- 1Department of Pediatrics and Child Health, School of Clinical Medicine, College of Health Sciences, University of KwaZulu Natal, Durban, South Africa
- 2Department of Paediatrics, Department of Medicine, Catholic University of Bukavu, Bukavu, Democratic Republic of Congo
- 3Department of Epidemiology, Infectious Diseases and Microbiology, Center for Global Health, University of Pittsburgh, Pittsburgh, PA, United States
- 4Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 5Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 6Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 7Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
- 8Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 9Centre for Tropical Diseases and Global Health and Faculty of Medicine, Catholic University of Bukavu, Bukavu, Democratic Republic of Congo
- 10Department of Paediatrics and Child Health, University of Kwazulu-Natal, Education for Health Africa, Durban, South Africa
Introduction: Despite the extra mortality associated with COVID-19 death globally, there is scant data on COVID-19-related paediatric mortality in Sub-Saharan Africa. We assessed predictors of critical care needs and hospital mortality in South African children with laboratory-confirmed SARS-CoV-2 infection in region with high HIV infection burden.
Methods: We conducted a secondary multicentre analysis of the AFREhealth cohort (a multinational, multicentre cohort of paediatric COVID-19 clinical outcomes across six African countries) of children admitted to the Inkosi Albert Luthuli, a quaternary hospital in KwaZulu-Natal, South Africa, with confirmed RT-PCR between March 2020 and December 2020. We constructed multivariable logistic regression to explore factors associated with the need for critical care (high care/ intensive care hospitalisation or oxygen requirement) and cox-proportional hazards models to further assess factors independently associated with in-hospital death.
Results: Of the 82 children with PCR-confirmed SARS-CoV-2 infection (mean ± SD age: 4.2 ± 4.4 years), 35(42.7%) were younger than one year, 52(63%) were female and 59(71%) had a pre-existing medical condition. Thirty-seven (45.2%) children required critical care (median (IQR) duration: 7.5 (0.5–13.5) days) and 14(17%) died. Independent factors associated with need for critical care were being younger than 1 year (aPR: 3.02, 95%CI: 1.05–8.66; p = 0.04), having more than one comorbidity (aPR: 2.47, 95%CI: 1.32–4.61; p = 0.004), seizure (aPR: 2.39, 95%CI: 1.56–3.68; p < 0.001) and impaired renal function. Additionally, independent predictors of in-hospital mortality were exposure to HIV infection (aHR: 6.8, 95%CI:1.54–31.71; p = 0.01), requiring invasive ventilation (aHR: 3.59, 95%CI: 1.01–12.16, p = 0.048) and increase blood urea nitrogen (aHR: 1.06, 95%CI: 1.01–1.11; p = 0.017). However, children were less likely to die from COVID-19 if they were primarily admitted to quaternary unit (aHR: 0.23, 95%CI: 0.1–0.86, p = 0.029).
Conclusion: We found a relatively high hospital death rate among children with confirmed COVID-19. During COVID-19 waves, a timely referral system and rapid identification of children at risk for critical care needs and death, such as those less than one year and those with comorbidities, could minimize excess mortality, particularly in high HIV-infection burden countries.
Introduction
The devastating impact of the COVID-19 pandemic has resulted in a staggering death toll of 6.9 million as of May 2023, according to the World Health Organization (WHO) (1). Alarming statistics reveal that the virus has claimed the lives of 17,400 individuals under the age of 20. However, these numbers only account for the direct impact of the virus, with the indirect effects garnering less attention. Current evidence supports that middle-income countries have been disproportionately affected by the indirect impact, with 81% of the 14.9 million excess deaths occurring in these regions between 2020 and 2021 (2). The impact of COVID-19 on healthcare systems has been significant, with economic implications that have hit low-income settings the hardest, where patients with additional comorbidities and limited access to appropriate care are most vulnerable (3, 4).
Moreover, despite the easing of lockdown measures, the emergence of novel variants of concern (VOCs) means that SARS-CoV-2 remains a global threat (3, 5, 6). However, previous data have shown limitations (direct and indirect impact) in generalising the evidence to under-represented African settings and other low to middle income countries, where healthcare limitations are already in play (7). A bibliometric analysis of COVID-19 research in Africa revealed that only 20.5% of studies included the African continent, with most of the research focusing on “country preparedness and response” (24.9%) and “the direct and indirect health impacts of the pandemic” (21.6%). Regrettably, very few studies focused on both the direct and indirect impacts on the paediatric population (8). The lack of primary research on children and adolescents who have been victims of varied experiences such as social isolation and who are at the intersection of a number of markers that cause inequity and asymmetry in health/disease interactions highlights a significant place in the generation of empirical evidence (9).
In our parent study, we explored clinical manifestations, outcomes, and factors associated with outcomes among children and adolescents hospitalized with COVID-19 in six countries in sub-Saharan Africa (The AFREhealth Cohort) (10). Our hypothesis was that the presence of any comorbidity and very limited access to high-quality paediatric intensive care could potentially affect the clinical course of SARS-CoV-2 in children living in Sub-Saharan Africa. We found that the morbidity and mortality rates among hospitalized children and adolescents with COVID-19 were substantially higher than those reported in non-African settings (11), and were associated with age younger than 1 year and selected non-communicable disease comorbidities (chronic kidney diseases, chronic lung diseases, haematological disorders, liver diseases and chronic neurologic diseases).
Our study postulated that children living with or exposed to HIV infection in regions with a significant HIV burden would face a higher risk of severe COVID-19 outcomes. Several potential explanations support this heightened risk (12–14), necessitating further investigation into outcomes for HIV-exposed children. Understanding determinants of severe COVID-19 outcomes in these resource-constrained settings could inform preventive strategies like vaccination and mitigate healthcare delays through enhanced referral systems and triage algorithms. Hence, we aimed to delve into factors tied to critical care needs and in-hospital mortality among children with confirmed SARS-CoV-2 infection in KwaZulu-Natal, South Africa, a region with a high HIV infection burden.
Methods
Settings, participants and study design
Detailed information about the AFREhealth Cohort (including participating health care facilities characteristics such as names, locations, urban vs. rural settings, and public vs. private status) is available in the published parent study (10). Briefly, this retrospective record review was a multi-country cohort study that included hospitalized children and adolescents between the ages of 0 and 19 with confirmed SARS-CoV-2 infection through reverse transcriptase polymerase chain reaction (PCR) testing. The study was approved by institutional and/or national research ethics committees and/or regulatory bodies in participating countries, and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (BREC/00002196/2020).
This study included all children and adolescents with confirmed SARS-CoV-2 infection who were admitted (directly or through transfer from a regional facility) to a quaternary facility in the province of Kwazulu-Natal, Inkosi Albert Luthuli Central Hospital (IALCH) between March 1, 2020 and December 31, 2020. IALCH receives referrals from all regional and tertiary hospitals in the province (Greys Hospital and its regional hospitals, Mahatma Gandhi Hospital, Prince Mshiyeni Hospital, RK Khan hospital, King Edward VIII Hospital, Addington Hospital). Data on race and ethnicity were not collected because the racial profile across was more than 78% Black or African descent, and the ethnic diversity across was too broad (South African and foreign ethnic groups combined) for meaningful categorization or analysis.
Independent and dependent variables
Independent variables included demographic and clinical data such as age, sex, pre-existing comorbidities, COVID-19 severity stage at admission, diagnosis of multisystem inflammatory syndrome in children (MIS-C) (1), and imaging, biological and microbiological assessments. They were extracted from national or institutional COVID-19 data sets and/or hospital records using WHO paediatric COVID-19 case report forms. Cases were characterized as suspected MIS-C when at least two required multisystem abnormalities were documented in the medical records and/or databases from which study data were extracted, in addition to fulfilling WHO criteria for MIS-C diagnosis and confirmation of COVID-19 through positive PCR. For a diagnosis of MISC in individuals aged 0–19 years, the patient must have a fever lasting more than three days and at least two of the following symptoms: rash or bilateral non-purulent conjunctivitis or muco-cutaneous inflammation signs (oral, hands or feet), hypotension or shock, features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including Echocardiogram/echo findings or elevated Troponin/NT-proBNP), evidence of coagulopathy (by Prothrombin Time /PT, Partial Thromboplastin Time /PTT, elevated d-Dimers), acute gastrointestinal problems (diarrhoea, vomiting, or abdominal pain), and elevated markers of inflammation such as Erythrocyte Sedimentation Rate/ESR, C-reactive protein, or procalcitonin. In addition, there should be no other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, and evidence of COVID-19 (RT-PCR, antigen test, or serology positive), or likely contact with patients with COVID-19.
Children were classified as HIV-exposed if their mother had a confirmed HIV infection during pregnancy or delivery or breastfeeding. Those who tested positive on an HIV PCR test were classified as living with HIV infection (HIV positive). The study's dependent variables comprised of the requirement for critical care, such as hospitalization with the need for oxygen supplementation, admission to the intensive care unit (ICU), invasive mechanical ventilation, and/or in-hospital mortality. Additionally, the length of hospital stay was measured as a secondary outcome.
Statistical analysis
We analysed the data using various statistical methods, including reporting frequencies and percentages for categorical variables, means and standard deviations (SDs), or medians and interquartile ranges (IQRs) for continuous variables as appropriated. To test for associations, we used chi-square (exact), t-tests, and Wilcoxon rank sum tests, where applicable. To identify independent predictors associated with the odds for the need for critical care and the hazard of death among PCR-confirmed children hospitalized at the quaternary hospital, we constructed multivariable regressions models. Considering that only a very number of selected patients might be admitted at a quaternary health care facility, different models exploring different factors associated with COVID-19 severity and susceptible of impacting on the clinical management such as sociodemographic characteristics, clinical presentation, history of comorbidity, biological markers, microbiology and imaging findings as well as type of treatment received were preferred to preserve the stability of models as well as the overall study power. The final models contained age (as continuous), sex, and other predictors of a priori clinical or epidemiological relevance. We represented the strength of the relationship as adjusted prevalence ratios (aPR) for modified Poisson regression (Poisson regression with log link and robust sandwich standard error) or adjusted hazard ratios (aHR) for Cox-proportional regression and corresponding 95% confidence intervals (CI). The utilization of Robust Poisson was deemed necessary due to convergence difficulties encountered with the binomial model utilizing the log link (log-binomial) (15, 16). We used Stata software version 14.1 (Stata, College Station, TX) for all data analysis, and reported p-values are exact and two-tailed, with values < 0.05 considered statistically significant. GraphPad Prism V 9.0 was used for visual representation.
The binomial model with the log link has convergence issues with many covariates. Therefore, the adjusted RR (ARR) or adjusted PR (APR) can be challenging to obtain in some situations. The modified Poisson regression (Poisson regression with log link and robust sandwich standard error) can be used as an alternative.
Results
Sociodemographic, clinical and radiologic characteristics of PCR-confirmed SARS-CoV-2 children by survival status
The study analysed data from 82 children aged 12 years and younger who were admitted directly from home to IALCH (57.3%) or transferred from tertiary and secondary referral facilities (42.7%). The age range of the cohort was 3 months to 12 years, with a median age of 4.19 years (SD 4.38 years) (Table 1). The majority of the cohort was female (52 females vs. 30 males) and there was a significant likelihood of death in females (13 vs. 1, p = 0.027). Most of the children (55%) required low care management in general wards, while 19/82 children required ICU admission, which was significantly associated with mortality (23%). One-third of the children had radiological signs of pneumonia, while 7/82 (8%) had clinical and laboratory findings suggestive of MISC. The overall mortality rate was significantly high at 17%. Symptoms at presentation associated with death were respiratory distress, seizures, floppiness/hypotonia, and hypotension. Children who presented with fever had a higher risk of death (30%), but this was not statistically significant. Half of the children had a comorbidity, with 17/50 (34%) having two or more comorbidities. One in five (22%) children had reported exposure to HIV infection and one-third of them died (33%). Three children tested positive for HIV infection, and one of them died.
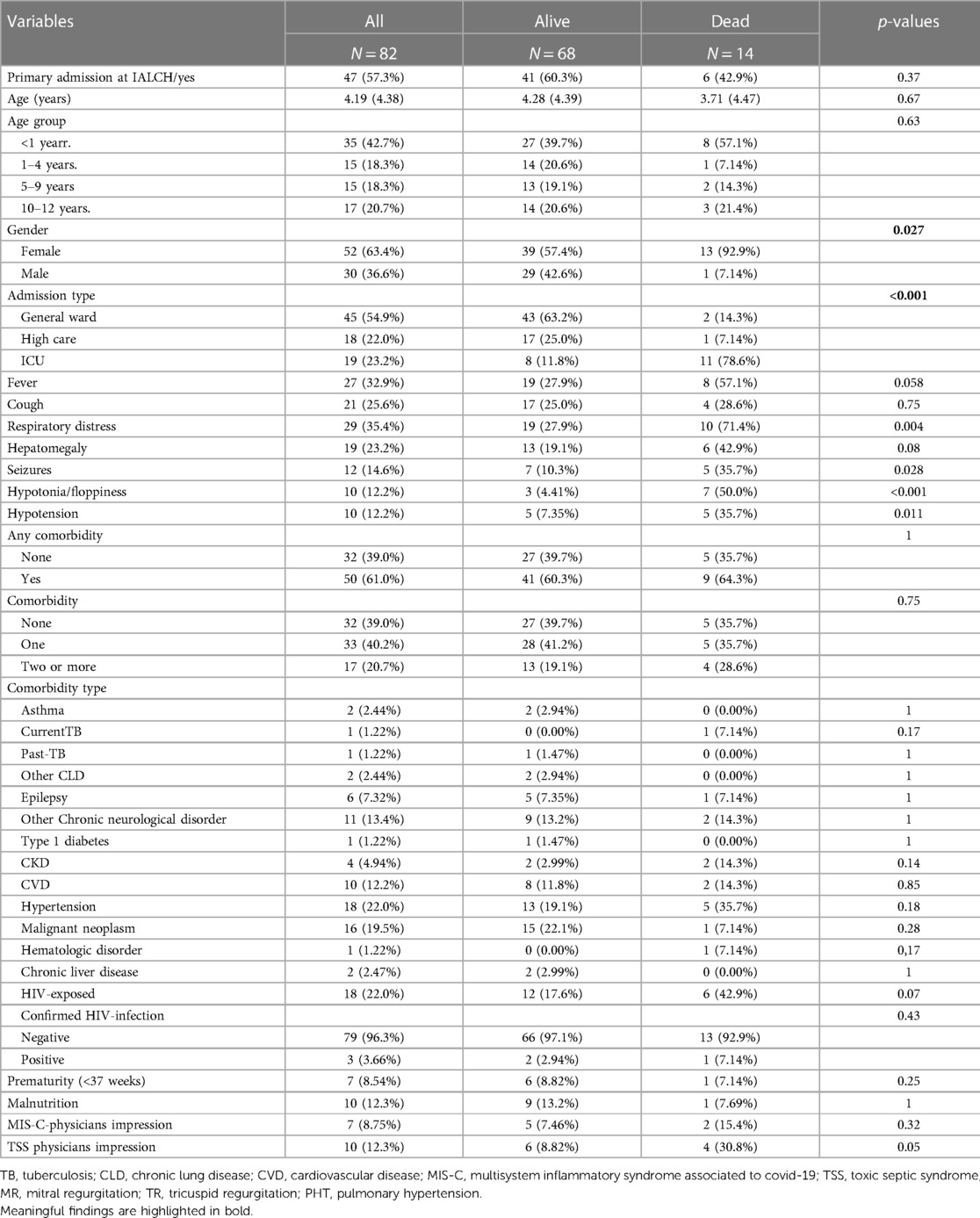
Table 1. Sociodemographic and clinical characteristics of PCR-confirmed SARS-CoV-2 children hospitalised at a quaternary health care facility in KwaZulu-Natal South Africa during the first and second waves of COVID-19 pandemic and classified by survival status.
Biological and microbiological characteristics, as well as treatment received, of PCR-confirmed SARS-CoV-2 children by survival status
At admission, children in this cohort who were at risk of death exhibited a significant impairment in coagulation, with low mean levels of fibrinogen (1.77 ± 0.87) and a decrease in platelet count (378 ± 244 vs. 233 ± 143 × 109/l, p = 0.006) (Figure 1 and Supplementary Table S1). Additionally, children who died had significantly higher mean levels of lactate (2.36 ± 1.53 vs. 4.87 ± 3.27, p = 0.053), and cardiac enzymes tended to be elevated in those at risk of death (Troponin 70.6 vs. 60.4 and Pro-BNP 714 vs. 1,387).
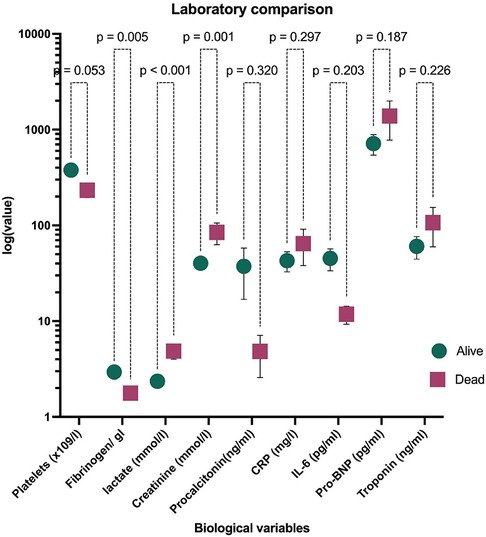
Figure 1. Laboratory findings of PCR-confirmed SARS-CoV-2 children hospitalised at a quaternary health care facility in KwaZulu-Natal South Africa during the first and second waves of COVID-19 pandemic and compared by survival status. Data are represented as median and IQR. The ordinate axis is at log 10 scale. Data at linear scale are in Supplementary Table S1. CRP, C reactive protein; IL-6, interleukin 6; Pro-BNP, pro B-type natriuretic protein.
The presence of a culture-proven infection in children was associated with a significant risk of death regardless of the type of pathogen or the source of the infection. In our cohort, 28/82 (34%) cases had confirmed health care associated infections, and 9 of those children died (Table 2). Compared to children who survived, those who died had a significant increase in the use of corticosteroids, antibiotics, oxygen therapy (35.3%), inotropic support (13.4%), blood transfusion (17%), and any type of advanced ventilatory support (27%). This difference in intensive care management was statistically significant.
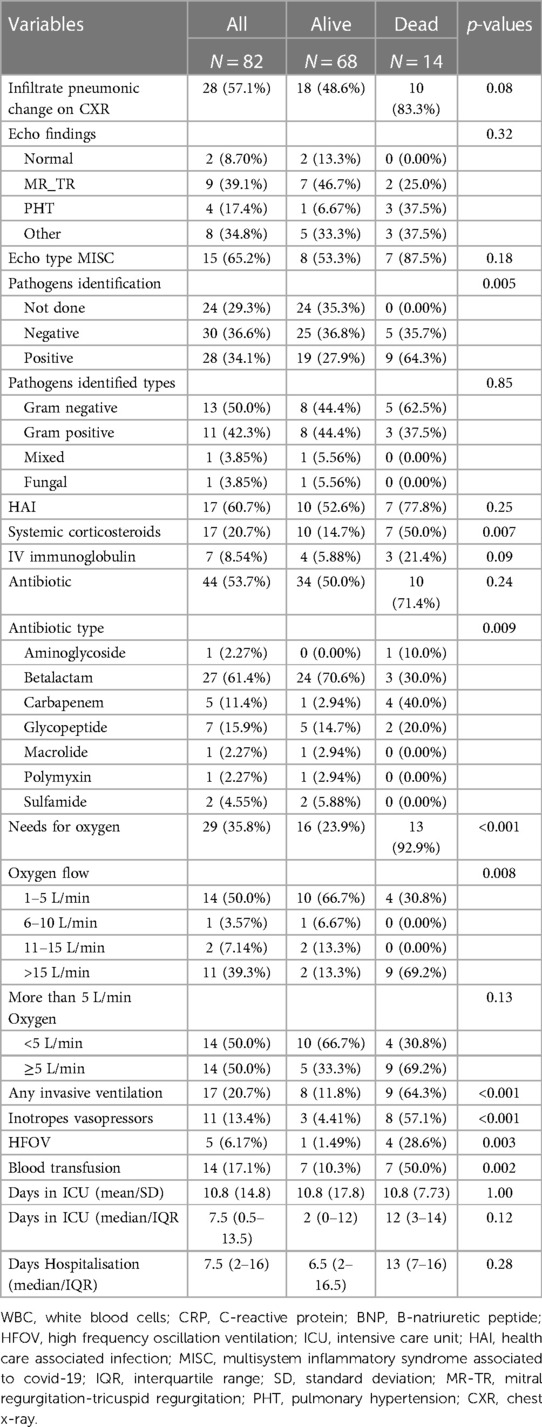
Table 2. Imaging and microbiology findings and type of treatment received of PCR-confirmed SARS-CoV-2 children hospitalised at a quaternary health care facility in KwaZulu-Natal South Africa during the first and second waves of COVID-19 pandemic and classified by survival status.
Predictors associated with need for intensive care in children with PCR-confirmed SARS-CoV-2
After stratification by age group and gender, children admitted to IALCH were 5.4 times more likely to be at risk of need for intensive care. This difference was statistically significant (p = 0.02) (Table 3). In a model that considered age, gender, and the presence of more than 1 comorbidity, every one-year increase in age reduced the risk of need for intensive care by 14%, with estimates ranging from 7% to 20%, p < 0.001. Children with more than one comorbidity had a 2.47-fold increased risk of need for intensive care (aPR:2.47, 95%CI: 1.32–4.61, p = 004). After adjusting comorbidities by age and gender, the impact of HIV exposure lost its statistical significance and was diluted. In this model, chronic kidney diseases (CKD) retained its statistical significance as a variable, as children diagnosed with CKD exhibited a 4.68-fold (95% CI: 1.30–16.86, p = 0.02) higher likelihood of requiring critical care. Further, an increase in creatinine and blood urea nitrogen (BUN) levels by 1 unit was significantly associated with a 10% (with estimates ranging from 1% to 15%, p < 0.001) and a 20% (with estimates ranging from 3% to 32%, p = 0.02) increase in the risk of need for intensive care, respectively, independently. Regardless of age, gender, and comorbidities, children presenting with seizures were at 2.39-fold increased risk of need for intensive care, with estimates ranging from 1.56 to 3.68, p < 0.001.
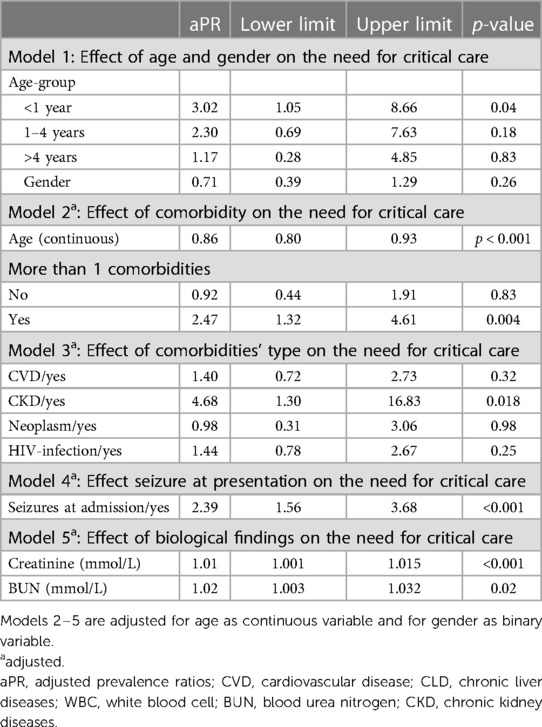
Table 3. Predictors associated with need for critical care among PCR-confirmed SARS-CoV-2 children hospitalised at a quaternary health care facility in KwaZulu-Natal South Africa during the first and second waves of COVID-19 pandemic.
Predictors associated with in-hospital death in children with PCR-confirmed SARS-CoV-2
The results of our study indicate that several factors are independently associated with in-hospital mortality among children with confirmed COVID-19 infection (Table 4). We found that mortality was significantly predicted by exposure to HIV infection, the need for invasive ventilation and increased in BUN, with adjusted hazard ratios (aHRs) of 6.8 (95%CI: 1.54–31.71, p = 0.011), 3.59 (95%CI: 1.01–12.16, p = 0.048) and 1.06 (95%CI: 1.01–1.11, p = 0.017), respectively. Nonetheless, after adjusting for age, we also observed that male children and those primarily admitted from home to the quaternary hospital were less likely to die from COVID-19, with aHRs of 0.12 (95%CI: 0.01–0.95, p = 0.05) and 0.23 (95%CI: 0.1–0.86, p = 0.029), respectively. Additionally, decreased platelet, increased creatinine levels, history of CKD and respiratory distress at admission were also associated with mortality at a borderline statistical or clinical significance.
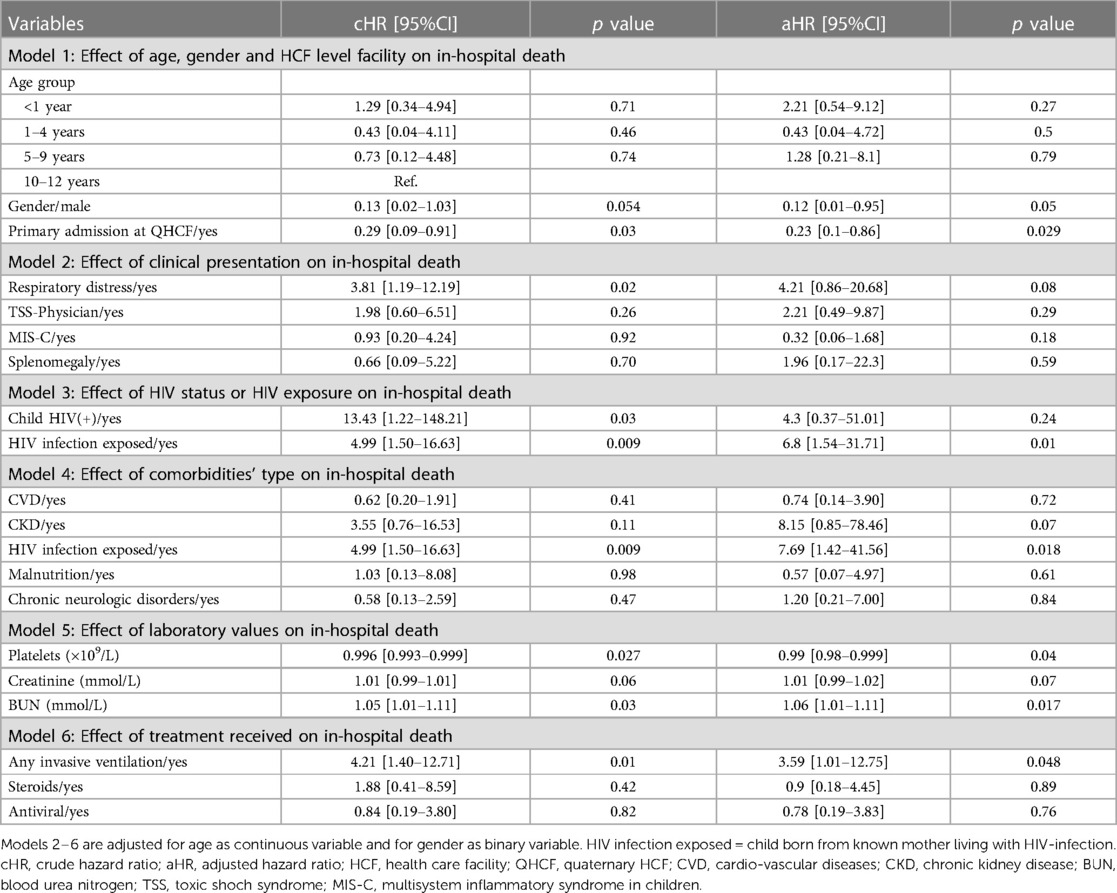
Table 4. Predictors associated with in-hospital mortality among PCR-confirmed SARS-CoV-2 children hospitalised at a quaternary health care facility in KwaZulu-Natal South Africa during the first and second waves of COVID-19 pandemic and classified by survival status.
Discussion
Our study presents a summary of the results obtained from a nested study conducted within a large cohort in various African countries. The study focused on children who were hospitalized with COVID-19 infection between March 2020 and December 2021 in KwaZulu-Natal South Africa. We analysed the 10-day outcome of these children, with a particular focus on risk of ICU admission, oxygen supplementation and in-hospital mortality. The median age of the study population was 4.1 years, and there was a slight female predominance (1.7:1). Our findings showed that 23.1% of the children required ICU admission, 35.3% needed oxygen supplementation, and among these children, 27% received some form of ventilation. The overall mortality rate was significantly high at 17% which highlights the severity of COVID-19 in children with underlying medical conditions. Furthermore, the findings indicated that children with pre-existing medical conditions were at higher risk of requiring critical care and death, with exposure to HIV-infection and younger age being among significant predictors.
Our cohort reported a higher mortality rate compared to previous multicentre studies (17–22), with similar factors associated with ICU admission and death, except for the particularity of HIV exposure and referral (vs. primary) admission to a quaternary health care facility. As such, the higher mortality rate observed in our cohort could be attributed to delays in referral to appropriate facilities and seeking medical help. A study conducted in South Africa reported a relatively high mean ambulance transport time of 4.9 h from the district hospital to the regional hospital (23). Additionally delays in seeking care for children with pneumonia have been linked to factors such as distance to health facilities, lack of skills to recognize emergencies, lack of confidence in the health system, and self-medication practices (24). The inadequate healthcare delivery, including the lack of appropriate equipment including lack of ICU beds and staffing in the public sector, could also contribute to the high mortality rates during the COVID-19 pandemic (25). These findings constitute a call for reducing health inequity by expending universal health coverage to achieve a resilient health system in regions with variables resources and expansion of intensive care facilities closer to where patients live.
Furthermore, studies investigating the association between HIV infection and COVID-19 outcomes have reported mixed results. Some studies have found no significant association, while others have found that people living with HIV (PLWH) may be at an increased risk of severe COVID-19 disease. Recent studies conducted in South Africa and the United States have shown that PLWH, particularly those with comorbidities, Black or Hispanic ethnicity, and HIV-infected children, are at a greater risk of hospitalization and death due to COVID-19. Despite these findings, further research is needed to fully understand the relationship between HIV infection and COVID-19 outcomes (26–28). Although we did not observe a direct association between confirmed HIV infection and increased mortality (due to limited number in this category, n = 3), we did find that exposure to HIV-infection played an independent role in COVID-19 outcomes for children. This novel finding agrees with previous studies suggesting that HIV exposure may increase the risk of infectious diseases in children, even if they are uninfected with HIV. For example, a meta-analysis pooling data from 35 studies and aimed to compare the incidence of diarrhoea and pneumonia in HIV-exposed uninfected (HEU) children and HIV-unexposed uninfected (HUU) children (29). The study found that HEU children had a higher risk of diarrhoea and pneumonia than HUU children. These results suggest that HEU children may benefit from targeted interventions to reduce their risk of these infections. Another hypothesis generated is that this exposure is a proxy for delayed HIV diagnosis and healthcare access, which may contribute to poorer COVID-19 outcomes. Additionally, healthcare-associated infections were observed in 20% of the assessed children during their hospitalization, which could have potentially influenced their outcomes, although didn’t reach statistical significance, but still having clinical significance.
In our study, severe disease in children was characterized by a combination of independent clinical and biological indicators including respiratory failure requiring ventilation, myocarditis indicated by elevated cardiac enzymes, shock manifested as hyperlactaemia and hypotension, acute kidney failure indicated by elevated creatinine and urea, and signs of neurological involvement such as seizures and hypotonia. These findings are consistent with those reported in previous studies of children from various populations (30–33). The fact that 27% of children who received oxygen supplementation required some form of ventilation indicates the severity of the disease in these patients. This highlights the importance of ensuring that adequate medical resources, such as ventilators, are available in healthcare facilities caring for children with COVID-19 in the African region. In the studied population, interim ventilation facilities are available outside an ICU setting, which may reflect the quality of care that is provided to these children outside of a formal intensive care unit. Another significant finding was the association between age and COVID-19 outcomes in children. The study found that younger children (less than 1 year old) were at a higher risk of requiring critical care. This finding is consistent with other studies that have found that children under 1 year of age are at a higher risk of severe COVID-19 outcomes (34, 35). The reported median age of 4.1 years is consistent with early studies conducted in Europe (17–20) as well as studies in Africa and Latin America, while studies in China and the United States reported higher median ages (22, 36).
Furthermore, the association between comorbidities and critical care need and death is consistent with previous studies that have shown comorbidities as a risk factor for severe COVID-19 in children (10, 30). While comorbidities such as CKD and signs of kidney failure identified as independent risk factors for severe COVID-19 outcomes in children, this was not the case for CVD and prematurity. This may be due to the lack of routine cardiological assessment during the pandemic's early stages in our setting, the important use of systemic corticoids and the limited numbers in our sample. Similarly, records of prematurity may have been missing in patient files. However, the study's findings regarding the association between male gender and a lower risk of death are somewhat conflicting, as some studies have reported a higher incidence of severe disease in boys (37), while others have found no significant difference (11).
Our findings will be interpreted in light of some limitations. Firstly, it was conducted in a single and quaternary health care facility and from a high HIV burden setting, which may introduce the risk of selection bias and limit the generalizability to other settings. Secondly, the sample size was relatively small, and the study may have been underpowered to detect some significant differences. Finally, due to the retrospective nature of the study design, there may have been some missing data or unmeasured confounding factors that could have influenced the results. This might include the lack of adjustment for circulating variant of concern among hospitalised children which could have influenced the severity of illness. Notably, our study was one of the first to investigate the impact of HIV exposure in a high-prevalence setting, demonstrating its significant association with mortality. Nationwide prospective cohort considering variant of concerns identification and more diverse populations are needed to better inform policy on the observed findings.
Conclusion
Our findings highlight the importance of providing special attention to children with underlying medical conditions in preventing and managing COVID-19 as they are at increased risk of dying with COVID-19. As such, when allocating intensive care resources during COVID-19 surges, age, and comorbidity, particularly for infants under one year old or those exposure to HIV infection, should be considered. Further, access to tertiary/quaternary facilities to overcome healthcare delivery challenges in Africa, such as delayed referral, insufficient equipment, lack of sufficient ICU beds and staffing shortage at different levels of care, which may contribute to high mortality rates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Committee (University of Kwazulu-Natal). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LB and RM: contributed to the study concept, protocol development, methodology, data collection, data analysis and manuscript preparation and revisions. AP and PJ: assisted with data collection, manuscript preparation and revisions. PK: assisted with data analysis, manuscript revision and edits. LZ: assisted with data collection, data analysis and manuscript revisions and review. JN: assisted with study concept, protocol development and final manuscript review and editing. LB, JN, AP, PK, PJ, LZ, RM: have agreed to be accountable for the content of the work and have all reviewed the final submitted work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1252886/full#supplementary-material
References
1. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available at: https://covid19.who.int/
2. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. Available at: https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021
3. Hebbani AV, Pulakuntla S, Pannuru P, Aramgam S, Badri KR, Reddy VD. COVID-19: comprehensive review on mutations and current vaccines. Arch Microbiol. (2022) 204:1–17. doi: 10.1007/s00203-021-02606-x
4. Lone SA, Ahmad A. COVID-19 pandemic—an African perspective. Emerg Microbes Infect. (2020) 9:1300–8. doi: 10.1080/22221751.2020.1775132
5. Dawood AA. Mutated COVID-19 may foretell a great risk for mankind in the future. New Microbes New Infect. (2020) 35:100673. doi: 10.1016/j.nmni.2020.100673
6. Rahman HS, Abdulateef DS, Hussen NH, Salih AF, Othman HH, Abdulla TM, et al. Recent advancements on COVID-19: a comprehensive review. Int J Gen Med. (2021) 14:10351. doi: 10.2147/IJGM.S339475
7. Number of COVID-19 orphans nears 150,000 in South Africa. Available at: https://www.unicef.org/southafrica/press-releases/number-covid-19-orphans-nears-150000-south-africa
8. Guleid FH, Oyando R, Kabia E, Mumbi A, Akech S. A bibliometric analysis of COVID-19 research in Africa. BMJ Glob Heal. (2021) 6:e005690.
9. Silva FSM, Machado SSF, de Sousa Moreira JL, Araújo JEB, de Araújo TI, Dionizio BS, et al. COVID-19 and HIV among children and adolescents: current inequalities. J Pediatr Nurs. (2022) 65:e9–e10. doi: 10.1016/j.pedn.2021.12.003
10. Nachega JB, Sam-Agudu NA, MacHekano RN, Rabie H, Van Der Zalm MM, Redfern A, et al. Assessment of clinical outcomes among children and adolescents hospitalized with COVID-19 in 6 sub-saharan African countries. JAMA Pediatr. (2022) 176:E216436. doi: 10.1001/jamapediatrics.2021.6436
11. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109:1088–95. doi: 10.1111/apa.15270
12. Global HIV & AIDS statistics — Fact sheet | UNAIDS. Available at: https://www.unaids.org/en/resources/fact-sheet
13. WHO warns that HIV infection increases risk of severe and critical COVID-19. Available at: https://www.who.int/news/item/15-07-2021-who-warns-that-hiv-infection-increases-risk-of-severe-and-critical-covid-19
14. Children, HIV and AIDS: How will progress be impacted by COVID-19?—UNICEF DATA. Available at: https://data.unicef.org/resources/children-hiv-and-aids-how-will-progress-be-impacted-by-covid-19/
15. Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. (2003) 3:1–13. doi: 10.1186/1471-2288-3-1
16. Methods for estimating prevalence ratios in cross-sectional studies—PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/19009156/
17. Götzinger F, Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
18. Parri N, Lenge M, Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. (2020) 383:187–90. doi: 10.1056/NEJMc2007617
19. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. Br Med J. (2020) 370.
20. Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. (2020) 55:2565–75. doi: 10.1002/ppul.24991
21. Damania R, Moore W, Viamonte HC, Kamat P, Basu RK. Severe acute respiratory syndrome coronavirus 2 infection and critically ill children. Curr Opin Pediatr. (2021) 33:286–91. doi: 10.1097/MOP.0000000000001019
22. Bhalala US, Gist KM, Tripathi S, Boman K, Kumar VK, Retford L, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med. (2022) 50:e40. doi: 10.1097/CCM.0000000000005232
23. Kong V. Understanding the reasons for delay to definitive surgical care of patients with acute appendicitis in rural South Africa. (2014). http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0038-23612014000100001
24. Pajuelo MJ, Huaynate CA, Correa M, Malpartida HM, Asayag CR, Seminario JR, et al. Delays in seeking and receiving health care services for pneumonia in children under five in the Peruvian Amazon: a mixed-methods study on caregivers’ perceptions. BMC Health Serv Res. (2018) 18:1–11. doi: 10.1186/s12913-018-2950-z
25. Maphumulo WT, Bhengu BR. Challenges of quality improvement in the healthcare of South Africa post-apartheid: a critical review. Curationis. (2019) 42. doi: 10.4102/curationis.v42i1.1901
26. Kollmann TR, Kampmann B, Mazza-Stalder J, et al. Impact of HIV infection on COVID-19 outcomes in children: preliminary findings from a South African cohortTitle. Lancet HIV. (2021).
27. Chow FC, Schechter M, Wei C, et al. Burden of COVID-19 hospitalizations among people with HIV in New York city. J Acquir Immune Defic Syndr. (2021) 3:265–71.
28. Huang SJ, Young J, Blumberg E, et al. COVID-19 outcomes in people living with HIV hospitalized in the United States: a multi-site surveillance study. J Int AIDS Soc. (2021) 7:e25773.
29. Brennan AT, Bonawitz R, Gill CJ, Thea DM, Kleinman M, Long L, et al. A meta-analysis assessing diarrhea and pneumonia in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. J Acquir Immune Defic Syndr. (2019) 82:1. doi: 10.1097/QAI.0000000000002097
30. Zhu F, Ang JY. COVID-19 Infection in children: diagnosis and management. Curr Infect Dis Rep. (2022) 24:51–62. doi: 10.1007/s11908-022-00779-0
31. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145:20200702. doi: 10.1542/peds.2020-0702
32. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. (2021) 78:536–47. doi: 10.1001/jamaneurol.2021.0504
33. Ye B, et al. Association between an increase in blood urea nitrogen at 24 h and worse outcomes in COVID-19 pneumonia. Ren Fail. (2021) 43:347–50.33583325
34. Liguoro I, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. (2020) 179:1029. doi: 10.1007/s00431-020-03684-7
35. Zheng F, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei. China Curr Med Sci. (2020) 40:275–80. doi: 10.1007/s11596-020-2172-6
36. Shekerdemian LS, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. (2020) 174:868–73. doi: 10.1001/jamapediatrics.2020.1948
Keywords: SARS-CoV-2, intensive care, comorbidity, death, sub-Saharan Africa
Citation: Byamungu LN, Nachega JB, Pillay A, Katoto PDMC, Jeena P, Zurba L and Masekela R (2023) Predictors associated with critical care need and in-hospital mortality among children with laboratory-confirmed COVID-19 infection in a high HIV infection burden region. Front. Pediatr. 11:1252886. doi: 10.3389/fped.2023.1252886
Received: 4 July 2023; Accepted: 11 August 2023;
Published: 7 September 2023.
Edited by:
San-Nan Yang, E-Da Hospital, TaiwanReviewed by:
Jaime Fernández-Sarmiento, Cardioinfantil Foundation, Institute of Cardiology, ColombiaMichael Fokuo Ofori, University of Ghana, Ghana
© 2023 Byamungu, Nachega, Pillay, Katoto, Jeena, Zurba and Masekela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Refiloe Masekela bWFzZWtlbGFyQHVrem4uYWMuemE=
 Liliane N. Byamungu
Liliane N. Byamungu Jean B. Nachega3,4,5,6
Jean B. Nachega3,4,5,6 Ashendri Pillay
Ashendri Pillay Refiloe Masekela
Refiloe Masekela