
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 15 November 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1273464
 Kishin Tokuyama1
Kishin Tokuyama1 Tsubasa Kitamura1
Tsubasa Kitamura1 Kazutaka Maruyama1,2
Kazutaka Maruyama1,2 Shun Toriumi1,2
Shun Toriumi1,2 Yayoi Murano1,2*
Yayoi Murano1,2* Daisuke Yoneoka3
Daisuke Yoneoka3 Tomoyuki Nakazawa1,2
Tomoyuki Nakazawa1,2 Toshiaki Shimizu2
Toshiaki Shimizu2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variants are now a pandemic. There are differences in clinical features in SARS-CoV-2 variants and we conducted this study to assess the clinical features of coronavirus disease (COVID-19) in children with SARS-CoV-2 omicron variants. The study included children with COVID-19 arrivedto Tokyo Metropolitan Toshima Hospital between January 2020 and October 2022. The clinical features of 214 children with SARS-CoV-2 non-omicron variants and 557 children with omicron variants were compared. In the SARS-CoV-2 omicron variant group, more patients had fever, sore throat, nausea and/or vomiting, and seizures and/or disorders of consciousness. In SARS-CoV-2 non-omicron variants, there was only one patient with seizure and/or unconsciousness whereas there were 92 children in omicron variants. Among these 92 patients, 46 (49%) were diagnosed with simple febrile seizures; 23 (25%), with complex febrile seizures; 10 (11%) with status epilepticus; and two (2%) with encephalopathy. Their mean age was 4.0 ± 3.0 years—a wider age distribution than that in other febrile seizures but similar to that in febrile seizures in patients with influenza. SARS-CoV-2 omicron variants are likely to cause seizures and unconsciousness in children and their age distribution was wider than other febrile seizures patients but similar to those in influenza patients. In clinical practice in patients with COVID-19 and influenza, clinicians should be aware of these features.
Coronavirus disease (COVID-19) caused a pandemic in 2019 and gradually becoming endemic. Since the beginning of the pandemic, children have been reported to experience milder symptoms than adults (1). However, the symptoms differ between variants (2), and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant, which is one of the “variants of concern,” appeared at the end of 2021 with high transmissibility. These variants have been reported to affect the younger population (3), and the pandemic is said to be an urgent matter (4). Also, some studies have reported seizures in children with COVID-19 infected with omicron variants (5–8); however, most are case reports. Moreover, although there are several studies from Japan comparing omicron variant mainly include adult participants (9–12), little are known in children.
Therefore, this study aimed to reveal the clinical features of the SARS-CoV-2 omicron variants among children.
The study included children aged 0–15 years who were diagnosed as COVID-19 by SARS-CoV-2 PCR test or antigen test and treated for COVID-19 at Tokyo Metropolitan Toshima Hospital between January 2020 and October 2022. Data were electronically extracted from their medical records. The data included age at arrival at our hospital, date of arrival at our hospital, sex, and symptoms (fever, cough, rhinorrhea, headache, sore throat, nausea, vomiting, diarrhea, abdominal pain, rash, taste and smelling disorder, joint pain, seizures, disorders of consciousness, and abnormal urinalysis results). Our hospital is a designated medical institution for infectious disease and accepted patients with COVID-19 from very first time of pandemic in Japan. We accepted all patients with COVID-19, and also there were admission to our hospital instructed by health care center during the early phase of pandemic. We collected additional detailed information on seizures and unconsciousness, which included the type of seizures and the final diagnosis of seizures and unconsciousness. The seizure occurred once and stopped within five minutes was defined as simple febrile seizure. Seizure occurred more than twice within 24 h was defined as complex seizure, and seizure continuing when arriving at the hospital was defined as status epilepticus.
At the beginning of study period, COVID-19 was classified as a Category 2 disease under the Infectious Disease Control Law, but in February 2021, it was classified as a new type of influenza and other infectious diseases.
The study participants were divided into two groups. The first group comprised children who visited our hospital between January 2020 and December 2021—the period corresponding to when omicron was not the dominant variant in Japan (non-omicron group). The second group comprised children who visited our hospital from January 2022, when omicron was the dominant variant in Japan (omicron group) (13).
To analyze the basic characteristics of the participants, we performed a chi-square test for categorical variables and a t-test for continuous variables. Using the χ2 test, we compared the clinical features between the non-omicron and omicron group. Unknown values were treated as missing values.
Statistical analyses were performed using Stata, version 15.1 (Stata Corp., College Station, TX, USA). Statistical significance was set at P < 0.05.
This study was approved by the ethical committee of Tokyo Metropolitan Toshima Hospital (Approve number: Jin 2–32). The requirement for patient consent was waived owing to the use of anonymous data.
During the study period, January 2020 to October 2022, there were 214 participants with non-omicron variant COVID-19 and 557 with an omicron variant.
Table 1 presents the participants' basic characteristics. There were 128 boys (59.8%) in the non-omicron group and 322 (57.8%) in the omicron group—the difference was not significant (p = 0.61). The mean [± standard deviation (SD)] age was 6.1 ± 4.7 years in the non-omicron group and 5.3 ± 4.4 years in the omicron group—the participants in the omicron group were significantly younger (p < 0.01). In the non-omicron and omicron group, 163 (76.2%) and 549 (98.6%) children had at least one symptom—this difference was significant (p < 0.001).
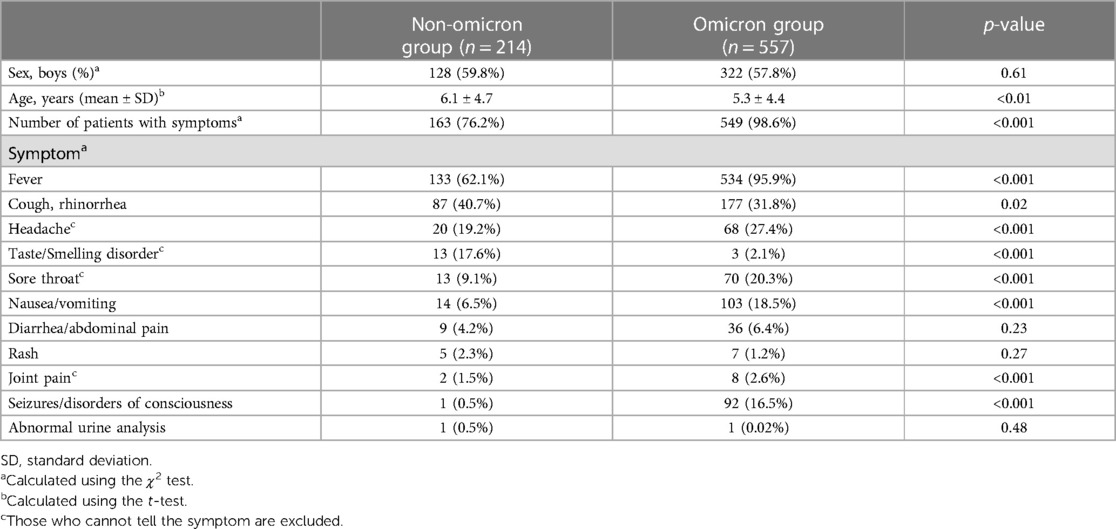
Table 1. Comparison of basic characteristics and symptoms of the participants between the non-omicron and omicron group.
Table 1 also presents the results of the comparison between the two groups stratified according to the participants' symptoms. The number of children with fever, headache, taste/smelling disorder, sore throat, nausea and/or vomiting, joint pain, and seizures and/or disorders of consciousness was significantly higher in the omicron group. In contrast, the number of patients with taste and/or smelling disorders was significantly higher in the non-omicron group. Other symptoms, including cough and/or rhinorrhea, diarrhea and/or abdominal pain, rash, and abnormal urinalysis results, were not significantly different between the groups.
Figure 1 shows the final diagnosis of patients with seizures and unconsciousness. Among the 92 children, 46 (50%) were diagnosed with simple febrile seizures (FS); 23 (25%), who had several seizures, with complex FS; 10 (11%) with status epilepticus, and two (2%) with encephalopathy. Of the remaining children, 10 were diagnosed with febrile delirium, and one with epilepsy. Their mean age was 4.0 ± 3.0 years (lowest: 2 months, highest: 11 years). There were 64 boys (69.6%).
Finally, the comparison of the two groups in presence of seizure or unconsciousness among children with fever and there was significant difference (p < 0.001): Among 133 febrile participants in non-omicron group, one had the symptom, whereas among 534 febrile participants in omicron group 92 had the symptom. The comparison of presence of simple or complex FS between the two groups also showed significant difference (p < 0.001): Among 133 febrile participants in non-omicron group, one had the symptom, whereas among 534 febrile participants in omicron group 69 had the symptom. All participants with seizure or unconsciousness were discharged without any complications.
In this study, we aimed to reveal the clinical features of the SARS-CoV-2 omicron variants among children, and revealed that, in comparison with other SARS-CoV-2 variants, the omicron variant was associated with more clinical symptoms, particularly seizures. The number of children with seizures and unconsciousness was significantly higher after than before the emergence of the SARS-CoV-2 omicron variant.
We considered the SARS-CoV-2 pandemic in terms of variants, where the non-omicron period extended over 24 months, from January 2020 to December 2022, and the omicron period extended over 10 months, from January 2022 to October 2022. Despite the omicron period having been shorter, there were more children with COVID-19 than there were during the non-omicron period. This result is consistent with that of one study that reported more children having been admitted during the SARS-CoV-2 omicron period (14) and another that reported an increased admission rate of children under one year (15). Based on the results of the present and previous studies, it can be concluded that SARS-CoV-2 omicron variants affect more children than do previous variants. Moreover, the lower mean age of the SARS-CoV-2 omicron group in our study supports these results.
We emphasize that there were many more children who had seizures and unconsciousness during the SARS-CoV-2 omicron period, because there was only one patient in the non-omicron group and 92 in the omicron group. In addition, the age of children with seizures and unconsciousness is noteworthy. FS are likely to occur between the ages of 6 months and 6 years (16). However, in our study sample, the age range was wider, and both younger and older patients had seizures and unconsciousness. This finding is similar to that for influenza, which has a higher tendency to cause FS in older age than do other diseases (17). During the COVID-19 pandemic, influenza has also started to cause an epidemic (18, 19) and clinicians should be aware of this tendency in their clinical practice.
Some studies have reported seizures in children with COVID-19 infected with omicron variants (5–8); however, most are case reports. In the present study, we statistically analyzed and determined the association of seizures and unconsciousness with SARS-CoV-2 omicron variants. Although the pathology is not well known, neurological manifestations of SARS-CoV-2 omicron variants in the adult population have been reported (20).
Our study has some limitations. First, we considered the SARS-CoV-2 pandemic in terms of variants; however, we did not confirm the variants with laboratory diagnostic tools. Nevertheless, the period that we defined as the SARS-CoV-2 pandemic is reported to be a pandemic of more than 90% omicron variants. Also, due to strict indication of neuroimage because of infection control, we could not diagnose encephalopathy and some participants may be categorized se status epilepticus. However, their clinical symptom do not require further investigation after isolation period and they had good prognosis. Second, patients' diagnostic thresholds have been changing. During the very early term of the pandemic, children in close contact were carefully screened, and more asymptomatic patients with COVID-19 were diagnosed compared to recently. As children are more likely to be asymptomatic (1), we cannot avoid selection bias in the study sample. On the other hand, there is a possibility that people came to hospital less frequently in the early phase of pandemic and the number of COVID-19 children are estimated lower. However, parents bring their children if they have seizure or unconsciousness, our conclusion still have impact on further clinical practice. Third, we could not obtain information about vaccination status. However, as they were introduced to Japanese children in later phase of pandemic with omicron, it can be said that even vaccination was done, there is still larger number of seizure in omicron variant infected children.
In conclusion, our study revealed that SARS-CoV-2 omicron variants are associated with increase in seizure and unconsciousness in children with COVID-19. As this study sample was drawn from a single institution, further studies including more institutions and larger sample sizes are required.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethical commitee of Toshima Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
KT: Data curation, Investigation, Methodology, Writing – original draft. TK: Data curation, Investigation, Methodology, Writing – original draft. KM: Data curation, Investigation, Methodology, Resources, Writing – original draft. ST: Data curation, Investigation, Methodology, Resources, Writing – original draft. YM: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Writing – original draft. DY: Data curation, Funding acquisition, Methodology, Software, Validation, Visualization, Writing – original draft. TN: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. TS: Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by JST, PRESTO grant no. JPMJPR21RC, Japan.
We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1273464/full#supplementary-material
1. Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. (2021) 93(2):1057–69. doi: 10.1002/jmv.26398
2. Florensa D, Mateo J, Spaimoc R, Miret C, Godoy S, Solsona F, et al. Severity of COVID-19 cases in the months of predominance of the alpha and delta variants. Sci Rep. (2022) 12(1):15456. doi: 10.1038/s41598-022-19125-4
3. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. (2021) 398(10317):2126–8. doi: 10.1016/S0140-6736(21)02758-6
4. Saxena SK, Kumar S, Ansari S, Paweska JT, Maurya VK, Tripathi AK, et al. Characterization of the novel SARS-CoV-2 omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. (2022) 94(4):1738–44. doi: 10.1002/jmv.27524
5. Ludvigsson JF. Convulsions in children with COVID-19 during the omicron wave. Acta Paediatrica. (2022) 111(5):1023–6. doi: 10.1111/apa.16276
6. Setiabudi D, Sribudiani Y, Hermawan K, Andriyoko B, Nataprawira HM. The omicron variant of concern: the genomics, diagnostics, and clinical characteristics in children. Front Pediatr. (2022) 10:898463. doi: 10.3389/fped.2022.898463
7. Iio K, Hagiwara Y, Saito O, Ishida Y, Horikoshi Y. Seizure in children with severe acute respiratory syndrome coronavirus 2 omicron variant infection. Pediatr Int. (2022) 64(1):e15255. doi: 10.1111/ped.15255
8. Thongsing A, Eizadkhah D, Fields C, Ballaban-Gil K. Provoked seizures and status epilepticus in a pediatric population with COVID-19 disease. Epilepsia. (2022) 63(8):e86–91. doi: 10.1111/epi.17293
9. Suzuki K, Ichikawa T, Suzuki S, Tanino Y, Kakinoki Y. Clinical characteristics of the severe acute respiratory syndrome coronavirus 2 omicron variant compared with the delta variant: a retrospective case-control study of 318 outpatients from a single sight institute in Japan. PeerJ. (2022) 10:e13762. doi: 10.7717/peerj.13762
10. Hujamberdieva LM, Chimed-Ochir O, Yumiya Y, Tanaka J, Ohge H, Kuwabara M, et al. Relationship between clinical symptom profiles and COVID-19 infection status during Delta-dominant period versus omicron-dominant period-analysis of real-world data collected in hiroshima prefecture, Japan. Int J Infect Dis. (2023) 136:92–9. doi: 10.1016/j.ijid.2023.09.007
11. Akaishi T, Kushimoto S, Katori Y, Sugawara N, Egusa H, Igarashi K, et al. COVID-19-related symptoms during the SARS-CoV-2 omicron (B.1.1.529) variant surge in Japan. Tohoku J Exp Med. (2022) 258(2):103–10. doi: 10.1620/tjem.2022.J067
12. Nakakubo S, Kishida N, Okuda K, Kamada K, Iwama M, Suzuki M, et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect Dis. (2023) 23:1244–56. doi: 10.1016/s1473-3099(23)00271-2
13. Overview of Variants in Countries 2023. Available at: https://ourworldindata.org/grapher/covid-variants-area?country=∼JPN (cited October 7, 2023).
14. Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. (2022) 6(5):294–302. doi: 10.1016/S2352-4642(22)00027-X
15. Torjesen I. COVID-19: omicron variant is linked to steep rise in hospital admissions of very young children. BMJ. (2022) 376:o110. doi: 10.1136/bmj.o110
16. Patel N, Ram D, Swiderska N, Mewasingh LD, Newton RW, Offringa M. Febrile seizures. BMJ. (2015) 351:h4240. doi: 10.1136/bmj.h4240
17. Hara K, Tanabe T, Aomatsu T, Inoue N, Tamaki H, Okamoto N, et al. Febrile seizures associated with influenza A. Brain Dev. (2007) 29(1):30–8. doi: 10.1016/j.braindev.2006.05.010
18. Zipfel CM, Colizza V, Bansal S. The missing season: the impacts of the COVID-19 pandemic on influenza. Vaccine. (2021) 39(28):3645–8. doi: 10.1016/j.vaccine.2021.05.049
19. Dhanasekaran V, Sullivan S, Edwards KM, Xie R, Khvorov A, Valkenburg SA, et al. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat Commun. (2022) 13(1):1721. doi: 10.1038/s41467-022-29402-5
Keywords: COVID-19, SARS-CoV-2, omicron variants, febrile seizure, seizure
Citation: Tokuyama K, Kitamura T, Maruyama K, Toriumi S, Murano Y, Yoneoka D, Nakazawa T and Shimizu T (2023) High number of seizures and unconsciousness in patients with SARS-CoV-2 omicron variants: a retrospective study. Front. Pediatr. 11:1273464. doi: 10.3389/fped.2023.1273464
Received: 6 August 2023; Accepted: 30 October 2023;
Published: 15 November 2023.
Edited by:
Diego Iacono, Neuroscience - Uniformed Services University of the Health Sciences (USU), United StatesReviewed by:
Chien-Yu Lin, Hsinchu Mackay Memorial Hospital, Taiwan© 2023 Tokuyama, Kitamura, Maruyama, Toriumi, Murano, Yoneoka, Nakazawa and Shimizu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayoi Murano eW11cmFub0BqdW50ZW5kby5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.