
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pediatr., 08 November 2023
Sec. Pediatric Infectious Diseases
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1271065
This article is part of the Research TopicClinical Challenges in Pediatric Transplant Infectious DiseasesView all 11 articles
Solid organ transplantation (SOT) candidates and recipients are at increased risk for morbidity and mortality from vaccine-preventable infections. Children are at particular risk given that they may not have completed their primary immunization series at time of transplant or have acquired natural immunity to pathogens from community exposures. Multiple society guidelines exist for vaccination of SOT candidate and recipients, although challenges remain given limited safety and efficacy data available for pediatric SOT recipients, particularly for live-vaccines. After transplant, individual patient nuances regarding exposure risks and net state of immunosuppression will impact timing of immunizations. The purpose of this review is to provide readers with a concise, practical, expert-opinion on the approach to vaccinating the SOT candidate and recipient and to supplement existing guidelines. In addition, pediatric-specific knowledge gaps in the field and future research priorities will be highlighted.
Despite efforts to optimize childhood immunizations, vaccine-preventable infections remain a significant cause of morbidity and mortality for solid organ transplantation (SOT) candidates and recipients (1–3). Young children are at particular risk given that they may not yet have acquired natural immunity to pathogens nor had the opportunity to complete their primary immunization series at the time of transplant. In fact, pediatric SOT recipients are at 87 times increased risk for hospitalization from a vaccine-preventable infection in the first 5 years after transplant compared to the general population (1). The impact of a vaccine-preventable infection in SOT recipients is associated with prolonged hospitalization, increased healthcare costs, and increased risk for death or graft loss (4, 5). Rates of vaccine-preventable infections in this vulnerable population may be higher than previously estimated in light of declining worldwide childhood immunization coverage as a consequence of the COVID-19 pandemic and vaccine hesitancy (6). Recent outbreaks of previously eliminated infections, such as measles, are a reminder for ongoing vigilance (7).
Pediatric SOT candidates should ideally receive all age-appropriate vaccines prior to transplantation, given their attenuated immune response to vaccines post-transplant (8). The need for ongoing immunosuppression to prevent organ rejection, diminished immunogenicity to vaccines, and waning vaccine titers contribute to a heightened infectious risk in the post-transplant period (9). Family members and close contacts should also ensure they are up-to-date on their vaccines (10). Oftentimes the urgency of transplant in young children and infants precludes their ability to complete the primary vaccine series even using an accelerated vaccine schedule (11). Incomplete vaccines and annual influenza and COVID-19 vaccine can be administered post-transplantation based on timing and net state of immunosuppression, although certain criteria should be met before administering live vaccines (12).
Pre and post-SOT immunization guidelines and recommendations are available through infectious disease and transplant societies such as the Infectious Diseases Society of America and the American Society of Transplantation (AST) (12–14). However, rates of immunizations in SOT candidates and recipients remain sub-optimal. Less than 30% of pediatric SOT candidates are up-to-date at time of transplant based on the Centers for Disease Control and Prevention (CDC) childhood immunization schedules (15). Factors contributing to low immunization rates in transplant candidates include lack of coordination between multidisciplinary teams, less frequent care from primary care physicians, gaps in knowledge about optional timing and safety of pre-transplant immunizations, and lack of centralized immunization records (16). Given nuances in each patient scenario, there is not likely a “one size fits all” approach. The purpose of this review is to provide a concise, practical expert-opinion approach to vaccinating the SOT candidate and recipient and to highlight knowledge gaps and priorities for future research.
You are asked to see a 4-month-old boy with hypoplastic left heart syndrome who underwent Stage 1 palliative cardiac surgical repair. He presents to the Emergency Department with poor feeding, increased work of breathing, and is admitted to the hospital in acute heart failure. A ventricular assist device (VAD) is placed and he is listed status 1A for heart transplantation. His parents report he has received his first set of childhood immunizations at 2 months of age. What should be checked as part of his pre-transplant evaluation and what vaccines would you recommend?
The pre-transplant screening visit is an opportunity to evaluate a candidate's risk for infection, including checking select vaccine serologies, and provide counseling about safe living after transplantation (10, 14, 17). The immunization history from the infant in Case 1 reveals that he has only received one set of routine vaccines 2 months ago, thus checking vaccine titers is not necessary, and he should receive routine vaccines now. If he is still waiting for a heart transplant in 4 weeks, the third set of primary childhood vaccines can be administered as part of the accelerated schedule (Table 1). In general, after review of records and serologic responses to certain vaccines, a vaccine roadmap should be developed and implemented as noted in Table 1 with additional considerations for asplenia or other conditions (Table 2).
For vaccinated children, serology can be checked for Hepatitis B (HBV), Hepatitis A (HAV), varicella (VZV), and measles/mumps/rubella (MMR) (14). To have maximal benefit, inactivated vaccines should be given at least 2 weeks prior to transplant. It is recommended to have at least 4 weeks before transplant for safety of administering live vaccines (14). Given that the infant in Case 1 is only 4 months of age and requires a VAD, he likely has passive antibody that would render live virus vaccines, such as MMR and varicella, useless; likewise, he is also listed status 1A for heart transplant with the potential for transplant to occur within 4 weeks, and thus live vaccines should not be given.
Family members and close contacts of the SOT candidate should ensure they are up-to-date on their immunizations as well, including seasonal influenza and COVID-19 vaccines. The latter two vaccines are of critical importance in infants too young to be vaccinated themselves, such as in Case 1. Live vaccines are not contraindicated in close contacts with the exception of the smallpox and oral polio vaccines; the latter is not available in the United States. Contact precautions and hand hygiene should be used if the rotavirus vaccine or live-attenuated influenza vaccines are given. Likewise, if a rash develops after varicella vaccine, direct contact with the rash should be avoided until complete crusting occurs (18). Household pets should also have annual check-ups to ensure they are healthy and fully immunized (14). Vaccine specific recommendations are outlined below.
Hepatitis B (HBV) infection is highly prevalent worldwide and universal vaccination is recommended for all infants, children, and adolescents to prevent progressive liver damage (14, 19). Immunity against HBV should be confirmed prior to transplant (anti-HBs >10 IU/ml) as antibody titers can wane, particularly in immunocompromised individuals or those receiving chronic dialysis (14, 20). Assessing HBV immunity became part of the Organ Procurement and Transplantation Network (OPTN) policy in 2021. Future analysis will help assess vaccine efficacy, particularly in cases of HBV core antibody positive donors. In a retrospective study of pediatric SOT candidates, approximately half had indeterminate, non-reactive, or unavailable HBV surface antibody titers, even amongst those who had completed the primary vaccine series (20). Documentation of immunity is of particular importance in SOT recipients as donor-derived infection has been reported and adequate titers has been shown to prevent de novo HBV infection post-transplant (21). In non-immune individuals (anti-HBs <10 IU/ml), revaccination can be done with the complete series, using high-dose (40 µg) vaccine, or with giving one dose and re-checking anti-HBs (14). An adjuvanted two-dose HBV vaccine (HepB-CpG) with increased immunogenicity compared to existing vaccine formulations is available for individuals older than 18 years of age (22).
All pediatric SOT candidates and recipients should receive diphtheria, tetanus and pertussis containing vaccines using an acellular pertussis component (DTaP or TdaP) (14). Infants and children should receive 5 doses DTaP vaccines with minimal intervals specified in Table 1. Combination vaccines are available and can be used to reduce the number of overall injections. A single booster dose of Tdap should be administered to adolescents and Tdap should be given every 10 years throughout life for ongoing tetanus prophylaxis (19).
Haemophilus influenza type B (Hib) infections have declined dramatically since the introduction of the Hib vaccine (19). Immunocompromised individuals particularly those with asplenia or impaired splenic function are at particular risk for invasive infection (23). After completion of the series vaccine titers are not routinely checked.
Inactivated polio vaccine (IPV) should be administered to all SOT recipients and can be safely administered post-transplant, with minimal intervals specified in Table 1 (19). Oral polio vaccine stopped being used in the United States since 2000 given risk for vaccine-associated paralytic poliomyelitis in vaccine recipients or close contacts. Being up to date on IPV is underscored with the resurgence of polio in the United States and other countries in unvaccinated people (24). After completion of the series vaccine titers are not routinely checked.
Invasive pneumococcal disease (IPD) causes significant morbidity and mortality in children and can lead to bacteremia, pneumonia, endocarditis or meningitis (25). SOT recipients are at significantly higher risk for IPD, particularly those with asplenia or infants heart transplant recipients (3). Two types of pneumococcal vaccines are available, protein-conjugated vaccines and the 23-valent polysaccharide vaccine used in certain high-risk individuals. In June 2022, the CDC recommended the use of 15-valent pneumococcal conjugate vaccine (PCV15) in children; PCV20 was subsequently approved in June 2023 (25). PCV15 contains two additional serotypes (22F, 33F) and PCV20 also contains (8, 10A, 11A, 12F and 15B). In a Phase III trial (NCT03921424) of PCV15 in children infected with Human Immunodeficiency Virus (HIV), PCV15 was more immunogenic for 8 shared serotypes compared to PCV13, although specific data in SOT recipients is lacking (25). Current recommendations are for the use of either PCV15 or PCV20 for all infants and children as the primary series (Table 3). For SOT recipients who have not received any dose of PCV13, PCV15, or PCV20, a single dose of PCV15 or PCV20 is recommended ≥8 weeks after the most recent dose of pneumococcal vaccine (26). In children who receive PCV20, additional administration of PPSV23 is not needed.
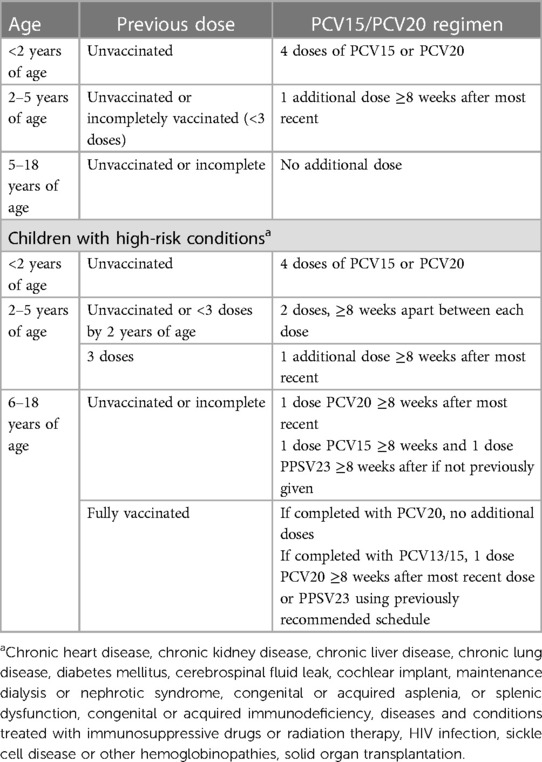
Table 3. Updated recommendations for pneumococcal vaccination with PCV15 or PCV20 in healthy children and those with high-risk conditions.
Meningococcal disease caused by Neisseria meningitidis can cause bacteremia, meningitis or invasive infection particularly in high-risk individuals (27). Three quadrivalent conjugate vaccines against serogroups A, C, W, and Y (MenACWY) and two serogroup B meningococcal vaccines (MenB) are available. Vaccination with MenACWY is recommended for adolescents 11 or 12 years of age and infants older than 2 months at increased risk, such as those with complement deficiency, receipt of complement inhibitor, anatomic or functional asplenia, travel to a high risk area, or those with HIV (27). Vaccine should ideally be given at least 2 weeks prior to splenectomy or receipt of complement inhibitor. In the United States, MenB vaccine is recommended starting at 10 years of age in individuals at high-risk for meningococcal infection with a booster dose for those who remain at increased risk. Canadian and European Guidelines start as early as 2 months of age. For asplenic individuals, booster doses of MenACWY should be given every 5 years and MenB every 2–3 years (27). Transplant recipients receiving terminal complement inhibitors to treat antibody-mediated rejection are at particular risk for meningococcal disease and should be vaccinated prior to treatment.
Hepatitis A virus (HAV) is a communicable disease transmitted fecal-orally (28). Periodic outbreaks occur typically from contaminated foods. Immunocompromised individuals and those with underlying liver disease are at risk for severe infection and fulminant hepatitis. HAV vaccine should be administered to all SOT candidates and recipients, particularly liver transplant candidates (14, 28). Serologic response to HAV should be checked to ensure seroconversion (14).
Human papillomavirus (HPV) is a common sexually transmitted infection which can lead to the development of cervical, anogenital, and oropharyngeal cancers. SOT recipients have higher rates of HPV cancers compared to the general population (29). Vaccination against HPV has been routinely recommended since 2006 for females and 2011 for males (19). Two or three-doses of HPV vaccine are recommended starting as early as 9 years of age. While the vaccine is safe to be given post-transplant, immunogenicity may be reduced, particularly in lung transplant recipients likely related to degree of immunosuppression (30). Because of this, efforts should be made to optimize HPV vaccine pre-transplant.
Influenza causes severe infection in immunocompromised individuals and annual vaccination is strongly recommended for SOT candidate and recipients 9 months and older and their close contacts (31). Live attenuated influenza vaccine should not be given to SOT recipients given the theoretical concern for viral replication. Although current recommendations are the same as those for the general public, SOT recipients likely have decreased immune response to the influenza vaccine and may benefit from alternative strategies, such as high-dose influenza vaccine or an additional booster dose (14, 32, 33). The optimal timing of influenza vaccine administration post-transplant remains a topic of interest. Current recommendations are for the influenza vaccine to be given 3 months post-transplant, although it is often given as early as 1 month post-transplant when there is community spread.
A number of COVID-19 vaccines are approved and the most up-to-date recommendations can be found on the CDC and AST websites (34, 35). Vaccination against SARS-CoV-2 is strongly recommended for everyone 6 months and older, and ideally should be completed prior to transplantation. However, it can be given as early as 1–3 months post-transplant depending on community circulation. Three doses of an mRNA vaccine is recommended as the primary series for SOT recipients given decreased humoral and cellular immune response with two doses, although some SOT recipients will fail to mount any detectable humoral response even after booster doses (36). Current recommendations include age-appropriate bivalent booster dose to be given at least 2 months after the last dose of vaccine, although the CDC website should be consulted for the most up to-date information given the evolving nature of the COVID-19 pandemic.
Varicella vaccine (VZV) is a live-attenuated vaccine routinely administered as a 2-dose series at 12–15 months and 4–6 years of age, although the second dose can be given as early as 4 weeks after the first. While efficacy is not assured, infants can receive a dose at 6 months with a second and third dose given if transplant has not occurred by 12 months of age (13, 14). Passive antibody from blood products may interfere with efficacy and timing of vaccination should be based on CDC recommended time intervals, unless serology is negative (12). Serology should be checked prior to transplant. Efforts should be made to administer both doses of VZV vaccine prior to transplant given concerns for safety of live vaccine administration post-transplant until immunosuppression can be lessened. If vaccine is given and an organ becomes available within 4 weeks, acyclovir can be administered to prevent vaccine virus infection. After SOT, VZV vaccine administration can be considered in select individuals (Table 4) (12).
The live-attenuated measles, mumps, rubella (MMR) vaccine is typically given as a two-dose series at 12–15 months and 4–6 years of age. Similar to VZV vaccine, the second dose can be given with only a 4-week interval and for infants a dose can be given as early as 6 months of age. This should strongly be considered in infants not anticipated to be transplanted within 4 weeks, as post-transplant administration is generally contraindicated or may be considerably delayed (12). Early administration may result in reduced immunogenicity from immature immune responses and interfering maternal antibodies, and thus the two-dose series should be re-initiated if transplant has not occurred by 12 months of age and is not anticipated to occur within 4 weeks. Vaccine should be delayed an appropriate interval if blood products or immunoglobulin has been given due to interference with immune response. MMR serology should be checked prior to transplant.
You are called about a 3-year-old girl who is now 2 years post-liver transplant without concerns for rejection. She remains on tacrolimus. Parents are concerned she will start preschool in the fall and may be exposed to additional germs from classmates. What vaccine recommendations can you provide to parents?
In general, inactivated vaccines are safe to administer 3–6 months post-transplant, although the optimal timing depends on the individual circumstances and net immunosuppression (14). If the individual in case 2 were not up-to-date on any of her primary vaccine series, it would be important to administer them in an expedited schedule at this time (Table 1). Live vaccines have not traditionally been recommended post-transplant, however can be considered in select individuals who meet clinical and immunologic criteria listed in Table 4. There is increasing evidence for the safety and immunogenicity of live vaccines given after SOT and expert guidelines have been developed for select kidney and liver transplant recipients (12). Live vaccines can considered in individuals who are clinically well, at least 1 year post-transplant without concern for rejection, on low-level immune suppression, and meet minimum immune criteria (12). If the individual in case 2 had normal immune parameters, Varicella and then MMR vaccines could be offered to the family. Results from a multicenter study containing 281 pediatric kidney and liver transplant recipients who received Varicella or MMR vaccines found live vaccines to be both safe and immunogenic (37).
While significant advances have been made in the field, uncertainty remains about optimal timing, durability of immune responses post-transplant and need for booster doses, and the safety and immunogenicity of new vaccines (38). In addition, the approach to live vaccines both pre and post-transplant is variable across institutions and warrants further study. In a small case series of 5 pediatric heart and liver recipients who received live vaccines 8–21 days prior to transplant, none developed vaccine-related adverse events or concern for viral illness (39). This suggests that a shorter interval may be safe, and transplant should not be delayed if live vaccines are inadvertently administered; although, it is important to note the sample size was small and four of the five individuals received post-exposure prophylaxis with IVIG and antiviral therapy.
There are a number of novel vaccine candidates in various stages of development, including candidates against respiratory syncytial virus (RSV) and cytomegalovirus. The first vaccine against RSV was recently approved by the US Food and Drug Administration for use in adults 60 years and older. RSV is a common cause of respiratory viral infections in infants and children and significant cause of severe infection in SOT recipients. There are a number of RSV vaccine candidates in late stages of clinical trials, and some already approved for pregnant women and the elderly with the hope for future trials in immunocompromised individuals.
Vaccine preventable infections remain a significant problem in pediatric SOT candidates and recipients. Efforts to optimize pre and post-transplant vaccines should be a priority for all clinicians taking care of SOT patients in order to optimize post-transplant outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CK: Writing – original draft, Writing – review and editing. MM: Writing – original draft, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported in part by the NIH/National Center for Advancing Translational Sciences (NCATS), grant UL1TR002345.
This project was funded in part by the Foundation for Barnes-Jewish Hospital and their generous donors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of hospitalization for vaccine-preventable infections in children following solid organ transplant and associated morbidity, mortality, and costs. JAMA Pediatr. (2019) 173(3):260–8. doi: 10.1001/jamapediatrics.2018.4954
2. Theodoropoulos NM, La Hoz RM, Wolfe C, Vece G, Bag R, Berry GJ, et al. Donor derived hepatitis B virus infection: analysis of the organ procurement & transplantation network/united network for organ sharing ad hoc disease transmission advisory committee. Transpl Infect Dis. (2021) 23(1):e13458. doi: 10.1111/tid.13458
3. Kumar D, Humar A, Plevneshi A, Green K, Prasad GV, Siegal D. Invasive pneumococcal disease in solid organ transplant recipients-10-year prospective population surveillance. Am J Transplant. (2007) 7(5):1209–14. doi: 10.1111/j.1600-6143.2006.01705.x
4. Walti LN, Mugglin C, Mombelli M, Manuel O, Hirsch HH, Khanna N, et al. Vaccine-preventable infections among solid organ transplant recipients in Switzerland. JAMA Netw Open. (2023) 6(4):e2310687. doi: 10.1001/jamanetworkopen.2023.10687
5. Feldman AG, Sundaram SS, Beaty BL, Kempe A. Hospitalizations for respiratory syncytial virus and vaccine-preventable infections in the first 2 years after pediatric liver transplant. J Pediatr. (2017) 182:232–238.e1. doi: 10.1016/j.jpeds.2016.12.021
6. Feldman AG, Hsu EK, Mack CL. The importance of prioritizing pre and posttransplant immunizations in an era of vaccine refusal and epidemic outbreaks. Transplantation. (2020) 104(1):33–8. doi: 10.1097/TP.0000000000002936
7. Abbasi J. Amid Ohio measles outbreak, new global report warns of decreased vaccination during COVID-19 pandemic. J Am Med Assoc. (2023) 329(1):9–11. doi: 10.1001/jama.2022.23241
8. Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. (2013) 8(2):e56974. doi: 10.1371/journal.pone.0056974
9. Knackstedt ED, Danziger-Isakov L. Infections in pediatric solid-organ transplant recipients. Semin Pediatr Surg. (2017) 26(4):199–205. doi: 10.1053/j.sempedsurg.2017.07.001
10. Avery RK, Michaels MG, AST Infectious Diseases Community of Practice. Strategies for safe living following solid organ transplantation-guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13519. doi: 10.1111/ctr.13519
11. CDC. Catch-up immunization schedule for children and adolescents who start late or who are more than 1 month behind (2023). Available at: https://www.cdc.gov/vaccines/schedules/hcp/imz/catchup.html (Accessed July 6, 2023).
12. Suresh S, Upton J, Green M, Pham-Huy A, Posfay-Barbe KM, Michaels MG, et al. Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Pediatr Transplant. (2019) 23(7):e13571. doi: 10.1111/petr.13571
13. Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. (2014) 58(3):309–18. doi: 10.1093/cid/cit816
14. Danziger-Isakov L, Kumar D, AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13563. doi: 10.1111/ctr.13563
15. Feldman AG, Sundaram SS, Beaty BL, Torres R, Curtis DJ, Kempe A. Immunization status at the time of liver transplant in children and adolescents. J Am Med Assoc. (2019) 322(18):1822–4. doi: 10.1001/jama.2019.14386
16. Feldman AG, Marsh R, Kempe A, Morris MA. Barriers to pretransplant immunization: a qualitative interview study of pediatric solid organ transplant stakeholders. J Pediatr. (2020) 227:60–8. doi: 10.1016/j.jpeds.2020.07.038
17. Malinis M, Boucher HW, AST Infectious Diseases Community of Practice. Screening of donor and candidate prior to solid organ transplantation-guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13548. doi: 10.1111/ctr.13548
18. CDC. General best practice guidelines for immunization: contraindications and precautions (2022). Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html (Accessed 2023, May 8).
19. Wodi AP, Murthy N, McNally V, Cineas S, Ault K. Advisory committee on immunization practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2023. Morb Mortal Wkly Rep. (2023) 72(6):137–40. doi: 10.15585/mmwr.mm7206a1
20. Ball M, Liverman R, Serluco A, Yildirim I. Vaccine-induced protection against hepatitis B in pediatric solid organ transplant patients. Pediatr Transplant. (2021) 25(3):e13920. doi: 10.1111/petr.13920
21. Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, et al. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. (2007) 7(1):195–200. doi: 10.1111/j.1600-6143.2006.01618.x
22. Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the advisory committee on immunization practices for use of a hepatitis B vaccine with a novel adjuvant. Morb Mortal Wkly Rep. (2018) 67(15):455–8. doi: 10.15585/mmwr.mm6715a5
23. Lee GM. Preventing infections in children and adults with asplenia. Hematology Am Soc Hematol Educ Program. (2020) 2020(1):328–35. doi: 10.1182/hematology.2020000117
24. Ryerson AB, Lang D, Alazawi MA, Neyra M, Hill DT, St George K, et al. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case - New York, March 9-October 11, 2022. Morb Mortal Wkly Rep. (2022) 71(44):1418–24. doi: 10.15585/mmwr.mm7144e2
25. Kobayashi M, Farrar JL, Gierke R, Leidner AJ, Campos-Outcalt D, Morgan RL, et al. Use of 15-valent pneumococcal conjugate vaccine among U.S. children: updated recommendations of the advisory committee on immunization practices - United States, 2022. Morb Mortal Wkly Rep. (2022) 71(37):1174–81. doi: 10.15585/mmwr.mm7137a3
26. Centers for Disease Control and Prevention. ACIP Updates: recommendations for use of 20-valent pneumococcal conjugate vaccine in children - United States, 2023. Morb Mortal Wkly Rep. (2023) 72(39):1072. doi: 10.15585/mmwr.mm7239a5
27. Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, et al. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. (2020) 69(9):1–41. doi: 10.15585/mmwr.rr6909a1
28. Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the advisory committee on immunization practices, 2020. MMWR Recomm Rep. (2020) 69(5):1–38. doi: 10.15585/mmwr.rr6905a1
29. Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. (2013) 13(12):3202–9. doi: 10.1111/ajt.12472
30. Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. (2013) 13(9):2411–7. doi: 10.1111/ajt.12329
31. Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. (2018) 67(9):1322–9. doi: 10.1093/cid/ciy294
32. Natori Y, Shiotsuka M, Slomovic J, Hoschler K, Ferreira V, Ashton P, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. (2018) 66(11):1698–704. doi: 10.1093/cid/cix1082
33. Odongo FCA, Braga PE, Palacios R, Miraglia JL, Sartori AMC, Ibrahim KY, et al. An open-label randomized controlled parallel-group pilot study comparing the immunogenicity of a standard-, double-, and booster-dose regimens of the 2014 seasonal trivalent inactivated influenza vaccine in kidney transplant recipients. Transplantation. (2022) 106(1):210–20. doi: 10.1097/TP.0000000000003702
34. CDC. COVID-19 vaccines for people who are moderately or severely immunocompromised (2023). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (Accessed May 10, 2023).
35. AST. COVID-19 vaccine FAQ sheet (2023). Available at: https://www.myast.org/sites/default/files/01012023%20AST%20Vaccine%20Prof%20FAQ%20FINAL.pdf (Accessed July 7, 2023).
36. Dulek DE, Ardura MI, Green M, Michaels MG, Chaudhuri A, Vasquez L, et al. Update on COVID-19 vaccination in pediatric solid organ transplant recipients. Pediatr Transplant. (2022) 26(5):e14235. doi: 10.1111/petr.14235
37. Feldman AG, Beaty BL, Ferrolino JA, Maron G, Weidner HK, Ali SA, et al. Safety and immunogenicity of live viral vaccines in a multicenter cohort of pediatric transplant recipients. JAMA Netw Open. (2023) 6(10):e2337602. doi: 10.1001/jamanetworkopen.2023.37602
38. Dulek DE, de St Maurice A, Halasa NB. Vaccines in pediatric transplant recipients-past, present, and future. Pediatr Transplant. (2018) 22(7):e13282. doi: 10.1111/petr.13282
Keywords: vaccines, immunizations, vaccine-preventable infections, solid organ transplant, pre-transplant evaluation
Citation: Kao CM and Michaels MG (2023) Approach to vaccinating the pediatric solid organ transplant candidate and recipient. Front. Pediatr. 11:1271065. doi: 10.3389/fped.2023.1271065
Received: 1 August 2023; Accepted: 27 October 2023;
Published: 8 November 2023.
Edited by:
Gabriela Maron, St. Jude Children’s Research Hospital, United StatesReviewed by:
Marc Foca, Albert Einstein College of Medicine, United States© 2023 Kao and Michaels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carol M. Kao a2FvY0B3dXN0bC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.