life (4 UC and 1 CD patients). VEO IBD was diagnosed also in 16 of 44 (36.3%) IBD patients without FMF, 1 (2%) of whom had infantile-onset IBD. However, there was no significant difference in VEO IBD frequency between these two groups of IBD patients with and without FMF, p = 0.82.
Interestingly, all 5 patients (4 UC and 1CD) with infantile-onset IBD and concomitant FMF carried the severe M694V mutation in different genotypes: 2 UC patients were homozygotes (M694V/M694V), 1 was compound heterozygote (M694V/V726A), and 1 heterozygote (M694V/0). In addition, 1 CD patient had the compound heterozygous genotype (M694V/M680I) (Table 9). They had atypical FMF manifestation with recurrent febrile colitis and/or episodes of diarrhea and abacterial hemocolitis or isolated febrile episodes during the first year of life. Later, typical FMF attacks with aseptic polyserositis (peritonitis, pleuritis, pericarditis) and recurrent arthritis and myalgia developed. All IBD patients with infantile-onset IBD and FMF developed severe courses of both UC and FMF and were partially resistant to conventional IBD treatment. After the diagnosis of FMF and starting maximal dosage of colchicine therapy (0.07–0.08 mg/kg/day), the remission of both diseases was achieved. The patient with infantile-onset CD and concomitant FMF, in addition to the severe enterocolitis, also developed perianal disease and recurrent arthritis. He was fully resistant to colchicine therapy and to immunosuppressive treatment and developed several complications.
4 Discussion
According to the data of the National Pediatric Centre for FMF (NPC FMF) of Arabkir MC—ICAH of Armenia, over the last 15 years (2005–2020), there has been a more than six times increase in the total number of FMF-diagnosed children (from 500 to 3250). The annual number of newly diagnosed pediatric FMF cases in NPC FMF is 350 per year. At the same time, according to the hospital-based data of the pediatric gastroenterology service of Arabkir MC—ICAH, over the last 12 years (2008–2020), the total number of pediatric IBD cases increased by three times (from 20 to 69). Among the 3250 FMF pediatric outpatients of the NPC FMF, 0.76% (25) are currently IBD patients, and 0.3% (9) of them have developed VEO IBD. At present, there are no available statistical data in Armenia about the rate of IBD in both adults and children compared to the healthy population.
To the best our knowledge, this is the first study that evaluates the frequency of MEFV gene mutations and FMF disease in 69 Armenian pediatric IBD patients and their influence on the IBD clinical course. The rate of MEFV gene mutations and the frequency of FMF disease were 53.6% and 36.2%, respectively. The highest frequency of MEFV mutations and FMF disease was recorded in UC patients (61.1% and 41.6%, respectively), followed by IBD-U patients (61.1% and 38.8%, respectively).
More than half of the IBD patients with MEFV mutations had heterozygous genotypes (54.0%), 32.4% had compound heterozygous genotypes, and 13.5% were homozygotes.
A high penetrance of the M694V mutation was the most common and presented among 56.7% of IBD patients. It was found to be more frequent in UC and IBD-U patients, mainly for M694V compound heterozygous and heterozygous genotypes (27% and 21.6%, respectively). As known, the M694V mutation is the most frequent FMF-causing mutation in Armenians (50.6%), but, at the same time, among the healthy Armenian population, M694V frequency is low at 4.7% (14). Similar data have been presented by authors from Turkey (1, 36).
The rate of MEFV mutations in UC patients was found to be twice high as in CD patients (P = 0.052), and MEFV mutation carriers were four times more likely to have UC than non-carriers. Thus, our data suggest that the presence of the MEFV mutation might be considered a risk factor for UC patients. Meanwhile, despite the same rate of MEFV mutations (and OR value) in the groups of UC (61.1%) and IBD-U patients (61.1%), the comparison of the frequency of these mutations between these patient groups (26.7% %) was statistically not significant (P = 0.104), probably due to their small sample sizes.
The majority of UC and IBD-U patients with MEFV mutations had heterozygous genotypes (59.0% and 45.4%, respectively), mainly for the M694V (27.7%) and V726A (22.2%) mutated alleles and more rarely for M680I (11.1%) and E148Q (2.7%). These data coincide with some other reports, which show that the M694V mutation is found not only among IBD patients with a coexisting typical FMF clinical pattern but also among IBD patients with an atypical FMF course or M694V mutation carriers. Therefore, in view of the frequent occurrence of FMF in the population at risk, several authors have called for the inclusion of genetic screening for FMF in the investigation of IBD children of Mediterranean ancestry who display unexplained recurrent isolated fever or other atypical FMF manifestations (repeated tonsilitis in early childhood and isolated arthralgia/myalgia, etc.) (20, 40).
The frequency of UC patients with coexisting FMF (41.6%) was significantly higher compared to CD patients with FMF (20%). It should be noted that the frequency of IBD-U patients with FMF was also rather higher (38.8%) than expected but without any significant difference compared to CD patients with FMF.
In the group of IBD patients with concomitant FMF, the M694V mutation was also the most frequent and observed in 50% of MEFV mutations detected in this group, followed by V726A (28.5%) and, more rarely, M680I (9.5%). That is, in case of IBD combined with FMF, the M694V mutation was detected almost twice more often but without any statistical significance.
However, in the study, when comparing IBD patients with and without FMF disease, we did not find associations either between any MEFV mutation and types of IBD or FMF coexistence (P = 0.28). That is, the presence of the MEFV mutation, including severe М694V mutation, did not depend on concomitant FMF disease, i.e., the M694V mutation in IBD patients with FMF was not detected significantly more often than other MEFV mutations.
We have supposed that the MEFV gene is not necessarily a susceptibility gene but most likely modifies the course of IBD. This was also indicated in some other studies, which noted that mutations do not have a high impact on the inflammatory response and clinical outcome of the disease (41). Salach S. et al. reported that although 88% of the investigated Egyptian children with IBD carried MEFV mutations (the V726A mutation being the commonest), no associations were found between MEFV mutations and the phenotypic characteristics of the IBD patients. They suggested that in populations with a high background of the carrier rate of MEFV variants, IBD patients should be screened for MEFV gene mutations, especially those diagnosed with determinate colitis (42).
Interestingly, in the group of our IBD patients who were MEFV mutation carriers without clinical symptoms of FMF, the V726A mutation was also revealed more frequently (36.4%) than M694V (27.3%). Moreover, the frequency of the E148Q mutation in this group of patients was the same as the M694V mutation (27.3%), although the rate of E148Q in healthy Armenian populations is low (3.4%) (8). In a Turkish study, the E148Q mutation was the most common mutation in patients with UC and CD and M694V in patients with IC, in contrast to other studies showing that M694V was the most common mutation detected in pediatric IBD patients (1, 24, 25, 36). Urgenci et al. studied 597 IBD children from 37 institutions from all over Turkey. They showed that E148Q was the most common mutation in patients with UC and CD and M694V in IC (intermittent colitis): 30,5%, 34,5%, and 47,1%, respectively (41). The E148Q mutation was reported as one of the most common mutations in adult IBD patients (20). Furthermore, a high frequency of some other mutations, particularly V726A and K695R, was found in other studies (36, 43)
Inflammatory bowel disease (IBD) and familial Mediterranean fever (FMF) are inflammatory diseases with complex interactions among genetic, immune, and environmental factors. Since there are similarities between FMF and IBD, the MEFV gene, which is responsible for FMF, has been introduced as a modifier gene for IBD. As reported in many studies, the MEFV gene, which frequently causes inflammation, may aggravate the clinical course of UC and CD (17–19, 23, 43). Moreover, some authors suggest that the environment also affects the phenotype of a monogenic disease of the innate inflammatory pathway (44). The concomitant presence of MEFV mutations in diseases other than FMF may modify their presence and severity (20, 36, 40, 43). Particularly, recent studies have revealed an increasing spectrum of rare monogenetic diseases, which can present with IBD or IBD-like intestinal inflammation, particularly with VEO IBD. These monogenic disorders also overlap with immunodeficiency and/or autoinflammatory disorders, including hereditary periodic fever syndromes, particularly with FMF (26, 39, 45–47).
In our study,36% of IBD patients with concomitant FMF developed VEO IBD during the first 6 years of life, while 20% of them had infantile-onset IBD within the first year of life. They carried the M694V mutation in different genotypes, had atypical FMF onset and a severe course of both UC/CD and FMF, and were partially resistant to conventional IBD treatment. After the diagnosis of FMF and starting with the colchicine therapy with the maximal dosage, remission of both diseases was achieved. VEO IBD was also diagnosed in 36.3% IBD patients without FMF, and 2% of them had infantile-onset IBD. The VEO IBD was equally frequent in IBD patients with and without FMF (p = 0.82). Some authors (24, 26, 28) have confirmed that the cases with unusual early onset of UC in infants and children in ethnically matched populations are alarming, and MEFV gene mutations must be evaluated as this association may influence the management of the disease. Particularly, U. Cucinotta et al. concluded that patients with VEO IBD may have a more severe disease course and a poorer response to steroids and anti-TNF-α agents, and they may require more frequent surgical treatment than P-IBD patients (30).
A recent study by Y.Furuta et al. (22) has shown that mutations in the MEFV gene may be associated with intestinal inflammation in Behçet's disease. Other authors (48) have proposed the MEFV gene-related enterocolitis concept for some cases diagnosed as IBD-U. Some of them (21) suggested that FMF patients may have accompanied enterocolitis, which could have a clinical course like that of CD.
Several studies have shown an increased rate of MEFV mutations and FMF among patients with chronic inflammatory diseases other than IBD, particularly with certain types of vasculitis (IgA vasculitis and PAN-like vasculitis), systemic onset of juvenile idiopathic arthritis (JIA), and spondyloarthropathies (37–39, 41, 49–52). According to the NPC FMF, in Armenian children with FMF, among concurrent pathologies, we found FMF-associated vasculitis (Henoch–Shönlein purpura and protracted febrile myalgia) in 4.3% of patients and, in some cases, higher-than-expected frequencies of JIA (4.7%) and non-amyloid kidney lesions (1.1%) as the first and the only manifestation of the disease, especially when the M694V mutation was present (53).
We consider that the limitations of our study are the small sample size of patients, convenient sampling, and conduction in a single center. Therefore, we suppose that further studies are needed.
In this study, despite the small cohort of Armenian pediatric IBD patients, the carrier frequency of MEFV gene mutations was rather high at 53.6%, and the frequency of FMF disease among them was 36.2%. The highest rate of them was among UC patients (61.1% and 41.6%, respectively), followed by IBD-U patients, compared to CD. Our data suggest that the presence of the MEFV mutation might be considered a risk factor for UC patients. The M694V mutation was found to be more frequent in UC and IBD-U patients, more often for the M694V heterozygous genotype. The presence of the MEFV mutation, including М694V mutation, did not depend on concomitant FMF disease. We suppose that the MEFV gene is not necessarily a susceptibility gene but most likely modifies the course of IBD, especially UC and U-IBD.
5 Conclusion
Considering the high prevalence of FMF in Armenians, MEFV genetic testing is recommended for pediatric IBD patients, especially for those with VEO UC and IBD-U, atypical IBD course, or resistance to conventional treatment. They should also be asked for isolated febrile attacks, recurrent arthritis, and family history, even in the absence of typical FMF symptoms, to rule out this monogenic disease. The early diagnosis of FMF and regular colchicine therapy may allow to improve the course of both diseases and prevent complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Committee of the Yerevan State Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
GA: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Formal Analysis, Software. TS: Investigation, Writing – review & editing. AT: Software, Formal Analysis, Writing – review & editing. CB: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Uslu N, Yüce A, Demir H, Saltik-Temizel I, Usta U, Yilmaz E, et al. The association of inflammatory bowel disease and Mediterranean fever gene (MEFV) mutations in Turkish children. Dig Dis Sci. (2010) 55(12):3488–94. doi: 10.1007/s10620-010-1178-5
2. Lidar M, Livneh A. Familial Mediterranean fever: clinical, molecular and management advancements. Neth J Med. (2007) 65:318–24.17954950
3. Mor A, Rivka G, Livneh A. Abdominal and digestive system associations of familial Mediterranean fever. Am J Gastroenterol. (2003) 98:2594–604. doi: 10.1016/j.amjgastroenterol.2003.07.007
4. Padeh S. Periodic fever syndromes. Pediatr Clin N Am. (2005) 52:577–609. doi: 10.1016/j.pcl.2005.01.005
5. Stojanov S, Kastner D. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. (2005) 17:586–99. doi: 10.1097/bor.0000174210.78449.6b
6. Rigante D, Torraca IL, Ansuini V, Compagnone A, Sallì A, Stabile A. The multi-face expression of familial Mediterranean fever in the child. Eur Rev Med Pharmacol Sci. (2006) 10:163–71.16910345
7. Torossyan Y, Aksentijevich I, Sarkisian T. Role of complex allels and gender in the susceptibility to FMF in the Armenian population. Am J Human Genetics. (2000) 64:1275.
8. Hayrapetyan A. Genetical Aspects of Familial Mediterranean Fever in Armenians. Dissertation. Yerevan State Medical University (2002).
9. Sarkisian T, Hayrapetyan H, Shahsuvaryan G. Molecular study of familial Mediterrenean fever patients in Armenia. Curr Drug Targets Inflamm Allergy. (2005) 4:113–6. doi: 10.2174/1568010053622885
10. Sarkisian T, Hayrapetyan H, Beglaryan A, Shahsuvaryan G, Yeghiazaryan A. Molecular diagnosis of familial Mediterrenean fever in Armenians. New Armenian Med J. (2007) 1:33–40.
11. Avagyan T, Amaryan G, Hayrapetyan H, Budumyan A. Tadevosyan a population based study of frequency of carrying FMF mutation among Armenian females. Pediatr Rheumatol. (2017) 15(Suppl 2):64. doi: 10.1186/s12969-017-0185-x
12. Avagyan TA, Budumyan AA, Amaryan GG, Hayrapetyan HS, Tadevosyan AE. Frequency of carrying of mutations of familial Mediterranean fever gene in Yerevan female population. Issues in theoretical and clinical medicine. J Scientific Prac Med. (2017) 20(114):58–61. Available at: https://tert.nla.am/archive/NLA%20AMSAGIR/ScientificPractical1998/2017/4.pdf
13. Avagyan TA. Frequency of carrying of mutations of familial Mediterranean fever gene in Armenian male population. Issues in theoretical and clinical medicine. J Scientific Prac Med. (2018) 21(118):34–7. Available at: https://tert.nla.am/archive/NLA%20AMSAGIR/ScientificPractical1998/2018/1.pdf
14. Hayrapetyan H, Amaryan G, Yeghiazaryan A, Sarkisian T. Clinical-genetic investigation of FMF in Armenia. Pediatric Rheumatology. (2013) 11(Suppl 1):A87. doi: 10.1186/1546-0096-11-S1-A87
15. Ben-Chetrit E, Hayrapetyan H, Yegiazaryan A, Shahsuvaryan G, Sarkisian T. Familial Mediterranean fever in Armenia in 2015: some interesting lessons. Clin Exp Rheumatology. (2015) 33(Supplement 94):S15–8.
16. Dervichian M, Courillon-Mallet A, Cattan D. Maladie periodique. Encyclopedie Medico Chirurgicale. (2003) 9-089:1–10.
17. Giaglis S, Mimidis K, Papadopoulos V, Thomopoulos K, Sidiropoulos P, Rafail S, et al. Increased frequency of mutation in the gene responsible for familial Mediterranean fever in a cohort of patients with ulcerative colitis: evidence for a potential disease-modifying effect? Dig. Dis. Sci. (2006) 51(4):687–92. doi: 10.1007/s10620-006-3192-1
18. Villani A-C, Lemire M, Louis E, Silverberg MS, Collette C, Fortin G, et al. Genetic variation in the familial Mediterranean fever gene (MEFV) and risk for crohn’s disease and ulcerative colitis. PLoS One. (2009) 4(9):e7154. doi: 10.1371/journal.pone.0007154
19. Cattan D, Notamicola C, Molinari N, Toutou I. Inflammatory bowel disease in non-Ashkenazi Jews with familial Mediterranean fever. Lancet. (2000) 355:378–9. doi: 10.1016/S0140-6736(99)02134-0
20. Sahin S, Gulec D, Günay S, Cekic C. Evaluation of the clinical effects and frequency of MEFV gene mutation in patients with inflammatory bowel disease. Gastroenterol Res Pract. (2021) 2021:5538150. doi: 10.1155/2021/5538150
21. Yokoyama Y, Yamakawa T, Ichimiya T, Kazama T, Hirayama D, Wagatsuma K, et al. Gastrointestinal involvement in a patient with familial Mediterranean fever mimicking Crohn’s disease: a case report. Clin J Gastroenterol. (2021) 14:1103–7. doi: 10.1007/s12328-021-01426-2
22. Furuta Y, Gushima R, Naoe H, Honda M, Tsuruta Y, Nagaoka K, et al. Possible association of mutations in the MEFV gene with the intestinal phenotype of Behçet’s disease and refractoriness to treatment J. Clin. Med. (2023) 12:3131. doi: 10.3390/jcm12093131
23. Fidder H, Chowers Y, Ackerman Z, Pollak RD, Crusius JBA, Livneh A, et al. The familial Mediterranean fever (MEVF) gene as a modifier of crohn’s disease. Am J Gastroenterol. (2005) 100(2):338–43. doi: 10.1111/j.1572-0241.2005.40810.x
24. Sari S, Egritas O, Dalgic B. The familial Mediterranean fever (MEFV) gene may be a modifier factor of inflammatory bowel disease in infancy. Eur J Pediatr. (2008) 167(4):391–3. doi: 10.1007/s00431-007-0508-x
25. Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore). (2005) 84(1):1–11. doi: 10.1097/01.md.0000152370.84628.0c
26. Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic aproach to monogenic very early onset inflammatory bowel disease. Gastroenterology. (2014) 147:990–1007. doi: 10.1053/j.gastro.2014.07.023
27. Moran CJ. Very early onset inflammatory bowel disease. Semin Pediatr Surg. (2017) 26:356–9. doi: 10.1053/j.sempedsurg.2017.10.004
28. Nambu R, Muise AM. Advanced understanding of monogenic inflammatory bowel disease. Front Pediatr. (2021) 8:618918. doi: 10.3389/fped.2020.618918
29. Uhlig HH, Charbit-Henriony F, Kotlarz D, Shouval DS, Schwerd T, Strisciuglio C, et al. Clinical genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the paediatric IBD porto group of European society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. (2021) 72(3):456–73. doi: 10.1097/MPG.0000000000003017
30. Cucinotta U, Arrigo S, Dipasquale V, Gramaglia S, Laganà F, Romano C, et al. Clinical course of very early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2023) 76(5):590–5. doi: 10.1097/MPG.0000000000003730
31. ESPGHAN Revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. (2014) 58(6):795–806. doi: 10.1097/MPG.0000000000000239
32. Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. (1997) 40:1879–85. doi: 10.1002/art.1780401023
33. Livneh A, Langevitz P. Diagnostic and treatment concerns in familial Mediterranean fever. Bailliere’s best practice & research. Clin Rheumatol. (2000) 14:477–98. doi: 10.1053/berh.2000.0089
34. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. (2007) 133(2):423–32. doi: 10.1053/j.gastro.2007.05.029
35. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric crohn’s disease activity index. J Pediatr Gastroenterol Nutr. (1991) 12:439–47.1678008
36. Beşer ÖF, Çullu Çokuğraş F, Kutlu T, Erginöz E, Gülcü D, Kasapçopur Ö, et al. Association of familial Mediterranean fever in turkish children with inflammatory bowel disease. Turk Pediatri Ars. (2014) 49(3):198–202. doi: 10.5152/tpa.2014.1998
37. Aksu K, Keser G. Coexistence of vasculitides with familial Mediterranean fever. Rheumatol Int. (2011) 31(10):1263–74. doi: 10.1007/s00296-011-1840-z
38. Ozdogan H, Arisoy N, Kasapçapur O, Sever L, Calişkan S, Tuzuner N, et al. Vasculitis in familial Mediterranean fever. J Rheumatol. (1997) 24(2):323–7.9034991
39. Sag E, Demir F, Ercin ME, Kalyoncu M, Cakir M. Neonatal ulcerative colitis associated with familial Mediterranean fever: a case report. Rheumatol Int. (2018) 38(1):137–40. doi: 10.1007/s00296-017-3848-5
40. Ekinci R.M K, Balci S, Altintaş DU, Yilmaz M. The influence of concomitant disorders on disease severity of familial Mediterranean fever in children. Arch Rheumatol. (2018) 33(3):282–7. doi: 10.5606/ArchRheumatol.2018.6488
41. Urgancı N, Ozgenc F, Kuloğlu Z, Yüksekkaya H, Sarı S, Erkan T, et al. Association between FMF and IBD familial Mediterranean fever mutation analysis in pediatric patients with İnflammatory bowel disease: a multicenter study. Turk J Gastroenterol. (2021) 32(3):248–60. doi: 10.5152/tjg.2021.20057
42. Salah S, El-Shabrawi M, Lotfy HM, Shiba HF, Abou-Zekri M, Farag Y. Detection of Mediterranean fever gene mutations in Egyptian children with inflammatory bowel disease. Int J Rheum Dis. (2016) 19(8):806–13. doi: 10.1111/1756-185X.12482
43. Somi MH, Bonyadi MJ, Mirinezhad SK, Esmaeli M, Laghaeian N, Faghihdinevari M, et al. Common mutatons detection in Iranian Azeri Turk patients with UC:a case–control study. Life Science J. (2013) 10(4s):149–52. Available at: http://www.lifesciencesite.com
44. Ozen S, Aktay N, Lainka E, Duzova A, Bakkaloglu A, Kallinich T. Disease severity in children and adolescents with familial Mediterranean fever: a comparative study to explore environmental effects on a monogenic disease. Ann Rheum Dis. (2009) 68:246–8. doi: 10.1136/ard.2008.092031
45. Kelsen JR, Sullivan KE, Rabizadeh S, Singh N, Snapper S, Elkadri A, et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the evaluation and management for patients with very early-onset inflammatory bowel disease. JPGN. (2020) 70:389–403. doi: 10.1097/MPG.0000000000002567
46. Shim JO. Recent advance in very early-onset inflammatory bowel disease. Intest Res. (2019) 17(1):9–16. doi: 10.5217/ir.2018.00130
47. Rialon KL, Crowley E, Seemann NM, Fahy AS, Muise A, Langer JC. Long-term outcomes for children with very early-onset colitis: implications for surgical management. J Pediatr Surg. (2018) 53:964–7. doi: 10.1016/j.jpedsurg.2018.02.023
48. Saito D, Hibi N, Ozaki R, Kikuchi O, Sato T, Tokunaga S, et al. MEFV Gene-Related enterocolitis account for some cases diagnosed as inflammatory bowel disease unclassified. Digestion. (2019) 101:785–93. doi: 10.1159/000502640
49. Jain A, Misra DP, Sharma A, Wakhlu A, Agarwal V, Negi VS. Vasculitis and vasculitis-like manifestations in monogenic autoinflammatory syndromes. Rheumatol Int. (2018) 38:13–24. doi: 10.1007/s00296-017-3839-6
50. Yildiz M, Adrovic A, Tasdemir E, Baba-zada K, Aydin M, Koker O, et al. Evaluation of co-existing diseases in children with familial Mediterranean fever. Rheumatol Int. (2020) 40(1):57–64. doi: 10.1007/s00296-019-04391-9
51. Salehzadeh F, Moghaddam AE. Coexisting diseases in patients with familial Mediterranean fever. Open Access Rheumatol: Res Rev. (2020) 12:65–71. doi: 10.2147/OARRR.S252071
52. Abbara S, Grateau G, Ducharme-Bénard S, Saadoun D, Georgin-Lavialle S. Association of vasculitis and familial Mediterranean fever. Front Immunol. (2019) 10:763. doi: 10.3389/fimmu.2019.00763
Keywords: children, inflammatory bowel disease, familial Mediterranean fever, clinical characteristics, genetic characteristics
Citation: Amaryan G, Sarkisian T, Tadevosyan A and Braegger C (2024) Familial Mediterranean fever in Armenian children with inflammatory bowel disease. Front. Pediatr. 11:1288523. doi: 10.3389/fped.2023.1288523
Received: 12 September 2023; Accepted: 27 December 2023;
Published: 12 February 2024.
Edited by:
Matthew Wyatt Carroll, University of Alberta, CanadaReviewed by:
Efimia Papadopoulou-Alataki, Aristotle University of Thessaloniki, GreeceOzgur Kasapcopur, Istanbul University-Cerrahpasa, Türkiye
© 2024 Amaryan, Sarkisian, Tadevosyan and Braegger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gayane Amaryan Z2F5YW5lYW1hcnlhbkB5YWhvby5jb20=; Z2F5YW5lYW1hcnlhbkBnbWFpbC5jb20=
Abbreviations Arabkir MC- ICAH, “Arabkir” Medical Complex- Institute of Child and Adolescent Health; CD, Crohn's disease; CI, confidence interval; CMG, Center of Medical Genetics; ESPGHAN, European Society for Paedriatic Gastroenterology, Hepatology, and Nutrition; FMF, Familial Mediterranean fever; HPFS, hereditary periodic fever syndromes; IBD, Inflammatory bowel disease; IBD-U, Unclassified colitis; MEFV, Mediterranean Fever; NPC FMF, FMF National Pediatric Centre for FMF; P-value, statistical significance; PCDAI, Pediatric Crohn's Disease Activity Index; PC, polymeraze chest reaction; PUCAI, Pediatric Ulcerative Colitis Activity Index; SD, standard deviation; VEO IBD, very early-onset inflammatory bowel disease; UC, ulcerative colitis; YSMU, Yerevan State Medical University.
 Gayane Amaryan
Gayane Amaryan Tamara Sarkisian
Tamara Sarkisian Artashes Tadevosyan5
Artashes Tadevosyan5