- 1Department of Pediatric Surgery, Binzhou Medical University Hospital, Binzhou, China
- 2Department of Gastroenterology, Binzhou Medical University Hospital, Binzhou, China
- 3Department of Gastrointestinal Surgery, Binzhou Medical University Hospital, Binzhou, China
- 4Department of Colorectal Surgery, Binzhou Medical University Hospital, Binzhou, China
- 5Department of General Surgery, Children’s Hospital Affiliated to Shandong University, Jinan, China
Background: This study aimed to explore the characteristics of pediatric upper gastrointestinal (UGI) perforations, focusing on their diagnosis and management.
Methods: Between January 2013 and December 2021, 30 children with confirmed UGI perforations were enrolled, and their clinical data were analyzed. Two groups were compared according to management options, including open surgical repair (OSR) and laparoscopic/gastroscopic repair (LR).
Results: A total of 30 patients with a median age of 36.0 months (1 day–17 years) were included in the study. There were 19 and 11 patients in the LR and OSR groups, respectively. In the LR group, two patients were treated via exploratory laparoscopy and OSR, and the other patients were managed via gastroscopic repair. Ten and three patients presented the duration from symptom onset to diagnosis within 24 h (p = 0.177) and the number of patients with hemodynamically unstable perforations was 4 and 3 in the LR and OSR groups, respectively. Simple suture or clip closure was performed in 27 patients, and laparoscopically pedicled omental patch repair was performed in two patients. There was no significant difference in operative time and length of hospital stay between the LR and OSR groups. Treatment failed in two patients because of severe sepsis and multiple organ dysfunction syndrome, including one with fungal peritonitis.
Conclusion: Surgery for pediatric UGI perforations should be selected according to the general status of the patient, age of the patient, duration from symptom onset, inflammation, and perforation site and size. Antibiotic administration and surgical closure remain the main strategies for pediatric UGI perforations.
Introduction
Upper gastrointestinal (UGI) perforation is a life-threatening condition. Although UGI perforation is uncommon in the pediatric population (1), its etiology is multifaceted and includes inflammation (2), congenital defects of gastric musculature or spontaneous rupture (3, 4), trauma (5), peptic or drug-induced ulcer (5, 6), ingestion of sharp foreign bodies or high-powered magnets (e.g., buckyballs) (7, 8), and iatrogenic injury (9–11).
Due to effective acid suppression, peptic gastroduodenal ulcer perforation has become less prevalent (11, 12), and endoscopic management of surgical gastrointestinal diseases has rapidly increased with advancements in endoscopic techniques over the last decade. Thus, the risk of iatrogenic perforation related to endoscopic procedures for UGI may increase (9–11).
The diagnosis of UGI perforation is based on medical history, physical examination, laboratory investigation, imaging studies, and endoscopy (13, 14). However, managing pediatric UGI perforation, including non-surgical and surgical treatments, remains a challenge in clinical practice. The general principles for these severe conditions are prompt diagnosis and effective treatment, although there is little standardization due to varied etiologies, different perforation sites and sizes, various clinical scenarios, and varied diagnostic methods (15, 16).
Herein, we retrospectively review the clinical data of UGI perforations in pediatric patients and focus on their etiologies, diagnostic methods, and management options. This study aimed to provide additional information on the diagnosis and management of pediatric UGI perforations.
Patients and methods
The present study included 30 pediatric patients with surgically confirmed UGI perforations at the Binzhou Medical University Hospital and Children's Hospital Affiliated to Shandong University from January 2013 to December 2021. Patients with UGI perforations of various etiologies were included in the study. The inclusion criteria are as follows: patients aged ≤18 years with gastroduodenal perforations confirmed by surgery or endoscopy. The exclusion criteria are as follows: patients in whom UGI perforations were clinically suspected but unconfirmed via surgery or endoscopy and aged >18 years.
The patients were then divided into two groups according to the different surgical approaches: laparoscopic repair and traditional open repair groups. Clinical data, such as age, sex, medical history, clinical signs, diagnostic modalities, surgical approach, intraoperative findings, operative time, postoperative complications, and length of hospital stay, were collected and compared between the groups. Postoperative complications were divided into grades I–IV according to the Clavien–Dindo classification (17).
The study adhered to the ethical principles of the Declaration of Helsinki and the local ethical and legal requirements. Written informed consent was provided by the legal guardians of the patients. According to the Institutional Review Board of Binzhou Medical University, formal approval was not required for retrospective archived studies.
Statistical analyses were conducted using descriptive statistics, Student's t-test, and the chi-squared test, where appropriate. Statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS (version 27.0; SPSS Inc., Chicago, IL, USA).
Results
As shown in Table 1, 30 patients with gastroduodenal perforations were included, of whom 21 were males (70.0%) and nine were females (30.0%), with a median age of 3.0 (0–17) years. Seventeen patients (56.7%) were 3 years of age. The etiologies included peptic ulcers (n = 9), foreign body ingestion (n = 11, including five patients with multiple buckyballs), congenital malformation (n = 2), trauma (n = 2), iatrogenic cause (possible electrosurgical knife induced injury, n = 1), necrotizing enterocolitis (NEC, n = 1), and unknown causes (n = 4). Of the nine patients with perforated peptic ulcer (PPU), the Helicobacter pylori test was conducted in four patients and was positive in two (both males, aged 12 and 15 years). Eighteen patients underwent laparoscopic repair, 11 underwent open repair, and one underwent gastroscopic closure. Eight (42.1%) and nine (81.2%) patients in the laparoscopic/gastroscopic repair (LR) and open surgical repair (OSR) groups (p = 0.034), respectively, were ≤3 years of age, indicating a tendency to undergo open repair in children aged ≤3 years.
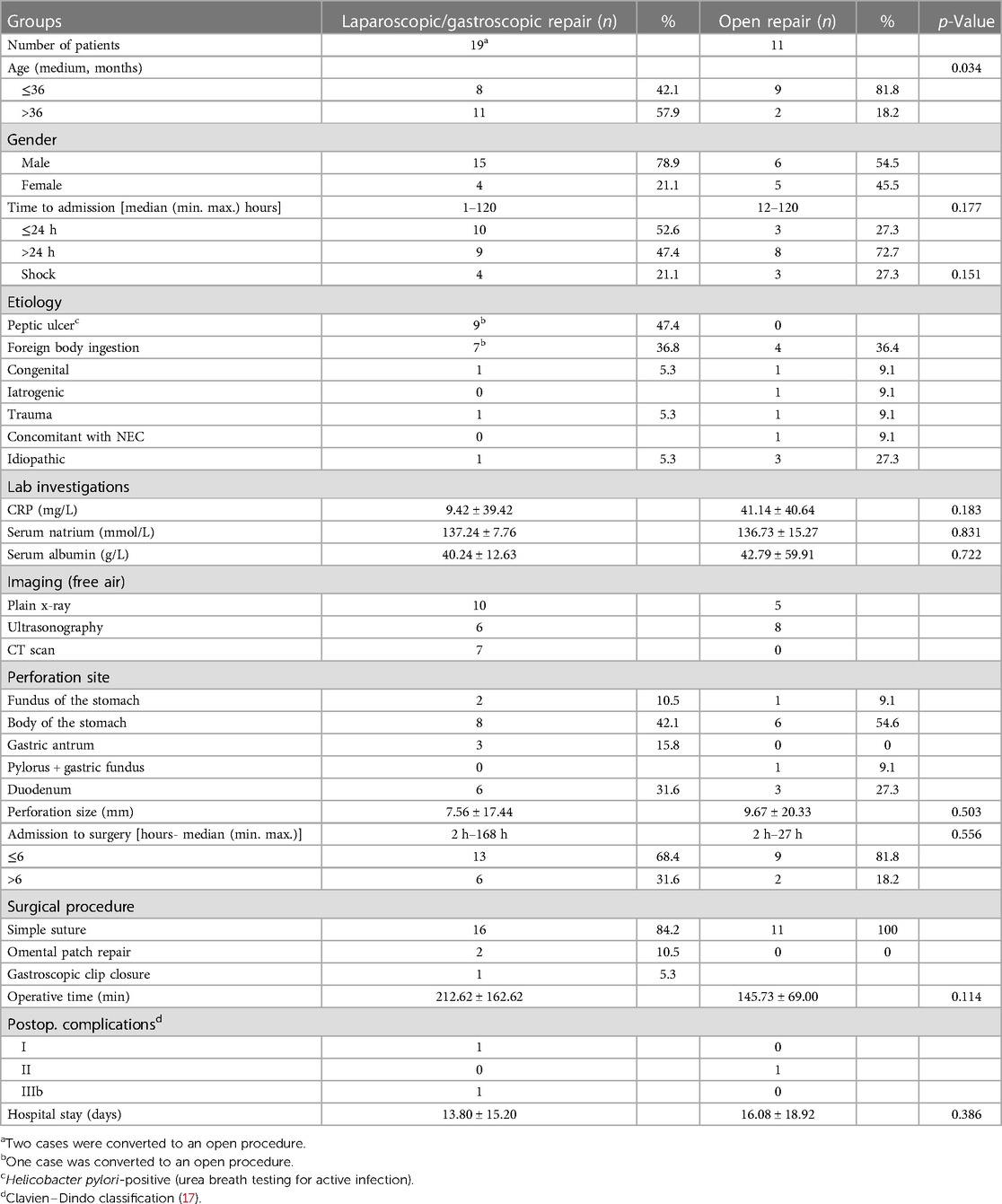
Table 1. Characteristics, diagnosis, management, and outcome of pediatric upper gastrointestinal perforation.
Almost all the patients presented with abdominal pain, tenderness, and rebound tenderness on palpation. Four and three patients experienced hypotension on admission in the LR and OSR groups (p = 0.151), respectively; the detailed data are shown in Table 2. The mean C-reactive protein level on admission was 9.42 ± 39.42 mg/L and 41.14 ± 40.64 mg/L in the LR and OSR groups (p = 0.183), respectively. The mean values of serum albumin and natrium levels were within the normal range between the two groups, although some patients had hypoalbuminemia and hyponatremia.
Plain radiography, ultrasonography, and CT scanning, or plain radiography plus ultrasonography/CT scan, were chosen for the two groups. The pneumoperitoneum sign helped make this diagnosis. In the LR group, the perforation sites were located on the fundus of the stomach (n = 2), body of the stomach (n = 8), gastric antrum (n = 3), and duodenum (n = 6); in the OSR group, the perforation sites were located on the fundus of the stomach (n = 1), body of the stomach (n = 7), and duodenum (n = 3). The two groups had no significant difference in the perforation sizes (7.56 ± 17.44 mm vs. 9.67 ± 20.33 mm, respectively, p = 0.503).
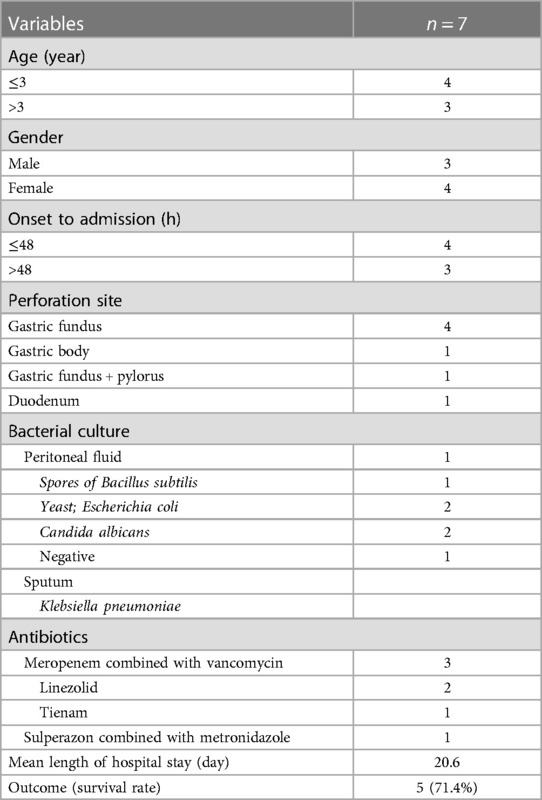
Of the seven patients with shock, three were males and four were females. Their ages ranged from 2 days to 11 years, with a median age of 2 years. The time until treatment initiation was <48 h (n = 4) or >48 h (n = 3). The perforation sites included the gastric fundus (n = 4), gastric body (n = 1), pylorus and fundus (n = 1), and duodenum (n = 1). Bacterial culture of the peritoneal fluid showed growth of Candida albicans (n = 2), spores of Bacillus subtilis yeast (n = 1), Escherichia coli (n = 1), and no growth (n = 2). Sputum cultures showed Klebsiella pneumoniae growth (n = 1). The patients were initially treated with antibiotics, including meropenem combined with vancomycin (n = 5), linezolid (n = 2), tienam (n = 1), and sulperazon combined with metronidazole (n = 2). The mean length of the hospital stay was 20.6 days. The survival rate of the patients with septic shock upon admission was 71.4%.
After initial fluid resuscitation, oxygen therapy, and intravenous antibiotics, surgical treatments were performed within 6 h of admission in 13 of 19 patients (68.4%) and nine of 11 (81.8%) patients in the LR and OSR groups (p = 0.556), respectively. In the LR group, perforation closure was performed via a simple suture (n = 16), omental patch repair (n = 2), and gastroscopic closure using clips (n = 1). In the OSR group, all 11 patients underwent simple suturing. The operative time was 212.62 ± 162.62 and 145.73 ± 69.00 min in the LR and OSR groups (p = 0.114), respectively.
Of the 28 patients who recovered, three patients experienced postoperative complications. Grades I (n = 1) and IIIb (n = 1) were observed in the LR group, and grade II (n = 1) was observed in the open repair group. The length of hospital stay was 13.80 ± 15.20 and 16.08 ± 18.92 days in the LR and OSR groups (p = 0.386), respectively. Treatment failed in two patients due to severe sepsis and multiple organ dysfunction syndrome (MODS), including one patient with intraperitoneal C. albicans infection.
Discussion
Pediatric UGI perforation is an uncommon but severe disorder that needs to be diagnosed and treated immediately to improve the outcomes of the patients (15). Many causes, such as high-power magnets, congenital malformations, trauma, peptic ulcers, H. pylori infection, non-steroidal anti-inflammatory drugs, and iatrogenic injury, can lead to UGI perforation (2–5, 7–10, 13). The etiology of UGI perforation in our case series included almost all the causes mentioned above.
Pediatric PPU typically occurs in adolescents. However, in our case series, among the nine PPU patients, four (44.44%) were younger than 7 years, suggesting a tendency for a younger-age population. In the present study, UGI perforation predominantly occurred in boys (70%), which is similar to that reported in the literature (18). However, four of the seven patients with shock on admission were females (57.14%). This phenomenon requires further investigation of sex-based differences in clinical presentation and management strategies for pediatric UGI perforation (18, 19).
Regarding the option of imaging tools for diagnosing UGI perforation, pneumoperitoneum via plain abdominal radiography or CT scanning was observed in all patients, indicating a higher sensitivity of these two imaging modalities. Ultrasonography, a commonly used tool for differential diagnosis in pediatric acute abdomen, was performed in approximately half of the patients. The results showed a higher sensitivity of accurate diagnosis; this may be because highly skilled sonographers performed the ultrasonography (16). Ultrasonographic examination may reveal gas accumulation or free air in front of the liver, discontinuous gastroduodenal wall, swollen soft tissue, and free ascites (16, 20). If radiograms present no free gas, UGI perforation could not be ruled out. Point-of-care ultrasonography or double-contrast CT helps make a more accurate diagnosis (13, 16, 20, 21).
Regarding the management of UGI perforation, surgical intervention is relevant for the pediatric population, especially for those with intra-abdominal infections, sepsis, and unstable hemodynamics (1, 16, 22–24). In patients with unstable vital signs, emergent surgical intervention is performed after initial fluid resuscitation, oxygen therapy, and intravenous antibiotics. The postoperative survival rate was 71.4% in the patients with septic shock upon admission. This revealed that broad-spectrum antibiotics should be started immediately upon admission and continued during and after the surgical procedure (25–27). Antibiotics should be broad-spectrum, covering all possible pathogens. Multidrug regimens and appropriate dosing to ensure sufficient coverage and peak blood levels of antibiotics may play an important role in preventing the development of MODS (28–30). In the present study, the bacterial isolates were susceptible to vancomycin and linezolid. However, antibiotics can lead to fungal colonization and invasion across the mucosal barrier, causing fatal fungal peritonitis (31, 32). Empiric antifungal therapy is only considered in patients at a higher risk of fungal infection. Antifungals, such as echinocandins or liposomal amphotericin B, should also be considered in patients with a perforated abdominal viscus (29, 33). In this case series, fungal peritonitis was diagnosed in two patients, one of whom survived.
Conservative treatment is confirmed as safe and feasible for some selected cases of perforated peptic duodenal ulcers, including the duration of symptom onset being within 24 h of admission with a stable condition, localized peritoneal irritation signs, and mild ascites (15, 34, 35). A gastroduodenogram is usually needed to evaluate water-soluble contrast extravasation. If there is no contrast extravasation, it is suggestive of a self-sealed microperforation (12, 34). Endoscopic repair is another option for UGI perforation owing to its minimal invasiveness. However, upper endoscopic closure is generally performed within 24 h of duration from the symptom onset (20, 36–39). In the present study, only one patient (aged 2 years) with perforation of the stomach body due to misingestion of multiple magnet beans underwent endoscopic closure. We chose an endoscopic approach to seal the perforation because the lesion was induced by ingestion of multiple magnets, which is usually complicated by unmarked inflammation of the surrounding tissues. In a recent randomized controlled trial, Negm et al. (40) recommended that the indication for endoscopic repair of acute PPU is decontamination, that is, early chemical peritonitis and no septic shock. They concluded that endoscopic closure techniques, including clip technique accompanied by interventional radiological drainage, are effective for adult PPU. Bingener et al. (41) described a new natural orifice transluminal endoscopic surgery (NOTES) for closure of PPU in select cases with promising results, especially for the elderly and/or immunocompromised patients. Many challenges remain for endoscopic repair of GIT perforation; technical aspects and patient selection are still evolving (41). Endoscopic-guided GIT perforation repair may contribute to the future management of GIT perforation in the pediatric population, especially in small children. Prospective multicenter clinical trials are needed to prove the feasibility and efficiency of endoscopic closure in managing pediatric UGI perforation.
Surgical approaches include open or laparoscopic repair, which is based on the age of the patient (≤3 years), general condition, duration from symptom onset to diagnosis, hemodynamic status, comorbidities, perforation site and size, and experience and preference of the surgeon (21, 23, 36). In the LR group, the duration from symptom onset to operation in half of the patients was within 24 h, while in the OSR group, only 27.3% of the patients underwent operation within 24 h. Four patients with shock on admission underwent laparoscopic repair, and one patient had complications of postoperative gastric fistula and MODS. Unstable hemodynamics on admission may not be an absolute contraindication (42). Prospective studies are needed to provide available information on the safety and efficacy of laparoscopic repair in this group of patients (42–47).
The most common surgical procedure for UGI perforation is perforation closure via simple suturing (15, 19, 42). In the present case series, simple suturing was performed in most cases in both groups; only two patients underwent laparoscopically pedicled omental patch repair. The simple suture technique to repair the PPU is usually recommended because of its feasibility and low procedure time (12, 15, 42). Pedicled omental patch repair is another favorable technique, especially in cases with a friable edge or a large perforation, which cannot allow the approximation of perforation edges (20, 48–50). Regarding postoperative management for those PPU with H. pylori infection, proton pump inhibitors and eradication of H. pylori infection are essential to reduce peptic ulcer recurrence (40, 51, 52).
The limitations of the present study are as follows: the retrospective study design is flawed because of its unavoidable selection bias; the incidence of UGI perforation was low, and the sample size was small; prospective clinical trials using standardized diagnosis and management protocols are needed to improve the outcome of pediatric UGI perforations.
In conclusion, the etiology of pediatric UGI perforations is multifaceted. The diagnosis was mainly based on medical history, physical examination, laboratory and imaging investigations, including plain radiography, ultrasonography, and/or CT scanning. Treatment options vary according to the patient's general status, patient’s age, duration from symptom onset to diagnosis, pathological findings, and perforation sites and sizes. Surgeons are concerned about the safety of endoscopic perforation repair. Therefore, surgical assistance may be required in such cases. With increased experience in applying titanium clips and the nylon rope purse-suture technique, endoscopic repair with fewer complications can be accomplished in select cases (46, 53). Closure of UGI perforations via the simple suture technique combined with appropriate antimicrobials remains the mainstay of management in the pediatric population (54).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by Binzhou Medical University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the legal guardians/next of kin of the participants.
Author contributions
MW: Data curation, Investigation, Methodology, Writing—original draft. SS: Data curation, Investigation, Writing—original draft. QN: Data curation, Methodology, Writing—original draft. BH: Conceptualization, Investigation, Writing—review and editing. HZ: Investigation, Methodology, Writing—original draft. LG: Conceptualization, Investigation, Methodology, Writing—review and editing. TF: Conceptualization, Data curation, Project administration, Software, Supervision, Validation, Writing—original draft, Writing—review and editing. HQ: Conceptualization, Methodology, Supervision, Validation, Writing—review and editing. BZ: Conceptualization, Data curation, Methodology, Supervision, Writing—review and editing. HL: Conceptualization, Methodology, Supervision, Validation, Writing—review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brown AD, Traynor MD Jr, Potter DD Jr, Ishitani MB, Moir CR, Galardy PJ, et al. Evolution of pediatric gastrointestinal ulcer disease: is acute surgical intervention relevant? J Pediatr Surg. (2021) 56(10):1870–5. doi: 10.1016/j.jpedsurg.2021.02.010
2. Gunjaca I, Mlinac-Lucijanić M, Pavlović A, Gunjaca M. Inflammation of ectopic pancreatic tissue as unusual cause of duodenal perforation—a case report. Coll Antropol. (2010) 34(3):1119–22.20977114
3. Braunstein H. Congenital defect of the gastric musculature with spontaneous perforation; report of five cases. J Pediatr. (1954) 44(1):55–63. doi: 10.1016/S0022-3476(54)80092-8
4. Kothari P, Jiwane A, Kumar T, Deshmukh A, Kulkarni B. Spontaneous gastroduodenal disruption in neonate. Pediatr Surg Int. (2002) 18(2–3):181–3. doi: 10.1007/s003830100671
5. Lee JH, Kedia P, Stavropoulos SN, Carr-Locke D. AGA clinical practice update on endoscopic management of perforations in gastrointestinal tract: expert review. Clin Gastroenterol Hepatol. (2021) 19(11):2252–61.e2. doi: 10.1016/j.cgh.2021.06.045
6. Francavilla ML, Pollock AN. Perforated duodenal ulcer. Pediatr Emerg Care. (2017) 33(3):219–20. doi: 10.1097/PEC.0000000000001060
7. Ding G, Liu H, Zhou P, Niu Q, Wang W, Feng Z, et al. Pediatric multiple high-powered magnetic buckyballs ingestion-experience from six tertiary medical centers. Front Public Health. (2022) 10:892756. doi: 10.3389/fpubh.2022.892756
8. Ventura F, Candosin S, Barranco R, Bonsignore A, Andrello L, Tajana L, et al. A fatal case of coin battery ingestion in an 18-month-old child: case report and literature review. Am J Forensic Med Pathol. (2017) 38(1):43–6. doi: 10.1097/PAF.0000000000000297
9. Ledder O, Woynarowski M, Kamińska D, Łazowska-Przeorek I, Pieczarkowski S, Romano C, et al. Identification of iatrogenic perforation in pediatric gastrointestinal endoscopy. J Pediatr Gastroenterol Nutr. (2023) 77(3):401–6. doi: 10.1097/MPG.0000000000003852
10. Kang DH, Ryu DG, Choi CW, Kim HW, Park SB, Kim SJ, et al. Clinical outcomes of iatrogenic upper gastrointestinal endoscopic perforation: a 10-year study. BMC Gastroenterol. (2019) 19(1):218. doi: 10.1186/s12876-019-1139-1
11. Holmer C, Mallmann CA, Musch MA, Kreis ME, Gröne J. Surgical management of iatrogenic perforation of the gastrointestinal tract: 15 years of experience in a single center. World J Surg. (2017) 41(8):1961–5. doi: 10.1007/s00268-017-3986-7
12. Salman MA, Issa M, Salman A, Tourky M, Elewa A, Alrahawy M, et al. Surgical management of perforated peptic ulcer: a comparative meta-analysis of laparoscopic versus open surgery. Surg Laparosc Endosc Percutan Tech. (2022) 32(5):586–94. doi: 10.1097/SLE.0000000000001086
13. Ansari D, Torén W, Lindberg S, Pyrhönen HS, Andersson R. Diagnosis and management of duodenal perforations: a narrative review. Scand J Gastroenterol. (2019) 54(8):939–44. doi: 10.1080/00365521.2019.1647456
14. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. (2017) 390(10094):613–24. doi: 10.1016/S0140-6736(16)32404-7
15. Yan X, Kuang H, Zhu Z, Wang H, Yang J, Duan X, et al. Gastroduodenal perforation in the pediatric population: a retrospective analysis of 20 cases. Pediatr Surg Int. (2019) 35(4):473–7. doi: 10.1007/s00383-018-4420-4
16. Shen Q, Liu T, Wang S, Wang L, Wang D. Experience in diagnosis and treatment of duodenal ulcer perforation in children. BMC Pediatr. (2023) 23(1):144. doi: 10.1186/s12887-023-03957-8
17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Hua MC, Kong MS, Lai MW, Luo CC. Perforated peptic ulcer in children: a 20-year experience. J Pediatr Gastroenterol Nutr. (2007) 45(1):71–4. doi: 10.1097/MPG.0b013e31804069cc
19. Svanes C, Lie RT, Lie SA, Kvåle G, Svanes K, Søreide O. Survival after peptic ulcer perforation: a time trend analysis. J Clin Epidemiol. (1996) 49(12):1363–71. doi: 10.1016/S0895-4356(96)00278-8
20. Wang A, Yerxa J, Agarwal S, Turner MC, Schroder V, Youngwirth LM, et al. Surgical management of peptic ulcer disease. Curr Probl Surg. (2020) 57(2):100728. doi: 10.1016/j.cpsurg.2019.100728
21. Kim SH, Shin SS, Jeong YY, Heo SH, Kim JW, Kang HK. Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol. (2009) 10(1):63–70. doi: 10.3348/kjr.2009.10.1.63
22. Grosfeld JL, Molinari F, Chaet M, Engum SA, West KW, Rescorla FJ, et al. Gastrointestinal perforation and peritonitis in infants and children: experience with 179 cases over ten years. Surgery. (1996) 120(4):650–5. doi: 10.1016/S0039-6060(96)80012-2
23. Amouei A, Ehsani F, Zarch MB, Tabatabaei SM, Ghodratipour Z. Peritonitis following duodenal ulcer perforation in a newborn: a case report. J Clin Diagn Res. (2016) 10(11):PD10–1.28050434
24. Satoh K, Yoshino J, Akamatsu T, Itoh T, Kato M, Kamada T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol. (2016) 51(3):177–94. doi: 10.1007/s00535-016-1166-4
25. Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. (2017) 196(7):856–63. doi: 10.1164/rccm.201609-1848OC
26. Perner A, Gordon AC, De Backer D, Dimopoulos G, Russell JA, Lipman J, et al. Sepsis: frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. (2016) 42(12):1958–69. doi: 10.1007/s00134-016-4577-z
27. Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: guideline-based management. Cleve Clin J Med. (2020) 87(1):53–64. doi: 10.3949/ccjm.87a.18143
28. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. (2016) 62(4):e1–50. doi: 10.1093/cid/civ933
29. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47(11):1181–247. doi: 10.1007/s00134-021-06506-y
30. Högberg LD, Heddini A, Cars O. The global need for effective antibiotics: challenges and recent advances. Trends Pharmacol Sci. (2010) 31(11):509–15. doi: 10.1016/j.tips.2010.08.002
31. Zügel NP, Kox M, Lichtwark-Aschoff M, Gippner-Steppert C, Jochum M. Predictive relevance of clinical scores and inflammatory parameters in secondary peritonitis. Bull Soc Sci Med Grand Duche Luxemb. (2011) 1:41–71.
32. Esch SV, Krediet RT, Struijk DG. Prognostic factors for peritonitis outcome. Contrib Nephrol. (2012) 178:264–70. doi: 10.1159/000337889
33. Todd RT, Soisangwan N, Peters S, Kemp B, Crooks T, Gerstein A, et al. Antifungal drug concentration impacts the spectrum of adaptive mutations in Candida albicans. Mol Biol Evol. (2023) 40(1):msad009. doi: 10.1093/molbev/msad009
34. Fujii Y, Asato M, Taniguchi N, Shigeta K, Omoto K, Itoh K, et al. Sonographic diagnosis and successful nonoperative management of sealed perforated duodenal ulcer. J Clin Ultrasound. (2003) 31(1):55–8. doi: 10.1002/jcu.10125
35. Odisho T, Shahait AA, Sharza J, Ali AA. Outcomes of laparoscopic modified Cellan–Jones repair versus open repair for perforated peptic ulcer at a community hospital. Surg Endosc. (2023) 37(1):715–22. doi: 10.1007/s00464-022-09306-7
36. Kumar S, Youn YH, Lee JH. Life on a knife edge: the optimal approach to the management of perforations during endoscopic submucosal dissection (ESD). Expert Rev Gastroenterol Hepatol. (2020) 14(10):965–73. doi: 10.1080/17474124.2020.1791085
37. Vázquez JA A, Khodakaram K, Bergström M, Park PO. Stent treatment or surgical closure for perforated duodenal ulcers: a prospective randomized study. Surg Endosc. (2021) 35(12):7183–90. doi: 10.1007/s00464-020-08158-3
38. Tanaka Y, Nakamura T, Fujii S, Kusaka T. Successful treatment of a perforated duodenal ulcer with polyglycolic acid sheets. Gastrointest Endosc. (2017) 85(6):1299–300. doi: 10.1016/j.gie.2016.07.058
39. Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy. (2014) 46(8):693–711. doi: 10.1055/s-0034-1377531
40. Negm S, Mohamed H, Shafiq A, AbdelKader T, Ismail A, Yassin M, et al. Combined endoscopic and radiologic intervention for management of acute perforated peptic ulcer: a randomized controlled trial. World J Emerg Surg. (2022) 17(1):24. doi: 10.1186/s13017-022-00429-9
41. Bingener J, Loomis EA, Gostout CJ, Zielinski MD, Buttar NS, Song LM, et al. Feasibility of NOTES omental plug repair of perforated peptic ulcers: results from a clinical pilot trial. Surg Endosc. (2013) 27(6):2201–8. doi: 10.1007/s00464-012-2740-3
42. Quah GS, Eslick GD, Cox MR. Laparoscopic repair for perforated peptic ulcer disease has better outcomes than open repair. J Gastrointest Surg. (2019) 23(3):618–25. doi: 10.1007/s11605-018-4047-8
43. Cirocchi R, Soreide K, Di Saverio S, Rossi E, Arezzo A, Zago M, et al. Meta-analysis of perioperative outcomes of acute laparoscopic versus open repair of perforated gastroduodenal ulcers. J Trauma Acute Care Surg. (2018) 85(2):417–25. doi: 10.1097/TA.0000000000001925
44. Stepanyan SA, Petrosyan AA, Safaryan HH, Yeghiazaryan HH, Aleksanyan AY, Hakobyan VM, et al. Laparoscopic and open repair for perforated duodenal ulcer: single-center experience. Wideochir Inne Tech Maloinwazyjne. (2019) 14(1):60–9. doi: 10.5114/wiitm.2018.76281
45. Nguyen TH, Dang TN, Schnelldorfer T. Single-port laparoscopic repair of perforated duodenal ulcers. World J Surg. (2020) 44(5):1425–30. doi: 10.1007/s00268-019-05352-w
46. Wang C, Gao Z, Shen K, Cao J, Shen Z, Jiang K, et al. Safety and efficiency of endoscopic resection versus laparoscopic resection in gastric gastrointestinal stromal tumours: a systematic review and meta-analysis. Eur J Surg Oncol. (2020) 46(4 Pt A):667–74. doi: 10.1016/j.ejso.2019.10.030
47. Reusens H, Dassonville M, Steyaert H. Laparoscopic repair for perforated peptic ulcer in children. Eur J Pediatr Surg. (2017) 27(3):251–4. doi: 10.1055/s-0036-1586201
48. Mohamedahmed AYY, Albendary M, Patel K, Ayeni AA, Zaman S, Zaman O, et al. Comparison of omental patch closure versus simple closure for laparoscopic repair of perforated peptic ulcer: a systematic review and meta-analysis. Am Surg. (2022) 89(5):2005–13. doi: 10.1177/00031348211067991
49. Chan KS, Ng STC, Tan CHB, Gerard G, Oo AM. A systematic review and meta-analysis comparing postoperative outcomes of laparoscopic versus open omental patch repair of perforated peptic ulcer. J Trauma Acute Care Surg. (2023) 94(1):e1–13. doi: 10.1097/TA.0000000000003799
50. McCullers MR, Shin CC, Anderson CM. Laparoscopic graham patch for anterior duodenal perforation in a 6-year-old. Am Surg. (2023) 89(8):3618–20. doi: 10.1177/00031348231167406
51. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. (2019) 132(4):447–56. doi: 10.1016/j.amjmed.2018.12.009
52. Bose AC, Kate V, Ananthakrishnan N, Parija SC. Helicobacter pylori eradication prevents recurrence after simple closure of perforated duodenal ulcer. J Gastroenterol Hepatol. (2007) 22(3):345–8. doi: 10.1111/j.1440-1746.2006.04490.x
53. Seelig MH, Seelig SK, Behr C, Schönleben K. Comparison between open and laparoscopic technique in the management of perforated gastroduodenal ulcers. J Clin Gastroenterol. (2003) 37(3):226–9. doi: 10.1097/00004836-200309000-00007
Keywords: upper gastrointestinal perforation, peptic ulcer, diagnosis, surgical management, children
Citation: Wang M, Sun S, Niu Q, Hu B, Zhao H, Geng L, Fu T, Qin H, Zheng B and Li H (2023) Experience of management of pediatric upper gastrointestinal perforations: a series of 30 cases. Front. Pediatr. 11:1261336. doi: 10.3389/fped.2023.1261336
Received: 20 July 2023; Accepted: 22 September 2023;
Published: 11 October 2023.
Edited by:
Salvatore Oliva, Sapienza University of Rome, ItalyReviewed by:
Matjaž Homan, University Medical Centre Ljubljana, SloveniaVasiliki Spyropoulou, University Children’s Hospital Zurich, Switzerland
© 2023 Wang, Sun, Niu, Hu, Zhao, Geng, Fu, Qin, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingliang Fu ZHJmdXRsQHNpbmEuY29t Hong Qin eGlhb3hpYW9xaW5mZW5nQDEyNi5jb20= Bufeng Zheng NTk0NTMxNTEwQHFxLmNvbQ== Hesheng Li aGVzaGVuZ2xpMjAxNEAxNjMuY29t
 Mengqi Wang
Mengqi Wang Shuai Sun1
Shuai Sun1 Baoguang Hu
Baoguang Hu Tingliang Fu
Tingliang Fu Hong Qin
Hong Qin