- 1Center for Reproductive Medicine, Hunan Provincial Maternal and Child Health Care Hospital, Changsha, China
- 2Department of Laboratory Medicine, Hunan Children’s Hospital, Changsha, China
- 3Medical Laboratory, The Second Xiangya Hospital of Central South University, Changsha, China
- 4The Institute of Reproductive and Stem Cell Engineering, School of Basic Medical Science, Central South University, Changsha, China
- 5Department of Andrology, Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha, China
- 6Clinical Research Center for Reproduction and Genetics in Hunan Province, Changsha, China
- 7Scientific Research Department, Hunan Guangxiu Hi-Tech Life Technology Co., Ltd., Changsha, China
Since the coronavirus disease 2019 (COVID-19) pandemic began, several research groups in different countries have described cases of aplastic anaemia (AA) after COVID-19 or COVID-19 vaccination. Here, we present the case of a patient with new-onset AA in Changsha, China, that was presumably associated with preceding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We conducted an epidemiological assessment of the incidence rate of blood system diseases from July 1, 2022, to May 31, 2023, in the haematology department of the Second Xiangya Hospital of Central South University and Hunan Children's Hospital. The detection rates of AA and leukaemia in the first two months after the epidemic outbreak were higher than those before and during the outbreak. However, only the difference in the detection rate of leukaemia was statistically significant.
Introduction
Coronavirus disease (COVID-19) is a global infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the COVID-19 pandemic began, several research groups have described cases of aplastic anaemia (AA) after COVID-19 or COVID-19 vaccination in Japan (1, 2), South Korea (3), Italy (4, 5), China Taiwan (6), the United Kingdom (7) and the United State (8–10). AA, a rare life-threatening disorder, is a syndrome of bonemarrow failure characterized by peripheral blood pancytopaenia and empty bone marrow (11). Acquired AA is a rare disease that affects approximately 2 people per million per year in North America and Europe, which is two to three times greater than the rate in Asia. Adolescents, young adults and elderly individuals are the age groups that are most affected, and the rates are roughly the same for men and women.We believe this is the first new case of acquired bone marrow failure potentially caused by SARS-CoV-2 infection reported in China. In addition, we conducted an epidemiological assessment of the incidence rate of blood system diseases from July 1, 2022, to May 31, 2023, in the haematology department of the Second Xiangya Hospital of Central South University and Hunan Children's Hospital.
Patients and methods
A retrospective review of data from the Second Xiangya Hospital of Central South University and Hunan Children's Hospital, Changsha, Hunan, China, was performed. The number of patients with AA or leukaemia encounters was compared among half a year prior to the outbreak of COVID-19 (2022.7–2022.11), during the epidemic outbreak period (2022.12), the first two months after the outbreak of the epidemic (2023.1–2023.2) and later after the outbreak of the epidemic (2023.3–2023.5).Collection and retrospective analysis of anonymized data were approved by the institutional review board.
All statistical analyses were performed using IBM SPSS Statistics (v. 19.0; IBM Corp., Armonk, NY, USA).Categorical variables were summarized as frequency counts and percentages. Comparison of the detection rates of AA and leukaemia was performed using the chi-square test. All statistical tests were two-sided. P-values less than 0.05 were considered to indicate significance.
Results
Case presentation
A 14-year-old female was reported to have a previous history of good physical health, with menarche at age 11years and a menstrual cycle of 28–29 days and 4–5 days of ovulation. She was given two doses of the inactivated SARS-CoV-2 vaccine on August 14, 2021, and September 15, 2021, from Beijing Biotech and Chengdu Biotech, respectively. The complete blood count was normal in September 2022. There was no history of contact with toxins, nor was there a similar situation in the family. Amid the epidemic of the Omicron variant in December 2022 in Changsha, China, she tested positive for SARS-CoV-2 via polymerase chain reaction (PCR). She had a high fever of 39 degrees Celsius, lasting for 8–9 h, and was given oral acetaminophen (1 tablet at a time, 3 times per day) to reduce the fever. No routine blood test was performed at that time. On January 1, 2023, menstruation began and lasted for more than 10 days without a clean menstrual cycle. She fainted on January 11, 2023, and was admitted to the hospital. Blood tests showed pancytopaenia, and laboratory analysis revealed the following: a white blood cell (WBC) count of 0.3 × 109/L, a red blood cell (RBC) count of 2.88 × 109/L, an absolute neutrophil count (ANC) of 0.01 × 109/L, an absolute lymphocyte count (ALC) of 0.29 × 109/L, a haemoglobin (Hgb) level of 84 g/L, a reticulocyte count of 4.3 × 109/L, and a platelet count of 46 × 109/L. Her extensive workup, including for malignancy (including thoracic and abdominopelvic CT imaging), infection (HIV, viral hepatitis, Epstein‒Barr virus, and cytomegalovirus), and autoimmune aetiologies, was negative; vitamin B12 and folate levels were unremarkable. She was diagnosed with acquired AA according to laboratory criteria based on myelocytopaenia and total haemocytopaenia, excluding other causes of total haemocytopaenia and hereditary bone marrow failure, and after a first and second bone marrow biopsy, it was determined that her bone marrow cell counts were decreased by 10%. Extremely low bone marrow hyperplasia and adipose tissue hyperplasia were observed. Hematopoietic granulocytes and erythrocytes had low proliferation and were mainly composed of middle- and late-stage cells with a scattered distribution. No megakaryocytes were found on any slides. Individual lymphocytes and plasma cells were visible but scattered. Scattered tissue cells containing engulfed hemosiderin granules were clearly observed. No fibrosis was observed. Gomori staining results were MF-0 grade, and iron staining results were 2+. A comprehensive evaluation of inherited (including cytogenetic array, whole exome sequencing (WES), and copy number variation (CNV) sequencing) and acquired aetiologies of AA did not reveal the aetiology of this patient. During admission, this patient relied on oxygenated Hgb and platelet transfusion. Laboratory analysis on March 3, 2023, revealed the following: a WBC count of 0.4 × 109/L, a RBC count of 2.08 × 109/L, an ANC of 0.00 × 109/L, an ALC of 0.3 × 109/L, a Hgb level of 362.0 g/L, and a platelet count of 47 × 109/L. On the same day, we detected SARS-CoV-2 nucleic acid positivity in this patient's blood cells and plasma. At the same time, six women who had never been infected with SARS-CoV-2 and six women who had recovered from COVID-19 in mid-December 2022 served as negative control groups, and their blood cells and plasma test results were negative. She received a bone marrow transplant from her father on April 1, 2023. At the time of reporting, this patient is in remission with a good haematologic response.
Epidemiological assessment of the incidence rate of blood system diseasesbefore and during the outbreak in Changsha, China.
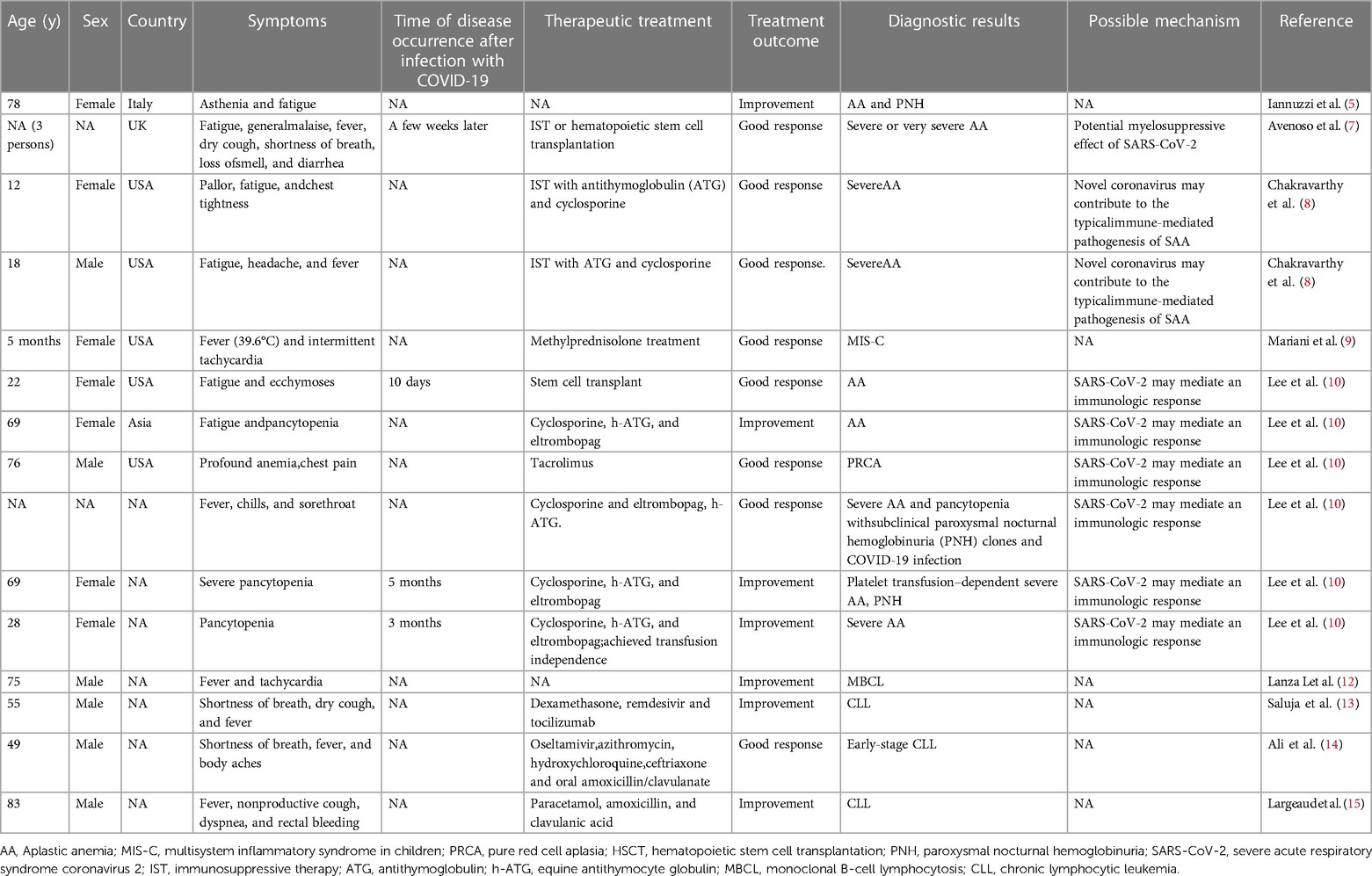
To our knowledge, only 9 studies have reported 17 cases of blood system disease acquired after COVID-19. We provide a brief review of these cases in Table 1. Therefore, it has become particularly important for us to monitor adverse events after COVID-19. Notably, the omicron variant outbreak rapidly spread around China beginning in December 2022. According to the Chinese Center for Disease Control and Prevention, the number and positive rate of SARS-CoV-2 nucleic acid tests in all provinces showed a trend of first increasing and then decreasing; the number of positive results peaked on December 22, 2022 (6.94 million), and the positive rate peaked on December 25, 2022 (29.2%). We conducted an epidemiological assessment of the incidence rate of blood system diseases from July 1, 2022 to May 31, 2023 in the haematology department of the Second Xiangya Hospital of Central South University and Hunan Children's Hospital (Table 2).The detection rates of AA and leukaemia in the first two months after the outbreak of the epidemic were higher than those before and during the outbreak. However, only the difference in the detection rate of leukaemia was statistically significant.
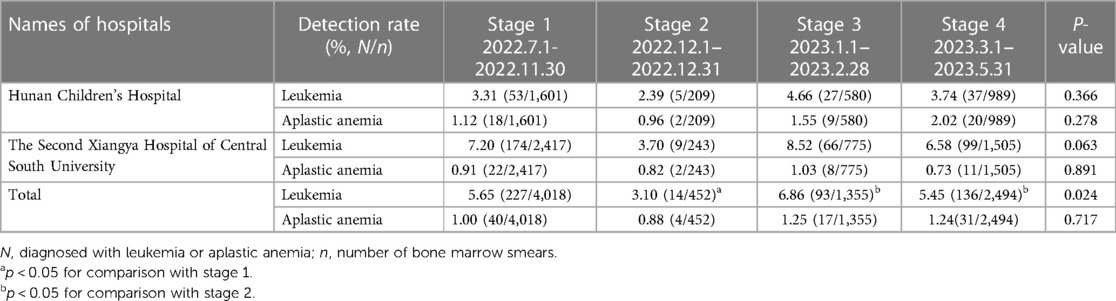
Table 2. Assessment of the incidence rates of leukemia and aplastic anemia in two hospitals in Changsha.
Discussion
According to some recent reports, SARS-CoV-2 infection precedes the occurrence of some autoimmune diseases and haematological diseases, including paediatric inflammatory multisystemic syndrome (PIMS), systemic lupus erythematosus (SLE), immune thrombocytopenia, chronic lymphocytic leukaemia and acquired haemophilia. Most of these reports have linked COVID-19 to the development of these diseases based on clinical observations of temporal associations. The possible main mechanisms linking COVID-19 and AA are as follows: 1. excessive production of inflammatory cytokines and the occurrence of cytokine storm in COVID-19 (16); 2. cytokine storm and the potential cytotoxicity of the virus (17, 18); 3. abnormal hematopoiesis caused by SARS-CoV-2 through the infection of bone marrow erythroid cells (19, 20); 4. an aberrant immune response triggered by the virus leading to depletion of the stem cell compartment and inducing bone marrow failure (7, 10); 5. a potential myelosuppressive effect of this virus (21, 22, 23); 6. manipulation of the hematopoietic stem cell replication process by impairing the expression of several vital proteins as well as disrupting the intracellular biochemical cascade (24, 25); 7. abnormal erythropoiesis caused by CD147on hematopoietic cellsacting as a receptor for virus entry (26); and 8. direct infiltration of the virus into the bone marrow (27).
To our knowledge, this is the first new case of acquired bone marrow failure reported in China. This patient had a very short time (15 days) between PCR positivity and total blood cell decline. This presentation is consistent with previous infectious disease data showing that SARS-CoV-2 infection leads to immune-mediated bone marrow failure, and the infection precedes pancytopenia by weeks to months. Considering the temporal correlation, it appears possible that COVID-19 had a direct role in the pathogenesis of AA.
Immunodeficient or immunocompromised persons are at risk of a prolonged viral phase, with a contagious period of shedding infectious viral particles that can even last for months compared to the typical 5-10 days reported for the general population. This patient remains persistently positive on PCR testing of blood cells and plasma even after 3 months of infection. This supports the theory that SARS-CoV-2 may be causally associated with AA.
However, due to the lack of cytokine studies or viral PCR analysis of bone marrow aspirates in our study, it has not been clarified whether the virus has a direct cytotoxic effect on haematopoietic stem cells or acts through a cytokine storm or aberrant immune dysregulation following the infection, which caused this patient to develop secondary AA. Further evaluations in large cohorts are warranted to elucidate the associations between blood system diseases and COVID-19.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. XW: Resources, Writing – review & editing. YM: Resources, Writing – review & editing. KW: Resources, Writing – review & editing. RM: Investigation, Writing – review & editing. XY: Investigation, Writing – review & editing. LH: Methodology, Writing – review & editing. GL: Resources, Writing – review & editing. GL: Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Hunan Province Municipal Natural Science Foundation (2022JJ30018 to WL), Hunan Province Health Commission Science Foundation (B202301037899 to WL), and Research Grant of CITIC-Xiangya YNXM-202302 to WL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J Autoimmun. (2022) 126:102782. doi: 10.1016/j.jaut.2021.102782
2. Yamamoto M, Keino D, Sumii S, Yokosuka T, Goto H, Inui A, et al. Severe hepatitis-associated aplastic anemia following COVID-19 mRNA vaccination. Intern Med. (2023) 62(12):1813–6. doi: 10.2169/internalmedicine.1308-22
3. Woo S, Kim B, Lee SC, Kim MS, Yoon YA, Choi YJ. Very severe immune aplastic anemia after mRNA vaccination against COVID-19 responds well to immunosuppressive therapy: clinical characteristics and comparison to previous reports. Hematology. (2022) 27:1191–5. doi: 10.1080/16078454.2022.2140986
4. Cecchi N, Giannotta JA, Barcellini W, Fattizzo B. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. (2022) 196:1334–6. doi: 10.1111/bjh.17947
5. Iannuzzi A, Parrella A, De Ritis F, Cammarota A, Berloco L, Paudice F, et al. Pancytopenia in a case of aplastic anaemia/paroxysmal nocturnal haemoglobinuria unmasked by SARS-CoV-2 infection: a case report. Medicina (Kaunas). (2022) 58(9):1282. doi: 10.3390/medicina58091282
6. Chen CY, Chen TT, Hsieh CY, Lien MY, Yeh SP, Chen CC. Case reports of management of aplastic anemia after COVID-19 vaccination: a single institute experience in Taiwan. Int J Hematol. (2023) 117:149–52. doi: 10.1007/s12185-022-03445-2
7. Avenoso D, Marsh JCW, Potter V, Pagliuca A, Slade S, Dignan F, et al. SARS-CoV-2 infection in aplastic anemia. Haematologica. (2022) 107:541–3. doi: 10.3324/haematol.2021.279928
8. Chakravarthy R, Murphy ML, Ann Thompson M, McDaniel HL, Zarnegar-Lumley S, Borinstein SC. SARS-CoV-2 infection coincident with newly diagnosed severe aplastic anemia: a report of two cases. Pediatr Blood Cancer. (2022) 69:e29433. doi: 10.1002/pbc.29433
9. Mariani R, Liu H. Severe transient pancytopenia with dyserythropoiesis and dysmegakaryopoiesis in COVID-19-associated MIS-C. Blood. (2020) 136:2964. doi: 10.1182/blood.2020009479
10. Lee NCJ, Patel B, Etra A, Bat T, Ibrahim IF, Vusirikala M, et al. SARS-CoV-2 infection associated with aplastic anemia and pure red cell aplasia. Blood Adv. (2022) 6:3840–3. doi: 10.1182/bloodadvances.2022007174
12. Lanza L, Koroveshi B, Barducchi F, Lorenzo A, Venturino E, Cappelli E, et al. A new diagnosis of monoclonal B-cell lymphocytosis with cytoplasmic inclusions in a patient with COVID-19. Am J Hematol. (2022) 97:1372–3. doi: 10.1002/ajh.26582
13. Saluja P, Gautam N, Amisha F, Safar M, Bartter T. Emergence of chronic lymphocytic leukemia during admission for COVID-19: cause or coincidence? Cureus. (2022) 14:e23470. doi: 10.7759/cureus.23470
14. Ali E, Badawi M, Abdelmahmuod E, Kohla S, Yassin MA. Chronic lymphocytic leukemia concomitant with COVID 19: a case report. Am J Case Rep. (2020) 21:e926062. doi: 10.12659/AJCR.926062
15. Largeaud L, Ribes A, Dubois-Galopin F, Memier V, Rolland Y, Gaudin C, et al. Major rise of a chronic lymphoid leukemia clone during the course of COVID-19. Int J Lab Hematol. (2021) 43:e82–3. doi: 10.1111/ijlh.13383
16. Alahyari S, Moradi M, Rajaeinejad M, Jalaeikhoo H. Post-COVID-19 hematologic complications: a systematic review. Expert Rev Hematol. (2022) 15(6):539–46. doi: 10.1080/17474086.2022.2080051
17. Dewaele K, Claeys R. Hemophagocytic lymphohistiocytosis in SARS-CoV-2 infection. Blood. (2020) 135(25):2323. doi: 10.1182/blood.2020006505
18. Hersby DS, Do TH, Gang AO, Nielsen TH. COVID-19-associated pancytopenia can be self-limiting and does not necessarily warrant bone marrow biopsy for the purposes of SARS-CoV-2 diagnostics. Ann Oncol. (2021) 32(1):121–3. doi: 10.1016/j.annonc.2020.09.020
19. Huerga Encabo H, Grey W, Garcia-Albornoz M, Wood H, Ulferts R, Aramburu IV, et al. Human erythroid progenitors are directly infected by SARS-CoV-2: implications for emerging erythropoiesis in severe COVID-19 patients. Stem Cell Rep. (2021) 16(3):428–36. doi: 10.1016/j.stemcr.2021.02.001
20. Gillespie MA, Palii CG, Sanchez-Taltavull D, Shannon P, Longabaugh WJR, Downes DJ, et al. Absolute quantification of transcription factors reveals principles of gene regulation in erythropoiesis. Mol Cell. (2020) 78(5):960–974.e11. doi: 10.1016/j.molcel.2020.03.031
21. Hartung HD, Olson TS, Bessler M. Acquired aplastic anemia in children. Pediatr Clin North Am. (2013) 60(6):1311–36. doi: 10.1016/j.pcl.2013.08.011
22. Shimano KA, Narla A, Rose MJ, Gloude NJ, Allen SW, Bergstrom K, et al. Diagnostic work-up for severe aplastic anemia in children: consensus of the North American pediatric aplastic anemia consortium. Am J Hematol. (2021) 96(11):1491–504. doi: 10.1002/ajh.26310
23. Rauff B, Idrees M, Shah SA, Butt S, Butt AM, Ali L, et al. Hepatitis associated aplastic anemia: a review. Virol J. (2011) 8:87. doi: 10.1186/1743-422X-8-87
24. King K.Y., Goodell M.A.. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–92. doi: 10.1038/nri3062
25. Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. (2020) 10(9):200160. doi: 10.1098/rsob.200160
26. Ulrich H, Pillat MM. CD147 As a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. (2020) 16(3):434–40. doi: 10.1007/s12015-020-09976-7
Keywords: COVID-19, SARS-CoV-2, acquired aplastic anaemia, leukaemia, China
Citation: Wu X, Mo Y, Wen K, Ming R, Yin X, Hu L, Liu G, Lin G and Li W (2023) Acquired aplastic anaemia after SARS-CoV-2 infection in China: a case report. Front. Pediatr. 11:1277540. doi: 10.3389/fped.2023.1277540
Received: 14 August 2023; Accepted: 25 October 2023;
Published: 7 November 2023.
Edited by:
Victor Aquino, University of Texas Southwestern Medical Center, United StatesReviewed by:
Bo-tao Ning, Shanghai Children’s Medical Center, ChinaDai Keino, Kanagawa Children’s Medical Center, Japan
© 2023 Wu, Mo, Wen, Ming, Yin, Hu, Liu, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weina Li bGl3ZWluYWtpbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
‡ORCID Weina Li orcid.org/0000-0002-8728-3167
 Xiyan Wu
Xiyan Wu Yi Mo2,†
Yi Mo2,† Weina Li
Weina Li