- Department of Pediatric Surgery, Dr. von Hauner Children's Hospital, University Hospital, LMU Munich, Munich, Germany
Background: Button battery (BB) ingestions may cause severe and possibly fatal complications, especially if the battery is located in the esophagus. The application of oral honey has recently been proposed by the National Capital Poison Center in the USA and in an ESPGHAN position paper in Europe, but clinical trials and experimental studies are limited. The goal of this systematic review was to analyze the evidence for this approach.
Materials and methods: A systematic review of clinical trials and experimental studies on the oral application of honey after BB ingestion in children was performed. Inclusion criteria according to the PICO format were patient age 0–18 years, ingestion of BB, oral administration of honey or other substances, all in vivo and in vitro studies, as well as reported complication rate, esophageal injury, and mortality. A manual search in the databases MEDLINE, Web of Science and Cochrane was performed to identify relevant search terms to form the following queries and to construct the extensive search. Furthermore, the search was extended by using snowballing on the reports reference lists. The review is registered at Research Registry. The identifying number is reviewregistry1581.
Results: We found four publications that investigated the effects of honey after button battery ingestion. Three of these presented experimental in vitro and in vivo results and one reported a clinical retrospective study of 8 patients.
Conclusion: Follow up studies are required to further elucidate the effectiveness of the treatment with honey. The time intervals in which the use of honey is effective is not clear. Furthermore, a physiological model is needed for in vitro testing, preferably mimicking peristalsis and dynamic flow of the applied substances. However, since it is easy to apply and of minimal risk in patients over one year of age, honey should be considered a possible treatment option during the interval between presentation and endoscopic removal of the retained BB.
Systematic Review Registration: https://www.researchregistry.com/browse-the-registry#registryofsystematicreviewsmeta-analyses/registryofsystematicreviewsmeta-analysesdetails/643e9df96750410027ee11b0/, identifier: reviewregistry1581.
1. Introduction
Button battery (BB) ingestion may lead to severe, sometimes fatal complications. Esophageal retention of a BB is associated with a particularly high rate of complications. The number of BB ingestions has been increasing rapidly throughout the past years following general technical advancement e.g., in the Unites States by 6.7 within 1985 through 2009, with almost two-thirds extracted from household devices by the patients (1, 2). Significant damage to the esophagus can occur as early as 2 h after ingestion, although ex vivo animal models showed macroscopically evident mucosal damage as early as 15 min after application of the BB (3). To minimize the associated risks, the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) formed a task force. In their position paper, they recommend the immediate localization of the battery and, if located in the esophagus, prompt removal within 2 h. If verification of the battery's localization is postponed >12 h, a computed tomography (CT)-scan is indicated to rule out possible vascular involvement (4). In an extensive metadata analysis, Varga et al. found BB ingestion associated with complications in 0.2%, with a mortality of 0.04%. Most complications affect the esophagus and can be subdivided in ulceration (22%), perforation (18%), trachea-esophageal fistula formation (15%), stricture/obstruction (14%), vascular involvement (6%), necrosis (5%), bilateral vocal cord palsy (2%), bronchopneumonia (0.4%), and spondylodiscitis (0.4%) (5). Patients under 6 years of age and ingestions of BB ≥ 2 cm in diameter bare the highest complication rate at 12% (1). The mechanism of injury is mainly due to pressure necrosis, electrical discharge, leakage of battery fluids and toxicity of the metal (5). Isothermal hydrolysis by the resulting alkaline solution causes alkaline injury to the surrounding tissue with colliquation necrosis (3).
A newly developed strategy to reduce any damage is the oral administration of honey during the interval between ingestion and retrieval of the battery. In 2018, Anfang et al. carried out in vitro experiments and in vivo animal trials regarding a possible protective effect of various substances including honey (6). As a result, the administration of honey has been implemented in the American guideline of the National Capital Poison Center. If button battery ingestion is suspected or confirmed in children older than 1 year of age [honey is associated with a risk of botulism in infants (7)] and the battery was swallowed less than 12 h ago, it recommends the administration of 10 ml honey every 10 min up to a total of 6 times (8). Further along the way, the European Society of Paediatric Gastroenterology, Hepatology and Nutrition issued a Position Paper which also recommends administering honey to children older than 1 year of age after BB ingestion (4). However, literature on the effects of honey after BB ingestion are rare, and these recommendations seem to be based on only three studies that have been published previously by Anfang et al. (6), by Gyawali et al. (9) and by Jia et al. (10). A small retrospective study points to a confirmation of the recommendation However, this study only included only 8 patients of whom only 2 received honey before button battery removal (11).
The objective of this systematic literature review was to identify all relevant literature regarding the use of honey in children (0–18 years) after esophageal button battery (BB) ingestion and analyze it regarding its potential protective effect. A structured literature search with evaluation of the resulting data was performed to establish whether the oral administration of honey after BB ingestion provides a benefit in the extent of esophageal injury, complication rate and mortality, compared to the administration of no or alternative substances.
2. Methods
We applied the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement standards and checklist for the literature review (12). The work has been reported in line with Assessing the methodological quality of systematic reviews (AMSTAR) Guidelines (13, 14). To minimize possible bias, a protocol was established before the search for suitable studies was initiated (see Supplementary Appendix) (15). MEDLINE (PubMed®), Web of Science and Cochrane Central Register of Controlled Trials (Central) were queried for literature on the use of honey in patients with potential or confirmed BB ingestion. Search terms included were the following: MEDLINE: (“Therapeutic Irrigation”[MeSH Terms] OR “Honey”[MeSH Terms] OR “Honey”[Text Word] OR “sirup”[Text Word] OR “glucos*”[Text Word] OR “sugar*”[Text Word] OR “therapeutic irrigat*”[Text Word]) AND (“electric power supplies/adverse effects”[MeSH Terms] OR “electric power supplies/methods”[MeSH Terms] OR “electric power supplies/trends”[MeSH Terms] OR “button batter*”[Text Word]). Web of Science: [ALL = (honey OR sirup OR glucos* OR sugar* OR “therapeutic irrigate*”)] AND ALL = (“button batter*”). Central: ([MeSH descriptor: (Honey) this term only] OR [MeSH descriptor: (therapeutic irrigation) this term only] OR Honey OR sirup OR glucos* OR “therapeutic irrigation” OR “therapeutic irrigations”) AND {[MeSH descriptor: (Electric power supplies) this term only] OR “button battery” OR “button batteries”}. All articles were reviewed independently by two investigators independently (YMS, DWK). Studies were reviewed in full-text detail when exclusion based on title/abstract was not possible. In- and exclusion criteria were strictly employed (see Supplementary Appendix). The research was conducted from February through September 2022.
3. Results
Our search strategy revealed three experimental and one clinical study assessing the use of honey in BB ingestions (see Tables 1, 2). In 2018, Anfang et al. investigated the administration of apple and orange juice, sports drinks, honey, maple syrup and sucralfate for a possible protective effect in case of BB ingestion. Initial ex vivo testing proofed honey and sucralfate to be neutralizing the batteries' effects. Subsequently, a transfer to porcine animal model verified the substances effect in vivo. Regarding optimal dosage and frequency, the authors followed physiological saliva production with appliance of 10 ml every 10–15 min (6). A second trial, published by Gyawali et al. in 2021 showed that BB previously covered with honey caused significantly less deep injury than an uncovered battery after 24 h in goat esophagi ex vivo (9). The third trial, published by Jia et al. in 2022 compared the effects of olive oil, honey and sucralfate in vitro and in vivo in a porcine model, finding honey and a mixture of honey and olive oil to reduce the injury to the tissue in comparison to the use of saline as a control (10).
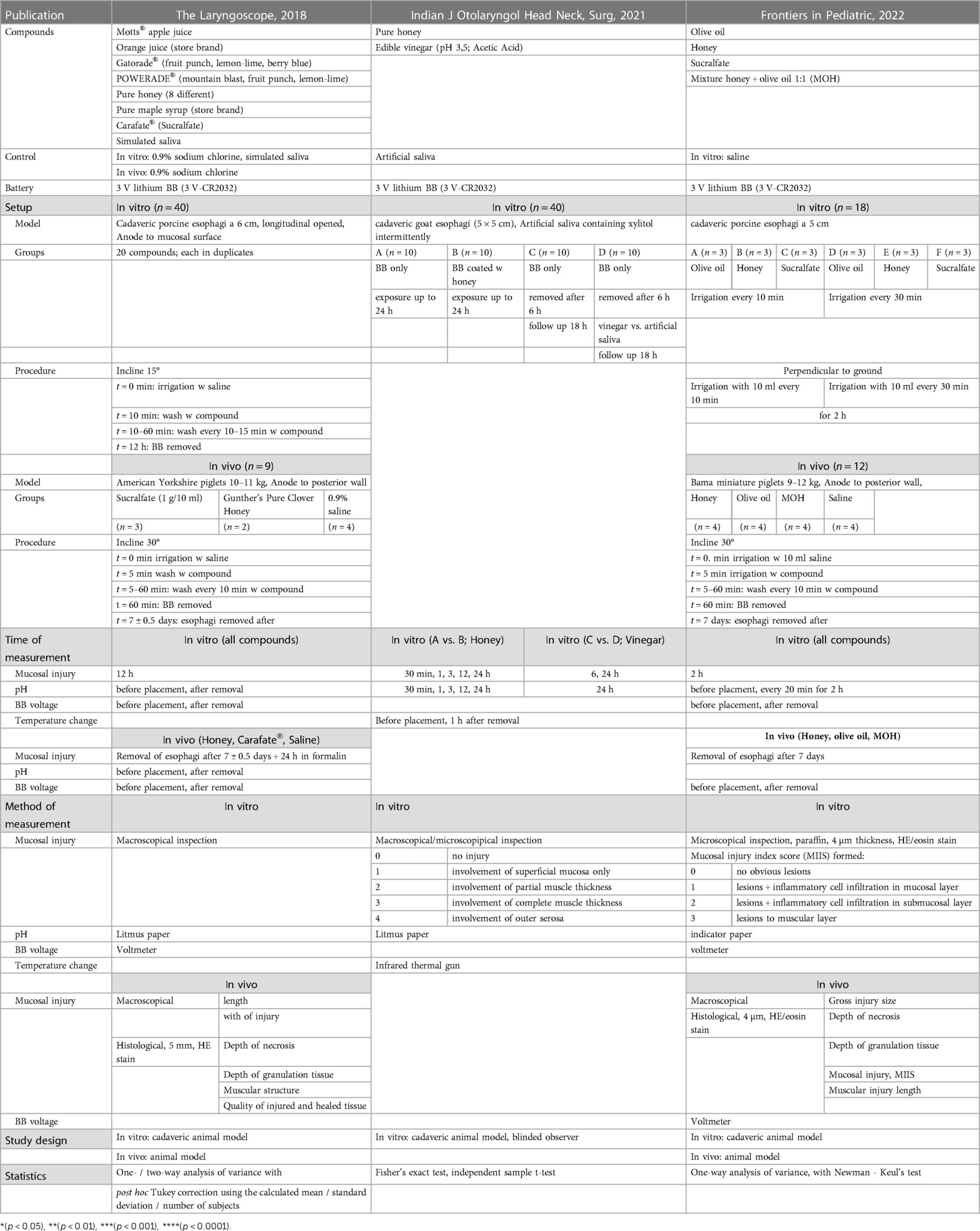
Table 1. Overview of study design, setup and methods of three experimental studies (Anfang et al., Gyawali et al., Jia et al.)
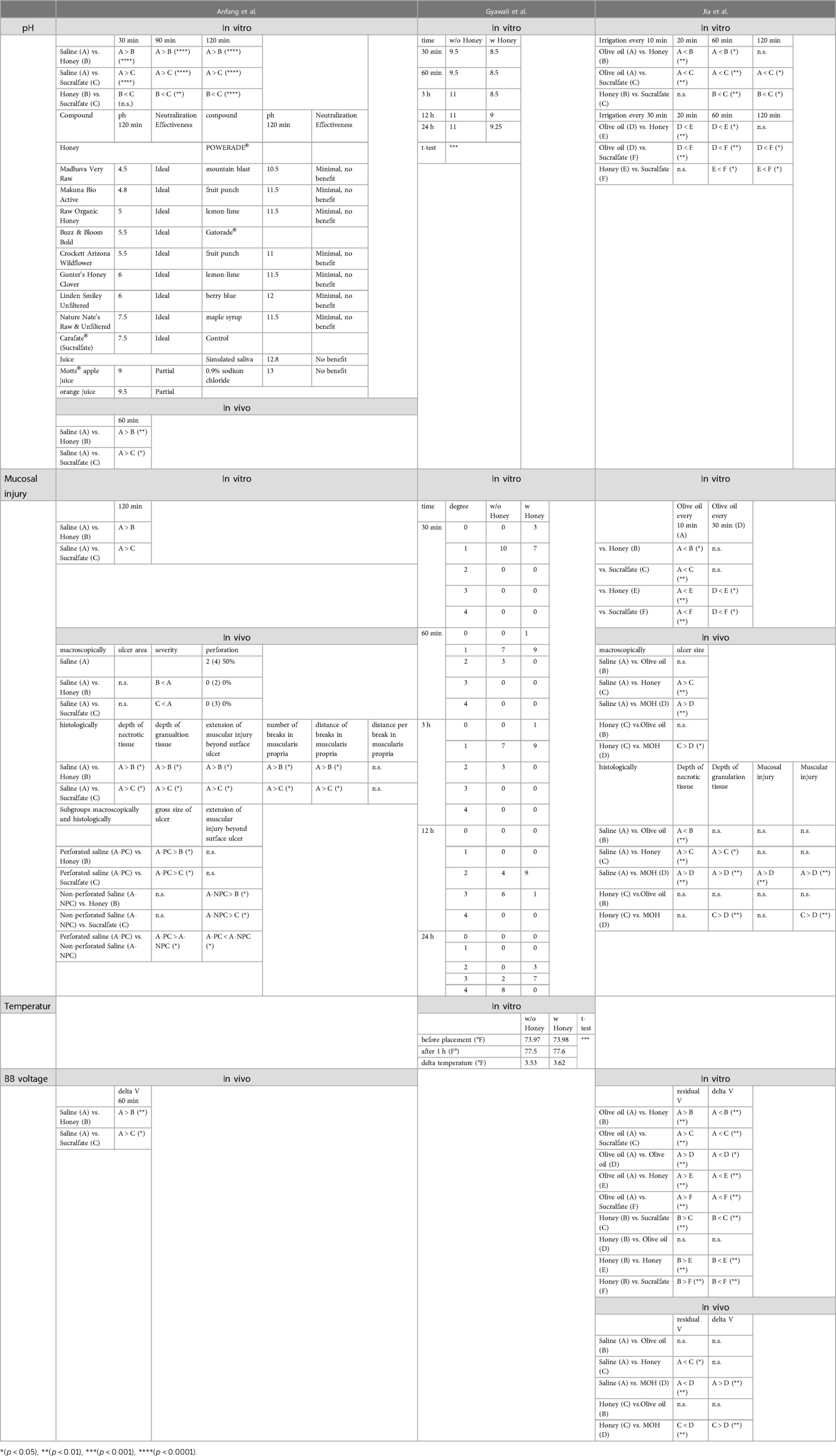
Table 2. Overview, categorization and comparison of collected results from three experimental studies (Anfang et al., Gyawali et al., Jia et al.).
The extend of tissue injury was measured in vitro in all three experimental studies with Anfang et al. and Gyawali et al. showing less damage to the tissue when applying honey compared to untreated or with saline treated tissue. Anfang et al. were able to demonstrate a protective effect for sucralfate as well. Depending on the interval of the irrigations, Jia et al. were able to show an equivalent or partly greater protective effect for the use of olive oil, compared to honey or sucralfate. However, all three experimental studies reported qualitative results only. Anfang et al. compared the extent and Gyawali et al. and Jia et al. evaluated the injury with their self-defined ordinal scale. To date, there are only two experimental studies assessing the in vivo effects of irrigations with honey in case of BB ingestion. In both studies, treatment with honey led to less injury of the esophagus with smaller ulcer size. There was no peroration when honey or sucralfate was used, in contrast to the piglets which were treated with saline only and developed perforation in 50% of the cases (6).
The histopathological examination of the esophagi as well revealed weaker extent of the BB induced damage when treated with honey or sucralfate (6) respectively honey or a mixture of honey and olive oil (MOH) (10). According to the authors, honey reduced the depth of the necrosis, the depth of granulation tissue, as well as the muscular injury induced by the BB. With regards to the in vitro results, Jia et al. saw perforation in all piglets treated with olive oil alone. This group also reported more favorable in vivo tissue protecting effects when using MOH rather than using honey alone.
The effects of honey on temperature in the affected area as another factor was examined by Gyawali et al. and showed no clinically relevant difference.
Anfang et al. and Jia et al. assessed the effects of their substances on the change of voltage within the used BB. In vivo they found honey and sucralfate to reduce the change in voltage across tissue (6), and honey and a MOH to reduce the loss of voltage, respectively the change of voltage when compared to saline.
The fourth study that we found was retrospective in nature and included 8 patients. The time to battery removal as well as the battery size varied, but the age was quite uniform between 1 and 3 years. Patients who were treated with honey did not develop any complications. These patients also received acetic acid after removal and had a shorter time to removal than most of the other patients so that we are facing a potential bias. No adverse effects of the application of honey after button battery ingestion were seen in this study (11).
4. Discussion
To our knowledge, this is the first systematic analysis of studies on the subject of applying honey to mitigate the effects of retained esophageal BB. Surprisingly, without much underlying data, this type of adjuvant therapy has made its way into guidelines on both sides of the Atlantic.
According to our review, the use of honey may be protective, not only by neutralizing the battery induced pH change, but also by forming a shielding film around the BB due to its higher viscosity.
Contrary to the assumption that exothermic neutralization could induce relevant thermal damage, no evidence of such was found and the observed rise in temperature in animal models was limited to 0–3°C (3, 16).
The results of the Anfang et al. trial have already been implemented into the recommendations of the National Capital Poison Center (United States), which advises the administration of honey in suspected or confirmed BB ingestion in children >1 year of age [risk of botulism in younger children, see above (7)] and swallowing of the battery <12 h ago. According to their recommendations, 10 ml of honey should be given orally every 10 min until recovery of the foreign body and maximum 6 times (8). Since esophageal perforation in BB ingestion is rare within the first 12 h (<2% of all perforations), potential adverse events should be negligible and administration of honey in the initial period therefore can be considered safe (17). The potentially increased risk of aspiration at induction of anesthesia due to oral intake (2.2/100.000 non-elective procedures) is neglectable in light of the small volume of honey ingested and in comparison, to the risk of a rapidly progressing, potentially fatal injury to the esophagus (18, 19).
The inclusion and exclusion criteria of our study led to a limited number of only four studies. However, the number of in vitro experiments in each study was sufficient to provide a basic knowledge of the time from BB ingestion to different extents of damage of the esophagus as well as the effects of various agents on the pH. In addition, the potential danger from high temperature seems to be ruled out. Nevertheless, the number of microscopic studies as well as in vivo studies was limited to a number n = 9 (Anfang et al.) respectively n = 12 (Jia et al.), and the application of honey was only investigated twice histologically and in vivo.
Therefore, prospective follow up studies are required to further elucidate the effectiveness of the treatment with honey or sucralfate. Furthermore, the time intervals in which the use of honey or sucralfate is effective is not clear since the studies applied the honey immediately with or even before the battery, which is not realistic in the clinical setting and the studies lack of testing a wide variety of potential intervals. Likewise, a more physiological model is needed for the in vitro testing, possibly mimicking the peristalsis and the flow of the applied substances which was lacking throughout the in vitro settings of the described studies. In addition, performance of clinical trials with a larger number of participants are needed in the future.
Due to heterogeneity of those studies in approach, setup, and analysis, the performance of a meta-synthesis or -analysis was only limited and descriptively possible. Nevertheless, these studies have provided a basic insight into the effects of honey after BB ingestion. In detail, the studies investigated the pH change, the extent of the mucosal injury, the temperature, and the voltage (see Tables 1, 2). In vitro the pH was decreased by honey more than by saline or sucralfate, and this effect increased over time. Furthermore, the pH was decreased by sucralfate more than by saline. Jia et al. also assessed the in vitro effect of olive oil, which lowered the pH more than honey or sucralfate. Neutralization effectiveness of honey and sucralfate after 120 min was ideal, whereas fruit juices and various Sports drinks as well as saliva and physiological sodium chloride solution did not recover the pH measured on the mucosa. In vivo pH testing was performed only by Anfang et al. and confirmed said observations with honey and sucralfate decreasing the pH more than saline did.
Our systematic review has several limitations. Due to heterogeneity of the assessed studies, a direct comparison of their results is limited. A statistical evaluation was therefore not reasonable and not performed. Studies published only in small databases might have been missed.
The most important limitation at this time is the lack of clinical comparative studies. These studies are difficult to perform, because the event incidence is low. Therefore, we are currently preparing a multicenter, prospective trial to objectively test the effect of honey in a systematic fashion. Nevertheless, the available studies suggest a positive effect, with minimal risks and disadvantages for the patients. Therefore, at this time, administering honey after suspected button battery injestion is advisable.
Our findings indicate that when BB ingestion is suspected or confirmed, a coordinated rapid approach to minimize the risk of complications is needed (20) and the oral administration of honey in the interval between ingestion and retrieval could potentially reduce complications. This approach should not delay the removal of the battery (1, 21).
In an experimental study that currently carried out at our clinic, we are addressing the above-mentioned weaknesses of previous studies. We are including, among other things, various battery types, as well as different application intervals and types of honey with different viscosities.
With only three experimental and one clinical study available so far, there is a great need for a large, prospective, and ideally multicenter study to carefully evaluate the effect of honey used in children with BB ingestion. This is the only way to assess whether the mentioned measures are not only safe, but also effective.
Other information/limitations
The review is registered at Research Registry. The identifying number is reviewregistry1581.
By limiting our research to only English publications, the possibility of a publication bias given.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft. OM: Conceptualization, Investigation, Project administration, Supervision, Validation, Writing – review & editing. DW: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1259780/full#supplementary-material
References
1. Litovitz T, Whitaker N, Clark L, White NC, Marsolek M. Emerging battery-ingestion hazard: clinical implications. Pediatrics. (2010) 125(6):1168–77. doi: 10.1542/peds.2009-3037
2. Litovitz T, Whitaker N, Clark L. Preventing battery ingestions: an analysis of 8648 cases. Pediatrics. (2010) 125(6):1178–83. doi: 10.1542/peds.2009-3038
3. Jatana KR, Rhoades K, Milkovich S, Jacobs IN. Basic mechanism of button battery ingestion injuries and novel mitigation strategies after diagnosis and removal. Laryngoscope. (2017) 127(6):1276–82. doi: 10.1002/lary.26362
4. Mubarak A, Benninga MA, Broekaert I, Dolinsek J, Homan M, Mas E, et al. Diagnosis, management, and prevention of button battery ingestion in childhood: a European society for paediatric gastroenterology hepatology and nutrition position paper. J Pediatr Gastroenterol Nutr. (2021) 73(1):129–36. doi: 10.1097/MPG.0000000000003048
5. Varga Á, Kovács T, Saxena AK. Analysis of complications after button battery ingestion in children. Pediatr Emerg Care. (2018) 34(6):443–6. doi: 10.1097/PEC.0000000000001413
6. Anfang RR, Jatana KR, Linn RL, Rhoades K, Fry J, Jacobs IN. pH-neutralizing esophageal irrigations as a novel mitigation strategy for button battery injury. Laryngoscope. (2019) 129(1):49–57. doi: 10.1002/lary.27312
7. Midura TF. Update: infant botulism. Clin Microbiol Rev. (1996) 9(2):119–25. doi: 10.1128/CMR.9.2.119
8. Center NCP. Button battery ingestion triage and treatment guideline. 2010–2022. Available at: https://wwwpoisonorg/battery/guideline
9. Gyawali BR, Guragain R, Gyawali DR. Role of honey and acetic acid in mitigating the effects of button battery in esophageal mucosa: a cadaveric animal model experimental study. Indian J Otolaryngol Head Neck Surg. (2022) 74(Suppl 3):5759–65. doi: 10.1007/s12070-021-02382-6
10. Jia W, Xu G, Xie J, Zhen L, Chen M, He C, et al. Electric insulating irrigations mitigates esophageal injury caused by button battery ingestion. Front Pediatr. (2022) 10:804669. doi: 10.3389/fped.2022.804669
11. Chandran D, Park S, Barker R, Burns H. Management of oesophageal impaction of button batteries in Queensland. ANZ J Surg. (2022) 92(9):2115–22. doi: 10.1111/ans.17638
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7:10. doi: 10.1186/1471-2288-7-10
14. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br Med J. (2017) 358:j4008. doi: 10.1136/bmj.j4008
15. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 349:g7647. doi: 10.1136/bmj.g7647
16. Jatana KR, Barron CL, Jacobs IN. Initial clinical application of tissue pH neutralization after esophageal button battery removal in children. Laryngoscope. (2019) 129(8):1772–6. doi: 10.1002/lary.27904
17. Soto PH, Reid NE, Litovitz TL. Time to perforation for button batteries lodged in the esophagus. Am J Emerg Med. (2019) 37(5):805–9. doi: 10.1016/j.ajem.2018.07.035
18. Hoagland MA, Ing RJ, Jatana KR, Jacobs IN, Chatterjee D. Anesthetic implications of the new guidelines for button battery ingestion in children. Anesth Analg. (2020) 130(3):665–72. doi: 10.1213/ANE.0000000000004029
19. Walker RW. Pulmonary aspiration in pediatric anesthetic practice in the UK: a prospective survey of specialist pediatric centers over a one-year period. Paediatr Anaesth. (2013) 23(8):702–11. doi: 10.1111/pan.12207
20. Russell RT, Griffin RL, Weinstein E, Billmire DF. Esophageal button battery ingestions: decreasing time to operative intervention by level I trauma activation. J Pediatr Surg. (2014) 49(9):1360–2. doi: 10.1016/j.jpedsurg.2014.01.050
Keywords: honey, button battery, ingestion, esophagus, pediatric
Citation: Schmidt YM, Muensterer O and Wendling-Keim D (2023) The use of honey in button battery ingestions: a systematic review. Front. Pediatr. 11:1259780. doi: 10.3389/fped.2023.1259780
Received: 16 July 2023; Accepted: 8 September 2023;
Published: 28 September 2023.
Edited by:
Andrea Conforti, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Riccardo Coletta, University of Florence, ItalyTutku Soyer, Hacettepe University, Türkiye
© 2023 Schmidt, Muensterer and Wendling-Keim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yannick Michael Schmidt eWFubmljay5zY2htaWR0QG1lZC51bmktbXVlbmNoZW4uZGU=
 Yannick Michael Schmidt
Yannick Michael Schmidt Oliver Muensterer
Oliver Muensterer Danielle Wendling-Keim
Danielle Wendling-Keim