
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 18 January 2024
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1266738
 Rafael Pricoco1
Rafael Pricoco1 Paulina Meidel1
Paulina Meidel1 Tim Hofberger1
Tim Hofberger1 Hannah Zietemann1
Hannah Zietemann1 Yvonne Mueller1
Yvonne Mueller1 Katharina Wiehler1
Katharina Wiehler1 Kaja Michel1
Kaja Michel1 Johannes Paulick1
Johannes Paulick1 Ariane Leone1
Ariane Leone1 Matthias Haegele1
Matthias Haegele1 Sandra Mayer-Huber1
Sandra Mayer-Huber1 Katrin Gerrer1
Katrin Gerrer1 Kirstin Mittelstrass1
Kirstin Mittelstrass1 Carmen Scheibenbogen2
Carmen Scheibenbogen2 Herbert Renz-Polster3
Herbert Renz-Polster3 Lorenz Mihatsch1*†
Lorenz Mihatsch1*† Uta Behrends1,4,†
Uta Behrends1,4,†
Background: Infectious mononucleosis after primary infection with Epstein-Barr virus (EBV-IM) has been linked to the development of myalgic encephalomyelitis/chronic fatigue-syndrome (ME/CFS) in children, adolescents, and young adults. Here, we present clinical phenotypes and follow-up data from a first German cohort of young people with ME/CFS following EBV-IM.
Methods: 12 adolescents and 13 young adults were diagnosed with IM-triggered ME/CFS at our specialized tertiary outpatient service by clinical criteria requiring post-exertional malaise (PEM) and a history of confirmed EBV primary infection as triggering event. Demographic information, laboratory findings, frequency and severity of symptoms, physical functioning, and health-related quality of life (HRQoL) were assessed and re-evaluated 6 and 12 months later.
Results: Young adults displayed more severe symptoms as well as worsening of fatigue, physical and mental functioning, and HRQoL throughout the study, compared to adolescents. After one year, 6/12 (54%) adolescents no longer met the diagnostic criteria for ME/CFS while all young adults continued to fulfill the Canadian consensus criteria. Improvement in adolescents was evident in physical functioning, symptom frequency and severity, and HRQoL, while young adults showed little improvement. EBV serology and EBV DNA load did not correlate with distinct clinical features of ME/CFS, and clinical chemistry showed no evidence of inflammation. Remarkably, the median time from symptom onset to ME/CFS diagnosis was 13.8 (IQR: 9.1–34.9) months.
Conclusions: ME/CFS following EBV-IM is a severely debilitating disease often diagnosed late and with limited responses to conventional medical care, especially in adults. Although adolescents may have a better prognosis, their condition can fluctuate and significantly impact their HRQoL. Our data emphasize that biomarkers and effective therapeutic options are also urgently needed to improve medical care and pave the way to recovery.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex and debilitating multi-system disease characterized by fatigue, post-exertional malaise (PEM) and additional symptoms, including unrefreshing sleep, cognitive impairment, orthostatic intolerance, and/or chronic pain. Up to 25% patients are severely affected and bound to home or bed (1, 2). ME/CFS has been identified as an important cause for long-lasting school absence (3–8) and is associated with a significant reduction of health-related quality of life (HRQoL) (7, 9–11).
Pre-pandemic global prevalence estimates for ME/CFS were 0.3%–0.5%, with age peaks at onset of 11–19 and 30–39 years (12). The prevalence reported for children and adolescents ranged from 0.1% to 1.9%, depending on case definitions, geographical region, and screening methods. Up to 95% of children with ME/CFS may remain undiagnosed (13). Adolescent girls represent the majority of pediatric ME/CFS patients, with a post-pubertal female-to-male ratio of 3–4:1 (8, 13).
Infectious triggers of ME/CFS account for 23%–90% pediatric cases (4, 14–16). 80% pediatric ME/CFS patients of a large Australian cohort recalled an initial infection, and 40% an infectious mononucleosis (IM) by Epstein-Barr virus (EBV) (7). ME/CFS was reported in 13%, 7%, and 4% adolescents in the US at 6, 12, and 24 months (17, 18), and in 23% college students at 3–6 months after EBV-IM, respectively (19). While EBV was the most prominent trigger of ME/CFS until 2019 (7, 18–30), it became outranked by severe acute respiratory coronavirus type 2 (SARS-CoV2), which was estimated to cause at least a doubling of ME/CFS cases worldwide, including Germany (31–33).
The pathomechanisms of ME/CFS remain unclear. Genetic polymorphisms might contribute to pathogenic immune dysregulation (34). Emerging evidence suggests vascular changes causing hypoperfusion of muscles and brain (35). Microbiome dysbiosis, defects in energy metabolism, dysregulated hormones, and vagus nerve dysfunction have been discussed (20, 36–38). A causative role of human herpes virus (HHV) reactivation was evaluated but has not been proven yet (39–44). We recently reported, that EBV (HHV4) might initiate autoimmunity by molecular mimicry (45).
Candidate risk factors for EBV-triggered ME/CFS include disease severity and days-in-bed during the acute phase, initial pain and autonomic symptoms, lower mental health scores, higher scores for anxiety, depression, and perceived stress, female gender, as well as distinct laboratory findings (e.g., elevated C-reactive protein and cytokine levels). However, different case definitions have been used and findings were inconsistent (19, 23, 27–29). Jason and colleagues found that baseline anxiety, stress, depression, or coping skills did not predict the development of ME/CFS after EBV-IM, while preceding symptoms of the ME/CFS spectrum increased the risk (19).
ME/CFS is diagnosed according to clinical case definitions and after thorough differential diagnosis (46). In adults the Institute of Medicine (IOM) criteria (47) are recommended for screening and the Canadian Consensus Criteria (CCC) (48) for diagnosis and research. For children and adolescents the CCC were adapted in a “pediatric case definition” by Jason and colleagues (PCD-J) (49) and a “clinical diagnostic worksheet” developed by Rowe and colleagues (CDW-R) (6). All four scores require PEM. Comorbidities can include autoimmune thyroiditis, hypermobile Ehlers Danlos syndrome (hEDS), and postural orthostatic tachycardia syndrome (PoTS) (6, 46).
No specific ME/CFS treatment is available yet. Consequent self-management with pacing was recommended together with non-pharmaceutical and pharmaceutical approaches to reduce the severity and frequency of symptoms. Psychosocial support may help with implementing coping strategies, and occupational therapy can support daily life and education (6, 46, 50). Promising experimental strategies are targeting the immune, vascular, and nervous system (51).
With adequate treatment, the course of disease seems to be better in children and adolescents compared to adults, with pediatric recovery rates of 5%–83% (4, 6–8, 14–16, 26, 52–58). Recovery rates in young people have been operationalized by measuring school attendance, symptom frequency and severity, as well as fulfillment of diagnostic criteria (53, 59). In an Australian pediatric cohort, one and two thirds of the patients recovered after 5 and 10 years, respectively, with a median disease duration of 5 (1–14) years in those who recovered (7).
However, in many cases the course of ME/CFS is fluctuating, with periods of deterioration (“crashes”), stabilization, improvement, or relapse-remitting cycles (6, 7, 22). About 40% of adult patients are estimated to improve over time, but only 5% fully recover (60, 61). Inferior outcomes might in part be due to inappropriate management resulting from inadequate disease-specific knowledge of medical staff (3, 52, 62, 63), to a lack of medical services and barriers to the health care system for patients with ME/CFS (64–66), and to stigmatization.
Here, we present a first German cohort of young ME/CFS patients diagnosed after confirmed EBV-IM at our MRI Chronic Fatigue Center for Young People (MCFC) and participating in our prospective MUC-CFS studies. The MCFC, so far, is Germany's sole pediatric university center specialized on ME/CFS research and care. The MUC-CFS studies offer comprehensive insights into patient demographics, clinical phenotypes, and health-related quality of life (HRQoL) at diagnosis and during follow-up. Our primary objective was to assess disease trajectories at 6 and 12 months after ME/CFS diagnosis to explore potential age-sepcific differences.
A cohort of 12 adolescents and 13 adults was diagnosed with ME/CFS after confirmed EBV-IM from March 2019 to November 2022 at our tertiary pediatric university hospital, enrolled in our single-center prospective MUC-CFS cohort studies, and reassessed at 6 and 12 months. Confirmed EBV-IM was defined as a combination of typical symptoms (e.g., fever, fatigue, sore throat, lymphadenopathy, and/or splenomegaly) and typical serology (positive IgM and/or IgG antibodies against EBV viral capsid antigen (VCA) without IgG antibodies against EBV nuclear antigen 1 (EBNA-1), in some cases with documented subsequent EBNA-1-IgG seroconversion). Diagnostic ME/CFS criteria were applied depending on age: For adults (≥18 years) the CCC were used. Adolescents needed to meet either the CCC or the less strict CDW-R criteria, with a disease duration of at least 3 or 6 months, respectively. PEM had to last for more than 14 h after mild exertion. All patients underwent a thorough differential diagnostic work-up (laboratory analyses, ECG, UCG, EEG, cMRI, pulmonary function analyses, psychological evaluation, additional investigations depending on symptoms) as recommended (6). A 10-minute passive standing test screened for orthostatic intolerance (OI), PoTS, or orthostatic hypotonia (OH). All patients received a symptom-oriented, non-pharmaceutical and/or pharmaceutical treatment, were guided on self-management, and were provided with psychosocial support, including adapted school education and home care if needed.
Clinical data were collected from clinical records and questionnaires. For personal or telephone follow-up visits, questionnaires were mailed to the families one month in advance. Five well-established patient-reported outcome measures (PROM) were used: (i) The Pediatric Quality of Life Inventory (PedsQL) was used to assess HRQoL in pediatric patients. It comprises 20 items and four subscales, namely physical, emotional, social, and school functioning, with good internal consistency and reliability (67). (ii) The Short Form-36 Health Survey (SF-36) is a well-validated 36-item questionnaire for measuring HRQoL in people older than 13 years, with eight subscales (physical functioning, role physical, general health, bodily pain, social functioning, vitality, role emotional, and mental health) ranging from 0 to 100. Lower scores indicate more impairment (68). (iii) The Chalder Fatigue Scale (CFQ) measures physical and mental fatigue and consists of eleven items on a Likert scale from 0 to 3. The total score ranges from 0 to 33, with 33 indicating most severe fatigue (69). (iv) The Charité Symptom Inventory (CSI), adapted from the CDC Symptom Inventory, rates frequency and severity of typical symptoms of ME/CFS within the month prior to the visit. Scales rate from 0 (not present) to 3 (severe) for severity and from 0 (not present) to 4 (always) for frequency of symptoms (70). (v) The Bell Score assesses the severity of ME/CFS by evaluating the impairment of daily activities (71); for adolescents the wording was adapted (e.g., “school” instead of “work”).
Statistical analyses utilized R version 4.2.1 (“Funny-Looking Kid”) (72). Categorial variables were compared using Fisher's exact test or Pearson's χ2 test. Numeric variables were compared between groups using the Wilcoxon rank-sum or Kruskal–Wallis test, as appropriate. Spearman's rank coefficient assessed correlations. Cox regression analysed association between independent variables and the time-to first presentation in the MCFC. Repeated measures correlation gauged within-subject PROMs' correlation (73). Due to small sample size and no adjustment for multiple testing, all P-values were considered exploratory. Significance level was set to α = 0.05.
Baseline characteristics are shown in Table 1. All 25 patients (80% female) had a history of EBV-IM with typival symptoms and documented serological findings indicating EBV primary infection at the time of disease onset. Adolescents (48%, median age at onset 15, IQR 13–15) did not differ from young adults (≥18 years) (52%, median age at onset 10, IQR: 18–21) with regard to demographics, medical and family history, and current medical care. The median time between EBV-IM and ME/CFS diagnosis at the first visit was 13.8 months (range 4–84), with no significant difference between males and females (P = 0.272), and/or adults and adolescents (P = 0.596). The time delay from symptom onset to diagnosis was less than 6, 12, and 24 months in 1/13 (8%), 5/13 (38%), and 7/13 (54%) adults as well as in 1/12 (8%), 5/12 (42%), and 10/12 (83%) adolescents (Supplementary Figure S1).
All adults met the CCC and all adolescents the CDW-R criteria and/or CCC, as required. Adults did not significantly differ from adolescents with regard to the baseline Bell Score or the SF-36 physical (PCS) and mental health component summary score (MCS). However, adults showed significantly higher CFQ scores (adults: 28 ± 4; adolescents: 22 ± 5; P = 0.006), and significantly lower PedsQL values (adults: 35 ± 11; adolescents: 54 ± 9; P = 0.002) compared to adolescents. At the time of diagnosis, all adolescents reported school absences, 2/11 (18%) received complementary home schooling and none had distance schooling. One patient reported a documented degree of disability, and none had received medical care at home.
24/25 (96%) patients showed comorbidities, with PoTS in 21/23 (83%) and allergies in 12/23 (48%) patients. Two patients droped out of the 10-min passive standing test due to severe OI symptoms. One patient presented with a diagnosis of anxiety disorder, and two with a mixed anxiety and depressive disorder. 17/24 (71%) patients took various nutritional supplements, and 11/24 (46%) prescription-only medications, including three patients on antidepressants. With regard to the family's medical history, in 2/25 (8%) cases ME/CFS was reported. 16/25 (64%), 10/25 (40%), and 18/25 (72%) patients remembered a family member with EBV-IM, autoimmune diseases, or either one.
The cohort had consulted several (median 6, range 1–11) private practice doctors across five different specialties (range 1–11) for ME/CFS symptoms. 11/20 (55%) patients had visited at least one hospital. 7/20 (35%) had consulted a psychotherapist/psychologist, 9/20 (45%) a naturopath, 6/20 (30%) traditional Chinese medicine, 4/20 (20%) homeopathy, and 5/20 (25%) osteopathy.
Laboratory findings at the time of diagnosis were primarily unremarkable, without significant differences between adolescents and adults (Table 2). Besides low vitamin D levels in 14/24 (58%) patients (range 7–29 ng/ml), the most frequent laboratory findings were elevated antinuclear antibodies (ANA) present in 14/25 (56%) (range 1:100–1:800), elevated IgE in 7/25 (28%) and mild anemia in 4/25 (16%) cases. ANA titers were in the range of <1:160 in 2/6 (33%) adolescents, of 1:160–1:640 in 2/6 (33%) adolescents and 8/8 (100%) adults, and of ≥1:640 in 2/6 (33%) adolescents, with higher ANA titers compared to adults (P = 0.015). ANA titers did not significantly correlate with disease severity (Bell Score: P = 0.452; SF-36 PF: P = 0.858), were not significantly different between males and females (P = 0.521), and not associated with any sign of connective tissue disorders. Herpes simplex virus coinfection was not more frequent in adults compared to adolescents (P > 0.999). Neither total immunoglobulin serum levels nor phenotypes of peripheral blood lymphocytes revealed any evidence of primary immunodeficiency (PID) (Supplementary Table S1).
Results from EBV serology and real-time PCR at the first visit are displayed in Table 3 and did not differ significantly between adolescents. No EBV DNA was detected in plasma. 8/20 (40%) patients showed EBV DNA in peripheral blood cells (5/8 very low titers, 1/8 17.7 Geq/105, 1/8 70.1 Geq/105, and 1/8 121.8 Geq/105), and 14/25 (66%) in throat washes. EBV DNA load in throat washes did not significantly correlate with disease severity (Bell Score: P = 0.686; SF-36 PCS: P = 0.871). All patients showed anti-EBV-VCA IgG as expected, 23/25 (92%) had detectable anti-EBNA-1 IgG and 6/25 (24%) anti-EBV-VCA IgM. The detection of anti-EBV-VCA IgM did not significantly correlate with disease severity (Bell Score: P = 0.877; SF-36 PCS: P = 0.788). Results of EBV immunoblots revealed IgG antibodies against early antigens (EA) p54 and p138, the immediate early antigen BZLF1, virus capsid antigens (VCA) p23 and p18, and EBNA-1 in 8/25 (32%), 5/25 (20%), 18/25 (72%), 23/25 (92%), 24/25 (96%), and 22/25 (88%) patients, respectively.
Follow-up data were available at 6 months after ME/CFS diagnosis from 22/25 (88%) patients, including 10/13 (77%) adults and 12/12 (100%) adolescents, and at 12 months from 20/25 (80%) patients, including 9/13 (69%) adults and 11/12 (92%) adolescents. Reasons for drop out were recovery (one adolescent), worsening of symptoms (one adult), or unknown (two patients). Changes in CCC and CDW-R criteria fulfilment are shown in Figure 1. Seven adults fulfilled the CCC at all three visits. One became CCC negative at 6 months but met the CCC criteria again at 12 months (Figure 1A). Six adolescents were still positive for the CDW-R criteria at 6 months and only four at 12 months follow-up. One patient became CDW-R negative at 6 months but met the CDW-R criteria again at 12 months (Figure 1B). By 6 months one and by 12 months three additional pediatric patients had turned 18 years old, and therefore the CDW-R criteria were not applicable anymore (indicated by N/A in Figure 1C). The CCC criteria were fulfilled by 8/12 (67%), 4/12 (33%), and 4/11 (36%) adolescents at the first visit, 6 months and 12 months. Two adolescents who were CCC positive at 12 months had been negative at the previous visits (Figure 1C). 7/12 (58%) adolescents met either the CCC or the CDW-R criteria at 6 months, and 5/11 (45%) either of both at 12 months (Figure 1D). Patients with partial recovery still presented with some of the symptoms. Two patients reported on OI only, one on fatigue with limitations in daily life and headaches, and three on several symptoms without fatigue. All patients in partial remission were adolescents (P = 0.005) and had a relatively short illness duration of less than three years (mean 24 months, range 15–34 months). They had significantly less fatigue (CFQ Likert score: P = 0.001) and higher HRQoL (PedsQL: P = 0.026) at diagnosis compared to patients without partial remission (Supplementary Table S2). Patients in partial remission did not significantly differ in any of the other baseline characteristics and laboratory parameters tested, including EBV antibodies and DNA (Supplementary Tables S2,S3).
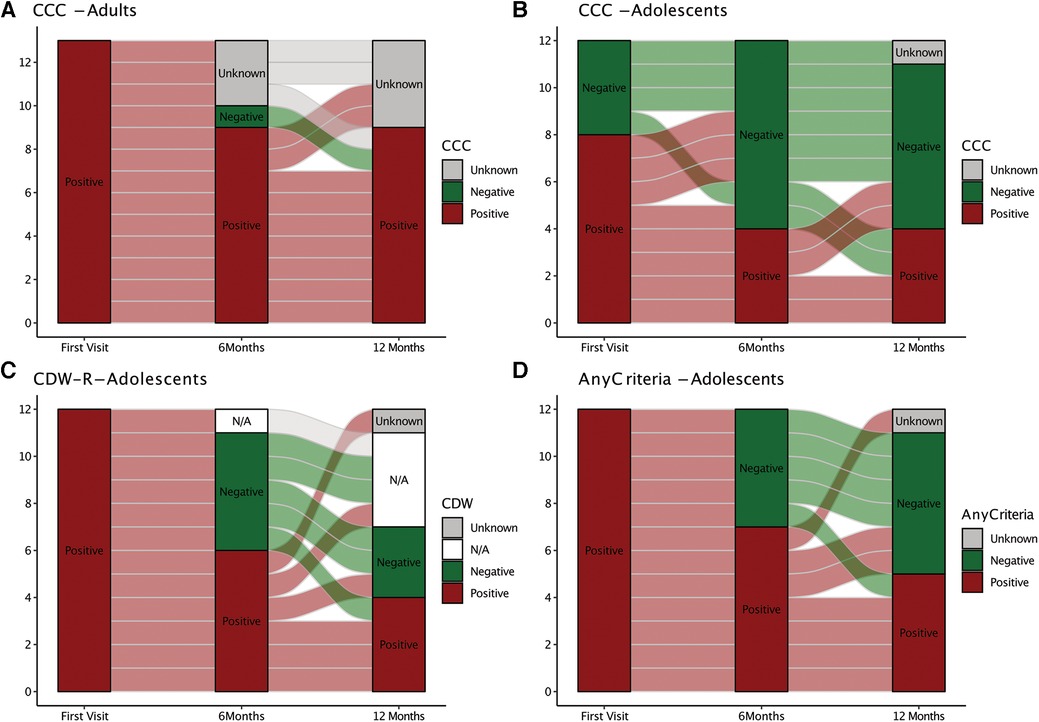
Figure 1. Alluvial chart illustrating ME/CFS diagnostic criteria fulfillment over time. The chart depicts diagnostic criteria fulfillment (red) or non-fullfillment (green) at the first visit and at 6 and 12 months. (Non-)fullfillment of the Canadian Consensus Criteria (CCC) is shown for adults (A). (Non)-fullfillment of CCC only (B), Rowe's diagnostic worksheet (CDW-R) criteria only, (C) or either of both (CCC or CDW-R) (D) is shown for adolescents. CDW-R criteria were not applicable anymore (N/A) when adolescents had turned 18 years.
At the baseline visit, patients presented with 27 ± 5 symptoms (mean ± SD), with 15 ± 5 occurring at least frequently (Figure 2). The symptoms reported at least frequently (3 or 4 on Likert scale) included fatigue (96%), limitations in daily life (96%), need for rest (92%) and PEM (83%). The most common severe (3 on Likert scale) symptoms were PEM (46%), stress intolerance (38%), fatigue (33%), limitations in daily life (33%), and unrefreshing sleep (33%). The number, severity, and frequency of individual symptoms did not significantly change between the first and follow-up visits (Supplementary Table S4). Adults reported slightly more symptoms (29 ± 3) than adolescents (25 ± 7, P = 0.084). Symptoms occurring at least frequently were more common in adults than adolescents (19 ± 6 vs. 12 ± 3, P = 0.006). This difference was also evident at the follow-up visits (Supplementary Table S5).
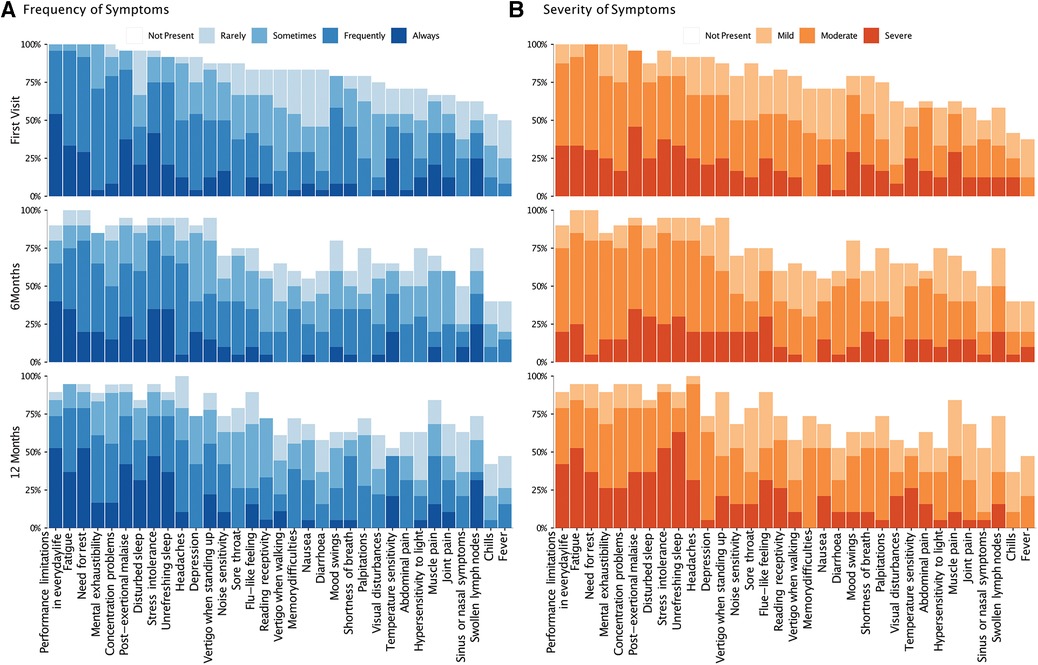
Figure 2. Frequency and severity of symptoms over time. The bar-chart displays individual symptoms on the x-axis. The y-axis shows the frequency (A) and severity (B) of symptoms on the left and right, respectively. The severity scale for each symptom ranged from 0 (not present) to 4 (severe), and the frequency scale from 0 (not present) to 5 (always present). At each time point, the chart shows the proportion of patients reporting the relevant symptom, with rating of severity and frequency rating, indicated by color-code.
At the first visit, the CFQ Likert score of the cohort was 25 ± 5 and did not significantly change over time. While adults showed a moderate worsening from the first (28 ± 4) to follow-up visits (28 ± 4 at 6months, 29 ± 4 at 12-months), adolescents demonstrated a moderate improvement (22 ± 5 at first visit, 19 ± 9 at 6 months, 18 ± 9 at 12-months) (Table 4 and Figure 3A). At all visits, adolescents had significantly less fatigue than adults (first visit: P = 0.006; 6-months: P = 0.016; 12 months: P = 0.003) (Supplementary Table S6).
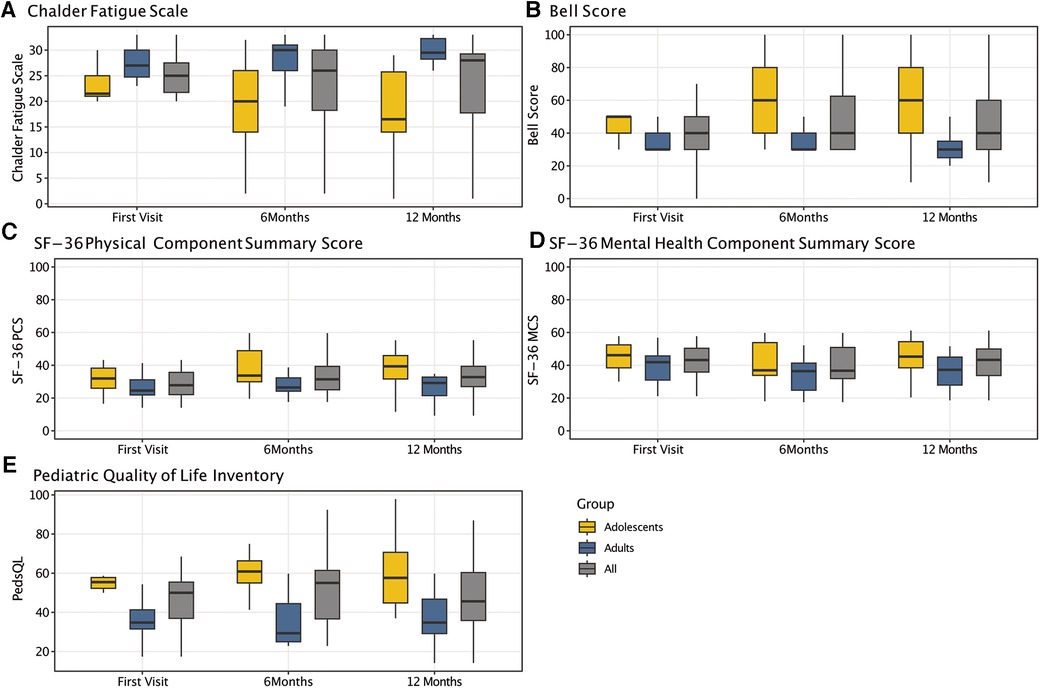
Figure 3. Results of patient-reported outcome measures over time. Boxplots displaying the dynamics of results from the chalder fatigue scale (CFQ) (A), the bell score (B), the SF-36 physical (C) (PCS) and mental health component summary score (D) (MCS), and the pediatric quality of life inventory (E) (PedsQL) for the entire cohort as well as for adolescents and adults only, respectively.
The median Bell Score was 40 (IQR: 30–50) and did not significantly change over time (P = 0.384), with a median adults' Bell Score of 30 at all visits. The adolescents' Bell Score moderately but not significantly improved from the first (median: 50, IQR: 40–50) to follow-up visits (both median: 60, IQR: 40–80) (P = 0.232) (Table 4 and Figure 3B). It was significantly better than adults' Bell Score at all visits (first visit: P = 0.019; 6 months: P = 0.019; 12 months: P = 0.007) (Supplementary Table S6).
The SF-36 summary and subscales did not significantly change between visits. However, adolescents had a significantly better PCS at the 12 months than adults (P = 0.013) (Table 4 and Figures 3C,D). Compared to adults, adolescents were significantly better at the first visit with regard to physical functioning (P = 0.039) and vitality (P = 0.012), at 6 months to physical functioning (P = 0.039), pain (P = 0.039), general health (P = 0.032), social (P = 0.025), and mental health (P = 0.025), and at 12 months to physical functioning (P = 0.019) and vitality (P = 0.010). There was no significant difference between adults and adolescents with regard to the MCS at any visit (Supplementary Table S6). At 12 monthst, 6/10 (60%) adolescents and none of the adults rated their general health at least somewhat better than in the previous year (Supplementary Table S7).
The PedsQL total score did not significantly change over time. The subscale scores were lowest for school and physical functioning, and highest for social functioning. Significant improvements over time were seen for adolescents' school functioning only (P = 0.03) (Table 4 and Figure 3E). Except for the school and emotional subscale, all subscales showed significant differences between adults and adolescents at all visits (Supplementary Table S6).
For all patients best correlations among PROMs were found for CFQ and PedsQL (r = −0.76, P < 0.001), indicating that more severe fatigue was associated with lower HRQoL (Figure 4). The most prominent difference between adults and adolescents was that adolescents' but not adults' CFQ and Bell Score correlated significantly (adults: r = −0.29, P = 0.209; adolescents: r = −0.77, P < 0.001).
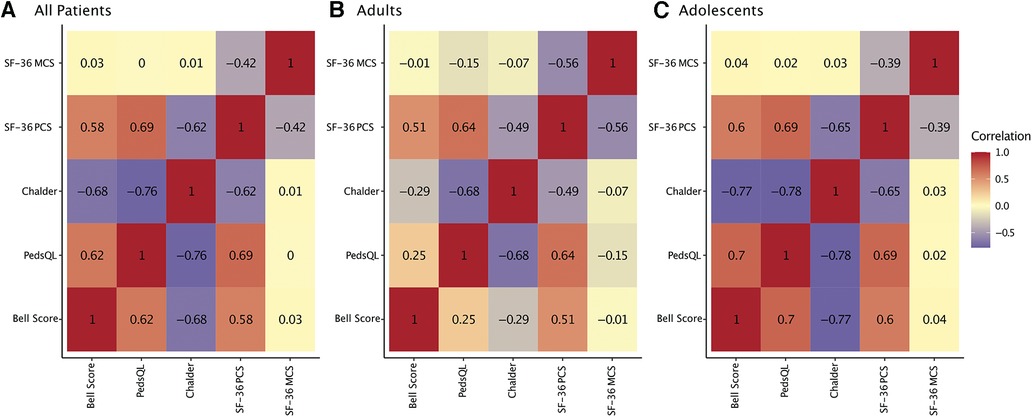
Figure 4. Correlation of patient-reported outcomes. Heatmap of repeated measures correlations between patient-reported outcomes (PROMs) for all patients (A), adults only (B), and adolescents only (C). Repeated measures correlations are a statistical tool to determine the overall within-patient correlation between a pair of variables.
At 12 months, results from PROMs for patients in partial remission vs. no remission were median Bell Score 80 (range 40–100) vs. 40 (range 20–80), mean CFQ Score 12.4 (SD 6.7) vs. 24.4 (SD 6.2), PedsQL total score 76.1 (SD 16.8) vs. 46.6 (SD 15.8), SF-36 PCS 44.7 (SD 7.7) vs. 28.9 (SD 11.5), and SF-36 MCS 50.9 (SD 7.1) vs. 42.9 (SD 10.4). These results again indicate that patients with partial recovery might still suffer from impairment of daily life.
This report contributes to rare follow-up data on young people with ME/CFS after EBV-IM. We present data on clinical phenotypes and HRQoL from a first German cohort of adolescents and young adults over time up to 12 months post ME/CFS diagnosis at our specialized tertiary pediatric center. So far, most data on pediatric and/or EBV-triggered ME/CFS originate from the US, the UK, and Australia, with no pediatric study from Germany (4, 6–8, 14–16, 26, 52–58). While some prospective pediatric studies examined ME/CFS with PEM after confirmed EBV-IM (17–19), to our knowledge, none compared adolescents and young adults with regard to symptom load and HRQoL over time.
Our youngest patient was 14 years-old, which was in line with the published ME/CFS age peak at 15–40 years (12, 46, 47). The observed female predominance (80%) is a widely recognized in post-pubertal ME/CFS patients (6, 20, 47). At the initial visit, all adults but only 8/12 (66%) met the CCC, supporting the use of more sensitive criteria for pediatric patients (52, 74, 75). Some pediatric follow-up studies employed the polythetic Fukuda criteria with the addition of mandatory PEM, while others used the broader Oxford criteria, potentially including individuals without ME/CFS (4, 7, 8, 14–16, 26, 53–58). To evaluate the CCC together with the CDW-R, the PCD-J, and the IOM criteria (47), we recently developed the Munich Berlin symptom questionnaire (MBSQ) (76).
The median diagnostic delay of more than one year was in line with most reports from other countries, indicating long and difficult patient journeys at any age (3, 52, 62, 63). We did not find any association of gender, age, or disease severity with time to diagnosis according to previous studies (62, 77). Published reasons for the diagnostic delay include insufficient knowledge by families and primary care providers, the requirement for comprehensive differential diagnosis, as well as negative attitudes and beliefs by primary care physicians and psychologists (52, 77, 78). A lack of ME/CFS specialists most likely exacerbates this issue. The young adults' longer disease duration prior to diagnosis possibly reflects challenges during transition from pediatric to adult health care services (79).
Comorbidities included PoTS (83%), allergies (48%), and psychiatric diagnoses (12%). The low prevalence of the latter alines with other reports on pediatric ME/CFS (6, 80, 81). PoTS has been reported in pediatric and adult ME/CFS cohorts with varying prevalence (6, 52). The large span of 5.7%–70% PoTS cases among adult ME/CFS patients (47) might in part be due to different PoTS tests and case definitions (82). Since PoTS is a frequent post-infectious phenomenon in adolescents (83) the high prevalence in our cohort was not unexpected. Since PoTS can significantly impair daily activities timely non-pharmaceutical and, if needed, pharmaceutical treatment is mandatory. In general, comorbidities are more prevalent in adult ME/CFS patients (79%–80%) (22, 81).
Only one of our patients had previously received a certificate of disability and none was supported by adequate medical devices or home care, reflecting poor medical care and barriers to specialized support (64–66). The large number of medical consultations prior to diagnosis, large proportion of our patients taking various dietary supplements and/or receiving complementary medical treatment, reflects the known lack of adequate, standard medical care and sets families at risk of financial challenges (7, 84).
All pupils in our study reported frequent school absences, and, remarkably, only a minority had received any educational assistance such as home or digital schooling. These findings align with earlier studies showing prolonged school absences and severely reduced social participation and education of young ME/CFS patients (3–8). This is particularly concerning, since pediatric patients with ME/CFS reported that remaining engaged in an education system that flexibly accommodated their illness and aspirations was crucial for their long-term functioning (7, 85, 86).
No established biomarker exist for ME/CFS, and standard laboratory tests typically yield unremarkable results (6, 52). Our patients mostly exhibited minor deviations, such as elevated ANA titers (56%), surpassing expectations for this age group (7). Elevated IgE levels were present in about a third, though previous studies found no clear associations with ME/CFS (87). Vitamin D deficiency was prevalent, yet it didn't seem directly linked to fatigue levels in another ME/CFS cohorts (88).
As expected, all patients showed anti-EBV VCA IgG as an indicator of previous EBV infection. Notably, undetectable EBNA-1 IgG and detection of anti-EBV VCA IgM, anti-EBV EA IgG, EBV DNA in throat washes weren't more common in our cohort than in the general population (89–92). Detectable EBV DNA in blood cells was more frequent than in a U.S. cohort without EBV-associated disorder (93). We found no significant correlation between EBV-specific results and disease severity or physical functioning, corresponding with earlier research that didn't establish a distinct pattern of EBV-specific virological results in ME/CFS patients (39, 41). However, our comprehensive EBV-specific immunological analyses suggest that EBV antigen mimicry might contribute to pathogenic autoimmunity (34, 43, 45, 94–98). While HHV, including EBV, are being discussed as potential causes or perpetuating factors of ME/CFS, no definite causal link has been established (39, 40, 41).
The majority of our adolescent patients partially recovered after 12 months, while all adults still met the CCC. The different health trajectories were also evident in the self-perceived health transition item of the SF-36 at 12 months, with 40% and 20% of adolescents rating their general health as much better or somewhat better, and 45% and 22% of adults much worse or somewhat worse than in the previous year, respectively. Over the whole study period symptom load (see below) and school functioning (PedsQL) significantly improved in adolescents but remained stable or worsened in adults.
These findings are in line with compelling evidence indicating a better ME/CFS prognosis of children and adolescence compared to adults, with pediatric studies reporting recovery of up to 83% (4, 6–8, 14–16, 26, 49–58). Dramatic improvement was reported to be more likely within the first four years (6). Accordingly, partial remission in our cohort was associated with illness duration of less than 3 years. A systematic review indicated that prognosis in adults is fairly poor, with only a minority of adult patients experiencing full recovery (60).
Only two pediatric patients were largely symptom-free (except OI) at their last visit. Additionally, we noticed fluctuations of disease load over time, with some patients not meeting the diagnostic criteria at 6 but again at 12 months. Remissions and relapses are frequent in pediatric ME/CFS and can follow overexertion or additional infectious illnesses (6). Our findings support the recommendation that patients should be monitored closely and adviced even after partial recovery. However, it remains challenging to measure recovery from ME/CFS, especially in young people, since what they consider as “recovery” can largely differ (7) and effective pacing might mask ongoing disease (61).
Candidate risk factors affecting the prognosis of ME/CFS include age, female gender, fatigue severity at disease onset, PEM severity, severity of ME/CFS symptoms, comorbidities, illness duration, life stressors, and lower socioeconomic status (6, 14, 15, 78, 99, 100), although findings remained inconclusive. Our findings suggest younger age, shorter disease duration, a better Bell Score, and milder fatigue (CFQ) at initial presentation could potentially indicate a more favorable disease course in adolescents compared to adults. The small patient sample size prohibits definite conclusions. The interpretation of published data on ME/CFS outcome is challenged by the fact that in many ME/CFS cohorts the initial trigger is less well characterized than in our cohort (7, 8, 14–16, 26, 54, 56).
Patients experienced a wide range of persisting symptoms with little change in severity or frequency over time, showing interindividual variability and intraindividual fluctuations throughout the year. Pediatric ME/CFS symptoms typically fluctuate more than symptoms in adults (6). Adolescents reported fewer symptoms at 6 and 12 months while adults' symptom count remained steady. Adults consistently reported more symptoms and nearly double the frequency of adolescents. Quantifying frequency and severity of symptoms was recommended to increase the specificity of ME/CFS diagnosis (101), since mild symptoms are common in the general population. Our novel MBSQ can be use to quantify the severity and frequency of ME/CFS symptoms in a 5-point Likert scale (76).
Previous studies revealed that ME/CFS profoundly affects social life, education, and HRQoL of children and young adults, showing poorer HRQoL compared to peers with various other chronic diseases (7, 9–11). Notably, our adolescent cohort's PedsQL results closely resembled those from other countries, depicting similar HRQoL distributions (9, 10, 102, 103), with worse HRQoL in physical and school function and better results in social and emotional functioning.
Over time, adolescents showed moderate improvements in total, physical, and psychosocial score, particularly in the school domain, although social and emotional aspects remained stable. These improvements exceeded suggested clinically meaningful differences in pediatric cohorts (104). Intensive school counseling might have contributed to better school situations and HRQoL changes. We found little evidence of improved HRQoL in young adults, except for some gains in emotional and social subdomains, likely due to specialized care. Compared to adolescents, young adults in our cohort reported significantly lower HRQoL, which aligns with general findings on adult ME/CFS patients consistently demonstrating very low HRQoL (105, 106). The transition from pediatrics to adult patient medicine can be particularly challenging for young people with ME/CFS, with uncertainties regarding health care, education, financials, and contact to peers. Unrevealing age-specific risk factors will be crucial for developing effective preventive strategies.
Few studies have investigated HRQoL in adolescents with ME/CFS. Factors contributing to low HRQoL were identified as high frequency of PEM, cognitive symptoms, regular school absence, delayed school progression, and attending physical therapy or rehabilitation. School support and attendance, along with leisure activities, correlated with better HRQoL (9, 10). Contradictory findings exist about the impact of depressive symptoms (9, 10, 103, 106). ME/CFS criteria requiring PEM might select patients with worse HRQoL compared to polythetic criteria (10), and this might be especially true for the complex CCC used to diagnose ME/CFS in our adult patients.
A strength of our study lies in providing long-term data on ME/CFS after serologically confirmed EBV-IM, supporting earlier reports on recovery (6, 7, 17). Confirming an infectious trigger of ME/CFS years later is challenging due to unreliable self-reports and to difficulties obtaining prior medical records. A second strength is the combined analyses of data from adolescents and young adults. The latter population often gets lost from pediatric as well as non-pediatric studies (107). Third and importantly, we provide data on ME/CFS cases that were diagnosed by clinical criteria requiring PEM as recommended by the European Network on ME/CFS research (EUROMENE) (46) and the Centers of Disease Control and Prevention (CDC) (79). Overall, our study adds to the current understanding of ME/CFS in young people and highlights the importance of an early diagnosis as well es of a thorough longitudinal evaluation of patients with ME/CFS following EBV-IM.
The study has limitations to be considered when interpreting the results. First, the low sample size and a potential selection bias limit the generalizability of results and may affect the statistical power. Second, although the drop-out rate of 20% at 12 months was deemed acceptable, it might contribute another bias. Third, the investigation of preexisting risk factors was limited, since patients were seen late after ME/CFS onset with potential recall bias. In addition, a longer follow-up period would be beneficial. Finally, the lack of a matched control group challenges the interpretation. Future studies with larger sample sizes, longer follow-up periods, and appropriate control groups are necessary to further validate and extend our findings.
In conclusion, ME/CFS after EBV-IM is a debilitating disease that results in severe functional impairment and poor HRQoL of both adolescents and young adults, with evidence of partial recovery in adolescents over time. Access to appropriate healthcare is a fundamental barrier for young people with ME/CFS in Germany as well as abroad. ME/CFS patients showed fluctuating symptoms, with adults reporting more symptoms, greater physical impairment, and worse HRQoL than adolescents. Laboratory findings did not provide any evidence for EBV replication perpetuating the disease. Further research is needed to clarify the responsible pathomechanisms, identify reliable biomarkers and risk-factors, and to develop effective strategies for ME/CFS treatments and prevention in young people.
The raw data supporting the conclusions of this article will be made available by the others upon reasonable request.
Patients and parents (of patients <18 years) provided informed written consent prior to inclusion. The study was approved by the Ethics Committee of the Technical University of Munich (529/18, 485/18) and conducted in accordance with the Declaration of Helsinki and its later amendments.
RP: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. PM: Conceptualization, Investigation, Validation, Writing – review & editing. TH: Conceptualization, Writing – review & editing. HZ: Investigation, Writing – review & editing. YM: Investigation, Writing – review & editing. KW: Investigation, Writing – review & editing. KaM: Writing – review & editing. JP: Investigation, Writing – review & editing. AL: Investigation, Writing – review & editing. MH: Writing – original draft, Writing – review & editing. SM-H: Formal Analysis, Writing – review & editing. KG: Conceptualization, Supervision, Writing – review & editing. KiM: Writing – review & editing. CS: Writing – review & editing. HR-P: Writing – review & editing. LM: Data curation, Formal Analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. UB: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work has been supported by the Lost Voices and Weidenhammer-Zoebele foundations.
We thank all patients who participated in this study and their parents for supporting their participation.
UB received research grants from Federal Ministry of Education and Research (BMBF), the Federal Ministry of Health (BMG), the Bavarian Ministry of Health and Care (StMGP), the Bavarian Ministry of Science and Arts (StMWK), the German Center for Infection Research (DZIF), the People for Children (Menschen für Kinder) Foundation, the Weidenhammer-Zöbele Foundation, the Lost-Voices Foundation, and the ME/CFS Research Foundation. CS was consulting Roche, Celltrend, and Bayer; she received support for clinical trials by Bayer, Fresenius, and Miltenyi, honoraria for lectures by Fresenius, AstraZeneca, BMS, Roche, Bayer, and Novartis, and research grants from the German Research Association (DFG), the BMBF, the BMG, the Weidenhammer-Zoebele Foundation, the Lost-Voices Foundation, and the ME/CFS Research Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1266738/full#supplementary-material
1. Unger ER, Lin JS, Tian H, Natelson BH, Lange G, Vu D, et al. Multi-site clinical assessment of myalgic encephalomyelitis/chronic fatigue syndrome (MCAM): design and implementation of a prospective/retrospective rolling cohort study. Am J Epidemiol. (2017) 185(8):617–26. doi: 10.1093/aje/kwx029
2. Pendergrast T, Brown A, Sunnquist M, Jantke R, Newton JL, Strand EB, et al. Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic Illn. (2016) 12(4):292–307. doi: 10.1177/1742395316644770
3. Crawley E, Sterne JA. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child. (2009) 94(10):752–6. doi: 10.1136/adc.2008.143537
4. Van Geelen SM, Bakker RJ, Kuis W, Van De Putte EM. Adolescent chronic fatigue syndrome. Arch Pediatr Adolesc Med. (2010) 164(9):810–4. doi: 10.1001/archpediatrics.2010.145
5. Nijhof SL, Maijer K, Bleijenberg G, Uiterwaal CSPM, Kimpen JLL, Van De Putte EM. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. (2011) 127(5):e1169–75. doi: 10.1542/peds.2010-1147
6. Rowe PC, Underhill RA, Friedman KJ, Gurwitt A, Medow MS, Schwartz MS, et al. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer. Front Pediatr. (2017) 5(121). doi: 10.3389/fped.2017.00121
7. Rowe KS. Long term follow up of young people with chronic fatigue syndrome attending a pediatric outpatient service. Front Pediatr. (2019) 7(21). doi: 10.3389/fped.2019.00021
8. Crawley EM, Emond AM, Sterne JA. Unidentified chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinics. BMJ Open. (2011) 1(2):e000252. doi: 10.1136/bmjopen-2011-000252
9. Similä WA, Halsteinli V, Helland IB, Suvatne C, Elmi H, Rø TB. Health-related quality of life in Norwegian adolescents living with chronic fatigue syndrome. Health Qual Life Outcomes. (2020) 18(1). doi: 10.1186/s12955-020-01430-z
10. Roma M, Marden CL, Flaherty MAK, Jasion SE, Cranston EM, Rowe PC. Impaired health-related quality of life in adolescent myalgic encephalomyelitis/chronic fatigue syndrome: the impact of core symptoms. Front Pediatr. (2019) 7:26. doi: 10.3389/fped.2019.00026
11. Kennedy G, Underwood C, Belch JJF. Physical and functional impact of chronic fatigue syndrome/myalgic encephalomyelitis in childhood. Pediatrics. (2010) 125(6):e1324–30. doi: 10.1542/peds.2009-2644
12. Bakken IJ, Tveito K, Gunnes N, Ghaderi S, Stoltenberg C, Trogstad L, et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008–2012. BMC Med. (2014) 12(1). doi: 10.1186/s12916-014-0167-5
13. Jason LA, Katz BZ, Sunnquist M, Torres C, Cotler J, Bhatia S. The prevalence of pediatric myalgic encephalomyelitis/chronic fatigue syndrome in a community-based sample. Child Youth Care Forum. (2020) 49(4):563–79. doi: 10.1007/s10566-019-09543-3
14. Josev EK, Cole RC, Scheinberg A, Rowe K, Lubitz L, Knight SJ. Health, wellbeing, and prognosis of Australian adolescents with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a case-controlled follow-up study. J Clin Med. (2021) 10(16):3603. doi: 10.3390/jcm10163603
15. Sankey A, Hill CM, Brown J, Quinn L, Fletcher A. A follow-up study of chronic fatigue syndrome in children and adolescents: symptom persistence and school absenteeism. Clin Child Psychol Psychiatry. (2006) 11(1):126–38. doi: 10.1177/1359104506059133
16. Bell DS, Jordan K, Robinson M. Thirteen-year follow-up of children and adolescents with chronic fatigue syndrome. Pediatrics. (2001) 107(5):994–8. doi: 10.1542/peds.107.5.994
17. Katz BZ, Jason LA. Chronic fatigue syndrome following infections in adolescents. Curr Opin Pediatr. (2013) 25(1):95–102. doi: 10.1097/MOP.0b013e32835c1108
18. Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. Br Med J. (2006) 333(7568):575. doi: 10.1136/bmj.38933.585764.AE
19. Jason LA, Cotler J, Islam MF, Sunnquist M, Katz BZ. Risks for developing myalgic encephalomyelitis/chronic fatigue syndrome in college students following infectious mononucleosis: a prospective cohort study. Clin Infect Dis. (2021) 73(11):e3740–6. doi: 10.1093/cid/ciaa1886
20. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. (2022) 28(5):911–23. doi: 10.1038/s41591-022-01810-6
21. Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Hanitsch LG, Zoller T, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. (2022) 13(1):5104. doi: 10.1038/s41467-022-32507-6
22. Chu L, Valencia IJ, Garvert DW, Montoya JG. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. (2019) 7:12. doi: 10.3389/fped.2019.00012
23. Pedersen M, Asprusten TT, Godang K, Leegaard TM, Osnes LT, Skovlund E, et al. Predictors of chronic fatigue in adolescents six months after acute Epstein-Barr virus infection: a prospective cohort study. Brain Behav Immun. (2019) 75:94–100. doi: 10.1016/j.bbi.2018.09.023
24. Moss-Morris R, Spence MJ, Hou R. The pathway from glandular fever to chronic fatigue syndrome: can the cognitive behavioural model provide the map? Psychol Med. (2011) 41(5):1099–107. doi: 10.1017/S003329171000139X
25. Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. (2009) 124(1):189–93. doi: 10.1542/peds.2008-1879
26. Gill AC, Dosen A, Ziegler JB. Chronic fatigue syndrome in adolescents: a follow-up study. Arch Pediatr Adolesc Med. (2004) 158(3):225–9. doi: 10.1001/archpedi.158.3.225
27. Candy B, Chalder T, Cleare AJ, Wessely S, White PD, Hotopf M. Recovery from infectious mononucleosis: a case for more than symptomatic therapy? A systematic review. Br J Gen Pract. (2002) 52(483):844–51.12392128
28. White PD, Thomas JM, Kangro HO, JBruce-ones WDA, Amess J, Crawford DH, et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet. (2001) 358(9297):1946–54. doi: 10.1016/S0140-6736(01)06961-6
29. Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med. (2000) 109(7):531–7. doi: 10.1016/S0002-9343(00)00560-X
30. White PD, Thomas JM, Amess J, Crawford DH, Grover SA, Kangro HO, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry. (1998) 173(6):475–81. doi: 10.1192/bjp.173.6.475
31. Mirin AA, Dimmock ME, Jason LA. Updated ME/CFS prevalence estimates reflecting post-COVID increases and associated economic costs and funding implications. Fatigue: Biomed Health Behav. (2022) 10(2):83–93. doi: 10.1080/21641846.2022.2062169
32. Komaroff AL, Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med. (2021) 7. doi: 10.3389/fmed.2020.606824
33. Roessler M, Tesch F, Batram M, Jacob J, Loser F, Weidinger O, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. (2022) 19(11):e1004122. doi: 10.1371/journal.pmed.1004122
34. Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun. (2012) 26(4):552–8. doi: 10.1016/j.bbi.2011.12.009
35. Wirth K, Scheibenbogen C. A unifying hypothesis of the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun Rev. (2020) 19(6):102527. doi: 10.1016/j.autrev.2020.102527
36. Renz-Polster H, Tremblay ME, Bienzle D, Fischer JE. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: the case for neuroglial failure. Front Cell Neurosci. (2022) 16:888232. doi: 10.3389/fncel.2022.888232
37. Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. (2021) 27(9):895–906. doi: 10.1016/j.molmed.2021.06.002
38. Cortes Rivera M, Mastronardi C, Silva-Aldana C, Arcos-Burgos M, Lidbury B. Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics. (2019) 9(3):91. doi: 10.3390/diagnostics9030091
39. Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. (2018) 16(1):268. doi: 10.1186/s12967-018-1644-y
40. Underhill RA. Myalgic encephalomyelitis, chronic fatigue syndrome: an infectious disease. Med Hypotheses. (2015) 85(6):765–73. doi: 10.1016/j.mehy.2015.10.011
41. Ruiz-Pablos M, Paiva B, Montero-Mateo R, Garcia N, Zabaleta A. Epstein-Barr virus and the origin of myalgic encephalomyelitis or chronic fatigue syndrome. Front Immunol. (2021) 12:656797. doi: 10.3389/fimmu.2021.656797
42. Loebel M, Strohschein K, Giannini C, Koelsch U, Bauer S, Doebis C, et al. Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS One. (2014) 9(1):e85387. doi: 10.1371/journal.pone.0085387
43. Shikova E, Reshkova V, Kumanova A, Raleva S, Alexandrova D, Capo N, et al. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J Med Virol. (2020) 92(12):3682–8. doi: 10.1002/jmv.25744
44. Komaroff AL. Chronic fatigue syndromes: relationship to chronic viral infections. J Virol Methods. (1988) 21(1-4):3–10. doi: 10.1016/0166-0934(88)90047-X
45. Sepúlveda N, Malato J, Sotzny F, Grabowska AD, Fonseca A, Cordeiro C, et al. Revisiting IgG antibody reactivity to Epstein-Barr virus in myalgic encephalomyelitis/chronic fatigue syndrome and its potential application to disease diagnosis. Front Med. (2022) 9:921101. doi: 10.3389/fmed.2022.921101
46. Nacul L, Authier FJ, Scheibenbogen C, Lorusso L, Helland IB, Martin JA, et al. European network on myalgic encephalomyelitis/chronic fatigue syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina. (2021) 57(5). doi: 10.3390/medicina57050510
47. Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. (2015) 313(11):1101–2. doi: 10.1001/jama.2015.1346
48. Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chronic Fatigue Syndr. (2003) 11(1):7–115. doi: 10.1300/J092v11n01_02
49. Jason LA, Jordan K, Miike T, Bell DS, Lapp C, Torres-Harding S, et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chronic Fatigue Syndr. (2006) 13(2-3):1–44. doi: 10.1300/J092v13n02_01
50. Bateman L, Bested AC, Bonilla HF, Chheda BV, Chu L, Curtin JM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. (2021) 96(11):2861–78. doi: 10.1016/j.mayocp.2021.07.004
51. Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, Wittke K, Stein E, Franke C, et al. Fighting post-COVID and ME/CFS – development of curative therapies. Front Med. 10. doi: 10.3389/fmed.2023.1194754
52. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Mil Med. (2015) 180(7):721–3. doi: 10.7205/MILMED-D-15-00085
53. Moore Y, Serafimova T, Anderson N, King H, Richards A, Brigden A, et al. Recovery from chronic fatigue syndrome: a systematic review—heterogeneity of definition limits study comparison. Arch Dis Child. (2021) 106(11):1087–94. doi: 10.1136/archdischild-2020-320196
54. Norris T, Collin SM, Tilling K, Nuevo R, Stansfeld SA, Sterne JA, et al. Natural course of chronic fatigue syndrome/myalgic encephalomyelitis in adolescents. Arch Dis Child. (2017) 102(6):522–8. doi: 10.1136/archdischild-2016-311198
55. Lim A, Lubitz L. Chronic fatigue syndrome: successful outcome of an intensive inpatient programme. J Paediatr Child Health. (2002) 38(3):295–9. doi: 10.1046/j.1440-1754.2002.00786.x
56. Rangel L, Garralda ME, Levin M, Roberts H. The course of severe chronic fatigue syndrome in childhood. J R Soc Med. (2000) 93(3):129–34. doi: 10.1177/014107680009300306
57. Krilov LR, Fisher M, Friedman SB, Reitman D, Mandel FS. Course and outcome of chronic fatigue in children and adolescents. Pediatrics. (1998) 102(2 Pt 1):360–6. doi: 10.1542/peds.102.2.360
58. Smith MS, Mitchell J, Corey L, Gold D, McCauley EA, Glover D, et al. Chronic fatigue in adolescents. Pediatrics. (1991) 88(2):195–202. doi: 10.1542/peds.88.2.195
59. Devendorf AR, Jackson CT, Sunnquist M, Jason LA. Approaching recovery from myalgic encephalomyelitis and chronic fatigue syndrome: challenges to consider in research and practice. J Health Psychol. (2017) 24(10):1412–24. doi: 10.1177/1359105317742195
60. Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med. (2005) 55(1):20–31. doi: 10.1093/occmed/kqi013
61. Collin SM, Crawley E. Specialist treatment of chronic fatigue syndrome/ME: a cohort study among adult patients in England. BMC Health Serv Res. (2017) 17(1):488. doi: 10.1186/s12913-017-2437-3
62. Knight S, Harvey A, Lubitz L, Rowe K, Reveley C, Veit F, et al. Paediatric chronic fatigue syndrome: complex presentations and protracted time to diagnosis. J Paediatr Child Health. (2013) 49(11):919–24. doi: 10.1111/jpc.12425
63. Solomon L, Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. (2004) 164(20):2241–5. doi: 10.1001/archinte.164.20.2241
64. Froehlich L, et al. Medical care situation of people with myalgic encephalomyelitis/chronic fatigue syndrome in Germany. Medicina. (2021) 57(7):646. doi: 10.3390/medicina57070646
65. Friedman KJ. Advances in ME/CFS: past, present, and future. Front Pediatr. (2019) 7:131. doi: 10.3389/fped.2019.00131
66. Sunnquist M, Nicholson L, Jason LA, Friedman KJ. Access to medical care for individuals with myalgic encephalomyelitis and chronic fatigue syndrome: a call for centers of excellence. Mod Clin Med Res. (2017) 1(1):28–35. doi: 10.22606/mcmr.2017.11005
67. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. (2001) 39(8):800–12. doi: 10.1097/00005650-200108000-00006
68. Morfeld M, Kirchberger I, Bullinger M. SF-36 Fragebogen zum Gesundheitszustand: Deutsche Version des Short Form-36 Health Survey. (2011).
69. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. (1993) 37(2):147–53. doi: 10.1016/0022-3999(93)90081-P
70. Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC symptom inventory for assessment of chronic fatigue syndrome. Popul Health Metr. (2005) 3(1). doi: 10.1186/1478-7954-3-8
71. Bell DS. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living With CFIDS. Boston: Addison-Wesley Pub. Co (1995).
72. R Core Team. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
73. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. (2017) 8. doi: 10.3389/fpsyg.2017.00456
74. Geraghty KJ, Adeniji C. The importance of accurate diagnosis of ME/CFS in children and adolescents: a commentary. Front Pediatr. (2019) 6. doi: 10.3389/fped.2018.00435
75. Collin SM, Nuevo R, van de Putte EM, Nijhof SL, Crawley E. Chronic fatigue syndrome (CFS) or myalgic encephalomyelitis (ME) is different in children compared to in adults: a study of UK and Dutch clinical cohorts. BMJ Open. (2015) 5(10):e008830. doi: 10.1136/bmjopen-2015-008830
76. Peo LC, Wiehler K, Paulick J, Gerrer K, Leone A, Viereck A, et al. Pediatric and adult patients with ME/CFS following COVID-19: a structured approach to diagnosis using the Munich Berlin symptom questionnaire (MBSQ). Eur J Pediatr. (2023). doi: 10.1007/s00431-023-05351-z
77. Webb CM, Collin SM, Deave T, Haig-Ferguson A, Spatz A, Crawley E. What stops children with a chronic illness accessing health care: a mixed methods study in children with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). BMC Health Serv Res. (2011) 11:308. doi: 10.1186/1472-6963-11-308
78. Ghali A, Lacout C, Fortrat J-O, Depres K, Ghali M, Lavigne C. Factors influencing the prognosis of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. (2022) 12:2540. doi: 10.3390/diagnostics12102540
79. White PH, Cooley WC. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. (2018) 142(5):e20182587. doi: 10.1542/peds.2018-2587
80. Loades ME, Sheils EA, Crawley E. Treatment for paediatric chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME) and comorbid depression: a systematic review. BMJ Open. (2016) 6(10):e012271. doi: 10.1136/bmjopen-2016-012271
81. Loades ME, Read R, Smith L, Higson-Sweeney NT, Laffan A, Stallard P, et al. How common are depression and anxiety in adolescents with chronic fatigue syndrome (CFS) and how should we screen for these mental health co-morbidities? A clinical cohort study. Eur Child Adolesc Psychiatry. (2021) 30(11):1733–43. doi: 10.1007/s00787-020-01646-w
82. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. (2011) 21(2):69–72. doi: 10.1007/s10286-011-0119-5
83. Katz BZ, Stewart JM, Shiraishi Y, Mears CJ, Taylor R. Orthostatic tolerance testing in a prospective cohort of adolescents with chronic fatigue syndrome and recovered controls following infectious mononucleosis. Clin Pediatr. (2012) 51(9):835–9. doi: 10.1177/0009922812455094
84. Porter NS, Jason LA, Boulton A, Bothne N, Coleman B. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. (2010) 16(3):235–49. doi: 10.1089/acm.2008.0376
85. Parslow R, Patel A, Beasant L, Haywood K, Johnson D, Crawley E. What matters to children with CFS/ME? A conceptual model as the first stage in developing a PROM. Arch Dis Child. (2015) 100(12):1141–7. doi: 10.1136/archdischild-2015-308831
86. Knight SJ, Politis J, Garnham C, Scheinberg A, Tollit MA. School functioning in adolescents with chronic fatigue syndrome. Front Pediatr. (2018) 6:302. doi: 10.3389/fped.2018.00302
87. Repka-Ramirez MS, Naranch K, Park YJ, Velarde A, Clauw D, Baraniuk JN. Ige levels are the same in chronic fatigue syndrome (CFS) and control subjects when stratified by allergy skin test results and rhinitis types. Ann Allergy Asthma Immunol. (2001) 87(3):218–21. doi: 10.1016/S1081-1206(10)62229-6
88. Earl KE, Sakellariou GK, Sinclair M, Fenech M, Croden F, Owens DJ, et al. Vitamin D status in chronic fatigue syndrome/myalgic encephalomyelitis: a cohort study from the North-West of England. BMJ Open. (2017) 7(11):e015296. doi: 10.1136/bmjopen-2016-015296
89. De Paschale M, Agrappi C, Manco MT, Mirri P, Viganò EF, Clerici P. Seroepidemiology of EBV and interpretation of the “isolated VCA IgG” pattern. J Med Virol. (2009) 81(2):325–31. doi: 10.1002/jmv.21373
90. Niller H-H, Bauer G. Epstein-Barr virus: clinical diagnostics, in Epstein Barr virus: methods and protocols. In: Minarovits J, Niller HH, New York, NY: Springer New York (2017). p. 33–55.
91. Ikuta K, Satoh Y, Hoshikawa Y, Sairenji T. Detection of Epstein-Barr virus in salivas and throat washings in healthy adults and children. Microbes Infect. (2000) 2(2):115–20. doi: 10.1016/S1286-4579(00)00277-X
92. Haque T, Crawford DH. PCR amplification is more sensitive than tissue culture methods for Epstein-Barr virus detection in clinical material. J Gen Virol. (1997) 78(12):3357–60. doi: 10.1099/0022-1317-78-12-3357
93. Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, Ambinder RF, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. (2016) 127(16):2007–17. doi: 10.1182/blood-2015-09-672030
94. Lerner AM, Beqaj SH, Deeter RG, Fitzgerald JT. Igm serum antibodies to Epstein-Barr virus are uniquely present in a subset of patients with the chronic fatigue syndrome. In Vivo. (2004) 18(2):101–6.15113035
95. Sairenji T, Yamanishi K, Tachibana Y, Bertoni G, Kurata T. Antibody responses to Epstein-Barr virus, human herpesvirus 6 and human herpesvirus 7 in patients with chronic fatigue syndrome. Intervirology. (1995) 38(5):269–73. doi: 10.1159/000150450
96. Lee JS, Lacerda EM, Nacul L, Kingdon CC, Norris J, O'Boyle S, et al. Salivary DNA loads for human herpesviruses 6 and 7 are correlated with disease phenotype in myalgic encephalomyelitis/chronic fatigue syndrome. Front Med. (2021) 8:656692. doi: 10.3389/fmed.2021.656692
97. Kerr JR. Epstein-Barr virus induced gene-2 upregulation identifies a particular subtype of chronic fatigue syndrome/myalgic encephalomyelitis. Front Pediatr. (2019) 7:59. doi: 10.3389/fped.2019.00059
98. Loebel M, Eckey M, Sotzny F, Hahn E, Bauer S, Grabowski P, et al. Serological profiling of the EBV immune response in chronic fatigue syndrome using a peptide microarray. PLoS One. (2017) 12(6):e0179124. doi: 10.1371/journal.pone.0179124
99. Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in chronic fatigue syndrome in children and adolescents. Clin Psychol Rev. (2014) 34(3):233–48. doi: 10.1016/j.cpr.2014.02.002
100. Rimes KA, Goodman R, Hotopf M, Wessely S, Meltzer H, Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics. (2007) 119(3):e603–9. doi: 10.1542/peds.2006-2231
101. Jason LA, Sunnquist M. The development of the DePaul symptom questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr. (2018) 6:330. doi: 10.3389/fped.2018.00330
102. Knight SJ, Harvey A, Hennel S, Lubitz L, Rowe K, Reveley C, et al. Measuring quality of life and fatigue in adolescents with chronic fatigue syndrome: estimates of feasibility, internal consistency and parent–adolescent agreement of the PedsQLTM. Fatigue: Biomed Health Behav. (2015) 3(4):220–34. doi: 10.1080/21641846.2015.1090816
103. Winger A, Kvarstein G, Wyller VB, Ekstedt M, Sulheim D, Fagermoen E, et al. Health related quality of life in adolescents with chronic fatigue syndrome: a cross-sectional study. Health Qual Life Outcomes. (2015) 13(1):96. doi: 10.1186/s12955-015-0288-3
104. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. (2003) 3(6):329–41. doi: 10.1367/1539-4409(2003)003%3C0329:TPAAPP%3E2.0.CO;2
105. Falk Hvidberg M, Brinth LS, Olesen AV, Petersen KD, Ehlers L. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS One. (2015) 10(7):e0132421. doi: 10.1371/journal.pone.0132421
106. Eaton-Fitch N, Johnston SC, Zalewski P, Staines D, Marshall-Gradisnik S. Health-related quality of life in patients with myalgic encephalomyelitis/chronic fatigue syndrome: an Australian cross-sectional study. Qual Life Res. (2020) 29(6):1521–31. doi: 10.1007/s11136-019-02411-6
Keywords: myalgic encephalomyelitis, chronic fatigue syndrome, infectious mononucleosis, Epstein-Barr virus, EBV, adolescents, ME/CFS, follow-up
Citation: Pricoco R, Meidel P, Hofberger T, Zietemann H, Mueller Y, Wiehler K, Michel K, Paulick J, Leone A, Haegele M, Mayer-Huber S, Gerrer K, Mittelstrass K, Scheibenbogen C, Renz-Polster H, Mihatsch L and Behrends U (2024) One-year follow-up of young people with ME/CFS following infectious mononucleosis by Epstein-Barr virus. Front. Pediatr. 11:1266738. doi: 10.3389/fped.2023.1266738
Received: 25 July 2023; Accepted: 20 December 2023;
Published: 18 January 2024.
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Andrew R. Lloyd, University of New South Wales, Australia© 2024 Pricoco, Meidel, Hofberger, Zietemann, Mueller, Wiehler, Michel, Paulick, Leone, Haegele, Mayer-Huber, Gerrer, Mittelstrass, Scheibenbogen, Renz-Polster, Mihtasch and Behrends. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenz Mihatsch bC5taWhhdHNjaEB0dW0uZGU=
†These authors have contributed equally to this work and share last authorship
Abbreviations ANA, antinuclear antibodies; CCC, Canadian consensus criteria; CDW-R, clinical diagnostic worksheet developed by Rowe and colleagues; CFQ, chalder fatigue scale; CSI, charité symptom inventory; EA, early antigen; EBV, Epstein-Barr virus; HHV, human herpes virus; HRQoL, health-related quality of life; IM, infectious mononucleosis; IOM, institute of medicine (IOM); MCFC, MRI chronic fatigue center for young people; MCS, mental health component summary score; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; PCD-J, pediatric case definition by Jason and colleagues; PedsQL, pediatric quality of life inventory; PEM, post-exertional malaise; PCS, physical health component summary score; PoTS, postural orthostatic tachycardia syndrome; PID, primary immunodeficiency; PROM, patient-reported outcome measures; SARS-CoV2, severe acute respiratory coronavirus type 2; SF-36, the short form-36 health survey; VCA, virus capsid antigen.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.