
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 04 September 2023
Sec. Pediatric Infectious Diseases
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1252495
Pediatric solid organ transplant is a life-saving procedure for children with end-stage organ failure. Viral infections are a common complication following pediatric solid organ transplantation (SOT), which can lead to increased morbidity and mortality. Pediatric solid organ transplant recipients are at an increased risk of viral infections due to their immunosuppressed state. The most commonly encountered viruses include cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), varicella-zoster virus (VZV), adenoviruses, and BK polyomavirus. Prevention strategies include vaccination prior to transplantation, post-transplant prophylaxis with antiviral agents, and preemptive therapy. Treatment options vary depending on the virus and may include antiviral therapy and sometimes immunosuppression modification. This review provides a Quick Algorithmic overview of prevention and treatment strategies for viral infectious diseases in pediatric solid organ transplant recipient.
Organ transplantation is a life-saving procedure for children suffering from end-stage organ diseases (1). Immunosuppressive therapy is necessary to prevent the rejection of transplanted organs in children; However, this therapeutic approach renders them highly susceptible to infections (2, 3). The probability of exposure to pathogens prior to transplantation and subsequent frequency of post-transplant infections can be influenced by the age of the patient at the time of the transplantation, the set of vaccinations received, and the intensity of immunosuppression (4, 5). The prevalence of seronegative recipients in the pediatric population is higher than in adults. Therefore, children are more sensitive to acquiring primary viral infections like CMV and EBV post-transplantation (6, 7).
Despite improvements in managing post-transplant infections during past 20 years, viral infections remain a significant factor affecting graft function and overall transplantation outcomes. Among this vulnerable population, delayed detection and management of viral infections can lead to significant levels of morbidity and mortality (8, 9). The most commonly observed viral infections following solid organ transplantation include CMV, EBV, HSV, VZV, adenoviruses, BK polyomavirus, and respiratory viruses like Influenza (10, 11).
Viral infections can have direct effects such as meningitis, pneumonia, encephalitis, and enteritis that adversely affect the patient's health condition. Moreover, viral infections can indirectly alter the immune system by inducing the release of chemokines and growth factors (12). Therefore, it is vital to closely monitor patients for identifying risk factors and providing preemptive therapy or prophylactic treatment in line with the center's policy (8).
This manuscript provides a quick, algorithmic overview on prevention and treatment of commonly encountered viral infections in post-solid organ transplant pediatric patients. With the implementation of these algorithms, we aim to reduce morbidity and mortality associated with these viral infections and to improve the long-term outcomes for pediatric organ recipients.
During the pre-prophylaxis period, the incidence of CMV infection and disease among SOT recipients was high, while a great percentage of them was invasive (13). Recently, the use of measures such as antiviral prophylaxis, preemptive therapy, or hybrid strategies has led to a reduction in the incidence of CMV disease in pediatric SOT recipients (14–16). Antiviral prophylaxis involves using maintenance doses of (Val)ganciclovir starting within 10 days after transplantation for a duration of 3–6 months. Preemptive therapy is based on routine CMV polymerase chain reaction (PCR) monitoring and the administration of antiviral medication once viremia goes above a certain threshold to prevent CMV disease (16, 17). The hybrid approach involves a short course of antiviral prophylaxis (2–4 weeks), followed by viral load surveillance (15, 18). There have been insufficient pediatric studies to compare the efficacy of these three methods. Retrospective data show equal support for these three preventative approaches, hence all three are suggested (17, 19). However, a key factor for selecting the preventative method is the serostatus of the donor and recipient at the time of transplantation. There is a challenge in determining donor and recipient serostatus in infants younger than 12 months because the passive transfer of maternal antibodies may result in false positive results. According to the Third International Consensus Guidelines, risk assessment in this age group should be based on the highest level of risk for the purposes of CMV prevention (Table 1) (19). In low-risk SOT patients (D−/R−), CMV prophylaxis is not recommended as long as patients are not treated with leukocyte-depleting drugs and receive CMV-negative blood products. These patients should be monitored for clinical symptoms. Preemptive therapy can also be considered as an alternative in this group of patients because they are more prone to acquiring de novo CMV infection from exposures in the community (19). In moderate to high-risk liver, kidney and heart transplant recipients (R+ or D+/R−), all three methods of prevention can be adopted, but some centers prefer prophylaxis with antiviral agents in high-risk patients (D+/R−) (20, 21). In high-risk lung transplant recipients (R+ or D+/R−) antiviral prophylaxis is recommended for longer duration (at least 6–12 months) (19). Prevention with either antivirals or preemptive therapy is also recommended during the use of lymphocyte-depleting agents or intravenous steroids for treatment of acute allograft rejection (Figure 1).
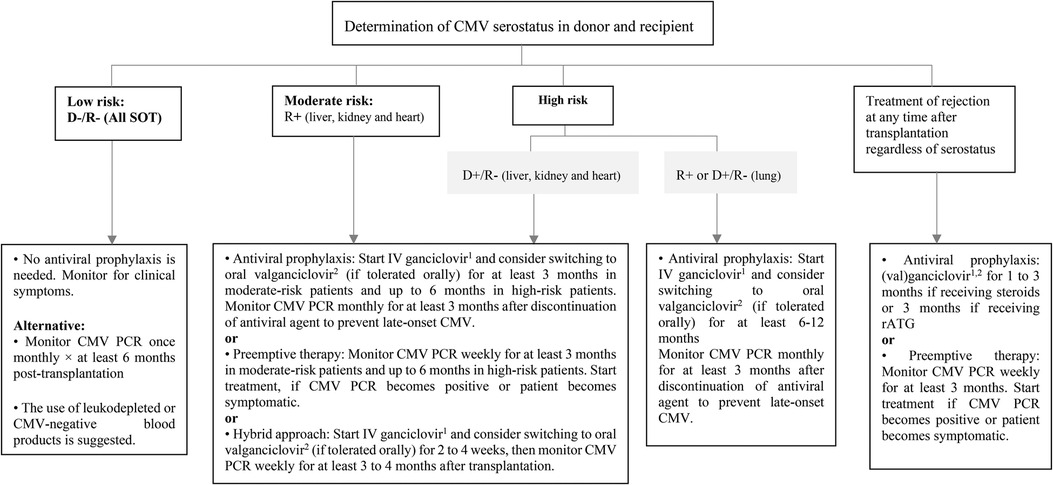
Figure 1. Prevention of CMV infection after pediatric SOT. CMV, cytomegalovirus; D, donor; IV, intravenous; PCR, polymerase chain reaction; R, recipient; rATG, rabbit antithymocyte globulin; SOT, solid organ transplantation. 15 mg/kg/dose once daily in normal kidney function. 2Dose (mg): 7 × BSA × CrCl once daily.
In patients with reliable gastrointestinal absorption, oral valganciclovir is preferred for mild to moderate CMV disease or asymptomatic CMV infection (22). However, in the situations of severe or life-threatening CMV disease, very high viral load and tissue-invasive CMV disease, intravenous ganciclovir is recommended to rapidly achieve optimal drug levels. In clinically stable patients with declining and well-controlled viremia and resolved or resolving clinical symptoms, intravenous therapy can be switched to oral valganciclovir (Figure 2) (17). The dose of intravenous ganciclovir as initial therapy is 5 mg/kg every 12 h, which should be adjusted according to the level of kidney function. A dosing algorithm based on body surface area (BSA) and kidney function is recommended for valganciclovir (23). Patient's monitoring during antiviral therapy includes complete blood counts to check for hematologic side effects, kidney function assessments to determine antiviral dose adjustment, and weekly quantitative CMV PCR to assess medication response (22). Antiviral therapy should be continued as long as all three following conditions are met (17):
• Clinical symptoms subside.
• Virologic clearance to below a negative value threshold based on weekly CMV PCR.
• Antiviral therapy has been given for a minimum of two weeks.
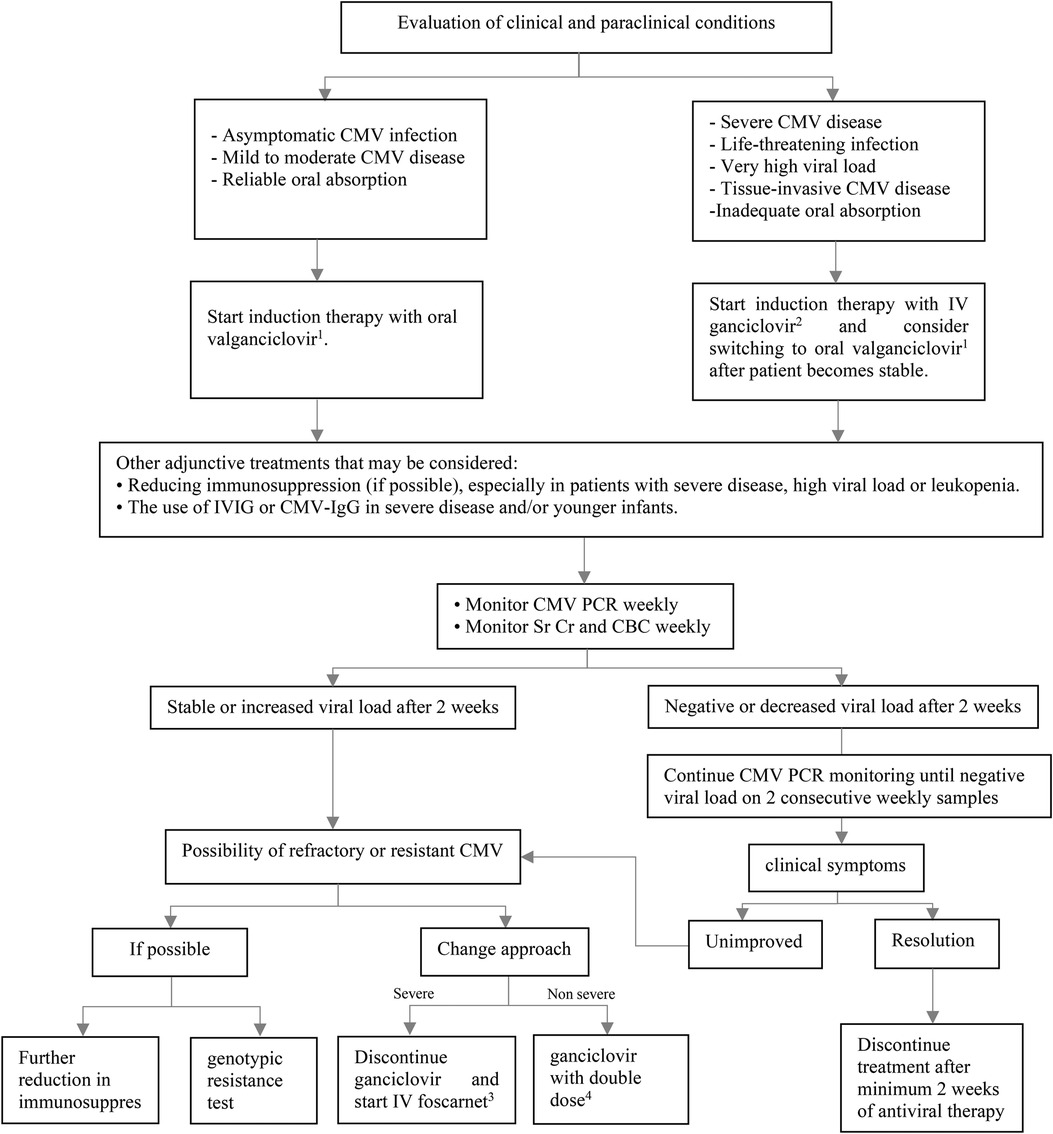
Figure 2. Treatment of CMV infection or disease after pediatric SOT. CBC, complete blood count; CMV, cytomegalovirus; IgG, immunoglobulin G; IV, intravenous; IVIG, intravenous immunoglobulin; PCR, polymerase chain reaction; SOT, solid organ transplantation; Sr Cr, serum creatinine. 1Dose (mg): 7 × BSA × CrCl twice daily. 25 mg/kg/dose twice daily in normal kidney function. 360 mg/kg/dose every 8 h OR 90 mg/kg/dose twice daily in normal kidney function. 410 mg/kg/dose twice daily in normal kidney function.
In general, CMV-immune globulin (IgG) or intravenous immunoglobulin (IVIG) is not advised but may be used in conjunction with intravenous ganciclovir to treat CMV disease in young infants and more severe forms of the disease (17).
Although secondary prophylaxis is not currently supported by good-quality data, some centers offer it after completion of treatment for 1–3 months to lower the chance of recurrence (24). Secondary prophylaxis may be helpful for children with recurrent CMV DNAemia or disease (≥2 episodes). The length of secondary prophylaxis is influenced by patient's age, immunosuppressive regimen, being infected with other opportunistic infections, and other risk factors (19).
According to pediatric cohorts studies the incidence of ganciclovir-resistance CMV is low. Empiric therapy includes doubling dose of ganciclovir or switching to full dose of foscarnet based on the severity of the disease (19). The UL97 kinase and UL54 DNA polymerase are two gene mutations that are associated with CMV drug resistance. Based on results of genotypic test, other antiviral agents such cidofovir and maribavir can be considered Maribavir is approved for treating post-transplant refractory CMV disease in pediatric patients who are at least 12 years old and weigh at least 35 kg (24).
In high-risk patients (D+/R−), a preemptive strategy is recommended. The standard schedule for monitoring EBV DNA is currently uncertain. The decision on how to monitor viral load should be personalized, taking into account various factors such as the type of organ and the level of ongoing immunosuppression. It is suggested to monitor EBV DNA weekly or every two weeks at least for first 3–6 months, then monthly until the end of the first year. In D−/R− patients, monthly EBV DNA monitoring should be considered due to the risk of community-acquired infection. Patients with changing immunosuppression, rejection events, or those without a viral “set point” may be candidates for ongoing surveillance after the first year after the transplantation. Regular viral load monitoring is not recommended for EBV seropositive SOT recipients, except for intestinal transplant patients and those who are undergoing retransplant following post-transplant lymphoproliferative disorder (PTLD) (Figure 3) (7). Administration of antivirals as a preventive strategy for EBV infection remains controversial. Some retrospective studies of SOT have shown that antiviral drugs are not effective in reducing the incidence of EBV infection and/or PTLD (25, 26). On the other hand, a number of studies have supported the use of antiviral medications after transplantation to reduce the risk of EBV infection (27–29) or EBV disease including PTLD (30). According to a meta-analysis, the incidence of PTLD was unaffected by the prophylactic and preemptive use of antivirals in EBV seronegative patients At the time of transplantation (31). The benefits of IVIG or adoptive immunotherapy using donor-derived cloned EBV-specific cytotoxic T cells for the prevention of PTLD are not clear, so the American Society of Transplantation (AST) does not recommend their use as universal prophylaxis measures (7).
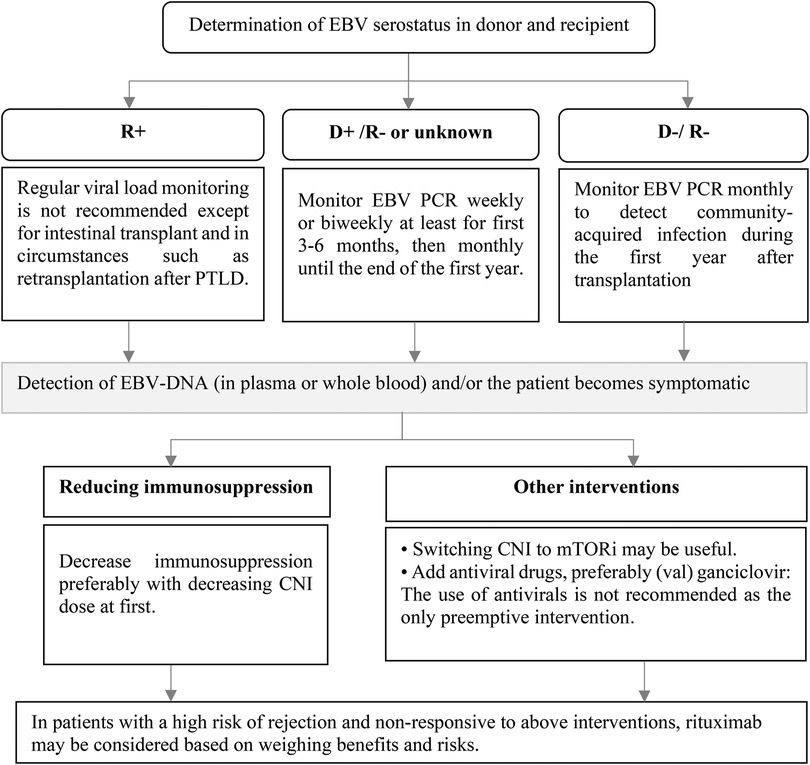
Figure 3. Prevention of EBV infection after pediatric SOT and preemptive intervention. CNI, calcineurin inhibitor; D, donor; DNA, deoxyribonucleic acid; EBV, Epstein–Barr virus; mTORi, mammalian target of rapamycin inhibitors; PCR, polymerase chain reaction; R, recipient.
If EBV-DNA is detected in plasma or whole blood during routine monitoring or whenever the patient becomes symptomatic, preemptive measures should be considered. Reduction in immunosuppression is the preferred intervention (7, 32). Although there is no specific protocol to reduce the patient's immunosuppression, it is suggested that the dose of calcineurin inhibitor (CNI) is reduced initially (33). Conversion from CNI to a mammalian Target Of Rapamycin (mTOR) inhibitor may also be effective due to their in vitro antiproliferative and antiviral properties, although clinical studies supporting their use are lacking (Figure 3) (7, 34). The use of antiviral agents as the only preemptive intervention is not recommended and it can be considered along with other treatments (7). Rituximab administration as preemptive therapy to prevent the consequence of EBV disease such as PTLD cannot be routinely recommended (32). Due to insufficient data, AST guideline does not make a recommendation for or against the use of rituximab in patients not responding to the reduction in immunosuppression (7). Most studies on the use of rituximab as a preemptive intervention have been conducted in hematopoietic stem cell transplant (HSCT) recipients (35, 36) and data on the usefulness of rituximab in SOT patients are limited. In a small retrospective study on 6 pediatric heart recipients who developed primary EBV DNAemia, administration of rituximab resulted in an 83.3% response rate (37). In another prospective single-center study on 8 heart transplantation recipients, a single dose of rituximab reduced PTLD incidence in patients with EBV infection who had a very high viral load or did not respond to reduction of immunosuppression compared with historical controls (38). When weighing the risks and benefits, rituximab administration may be considered in patients who are at high risk for rejection and are not responsive to the reduction of immunosuppression.
Patients taking antiviral medications to prevent CMV infection do not need additional intervention for HSV prophylaxis. Pediatric SOT recipients who are seropositive for HSV-1 and HSV-2 and are not administered antiviral agent for CMV prophylaxis should receive HSV-specific prophylaxis for a minimum of one month. In cases where the recipient is HSV seronegative but the donor is HSV seropositive, which is not uncommon in pediatric patients, some practitioners may consider administering antiviral prophylaxis. In addition, for patients receiving anti-rejection therapy with T-cell depleting drugs, an antiviral agent with activity against HSV can be resumed in the absence of anti-CMV prophylaxis (Figure 4) (39).
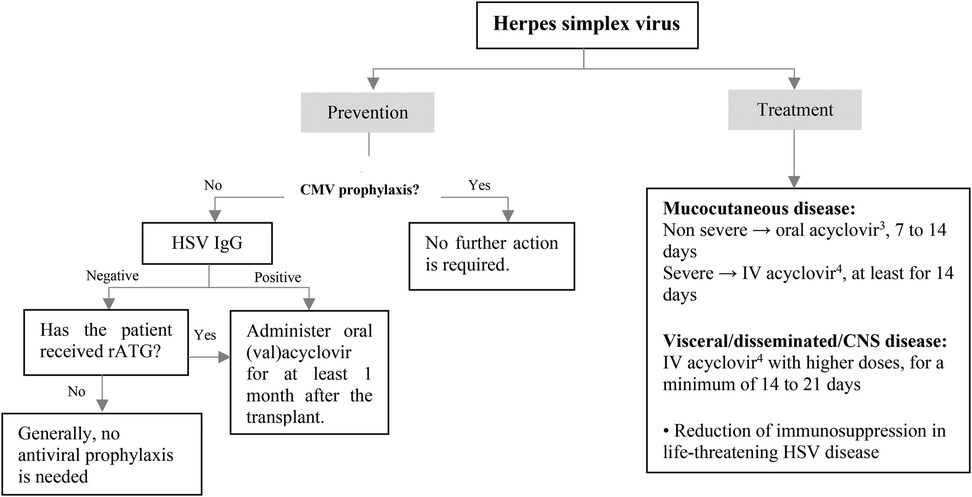
Figure 4. Prevention and treatment of HSV infection after pediatric SOT. CMV, cytomegalovirus; CNS, central nervous system; HSV, herpes simplex virus; intravenous; IgG, immunoglobulin G; rATG, rabbit antithymocyte globulin. 1Acyclovir: 200 mg/dose, 3–5 times/day in normal kidney function. 2Valacyclovir: <40 kg: 250 mg every 12 h, ≥40 kg: 500 mg every 12–24 h in normal kidney function. 320 mg/kg/dose every 6 h in normal kidney function. 410–15 mg/kg/dose every 8 h in normal kidney function.
HSV disease in immunocompromised children may be treated with oral or intravenous acyclovir. While both acyclovir and valacyclovir are considered equally effective when administered at the appropriate doses, there is still limited clinical experience with valacyclovir in pediatric patients. For non-severe mucocutaneous disease, oral acyclovir can be given for a period of 7–14 days. Patients with more serious mucocutaneous illness or those with disseminated/ central nervous system (CNS) disease should receive intravenous acyclovir with higher doses for a minimum of 14–21 days. In cases of life-threatening HSV disease, reducing immunosuppression should be considered (Figure 4).
Varicella vaccination should be considered in seronegative SOT candidates who do not have contraindications to receive live attenuated vaccine (40, 41). In order to improve response rates to varicella vaccination, two doses of the vaccine should be considered with a minimum interval of 4–6 weeks (40, 42, 43). Varicella vaccine should be given at least 4 weeks before the transplantation surgery (Figure 5). Live vaccines are contraindicated after the transplantation, but some selected seronegative kidney and liver transplant recipients with stable conditions may be eligible to receive the varicella vaccine, at least one year after transplantation [see ref (44) for more information]. A significant decrease in VZV antibody levels has been observed following transplantation, particularly among individuals who received fewer pre-transplant vaccine doses (45, 46). However, routine assessment of VZV serology to guide booster dosing is not recommended, as commercial assays have lower sensitivity in detecting vaccine-induced immunity (47).
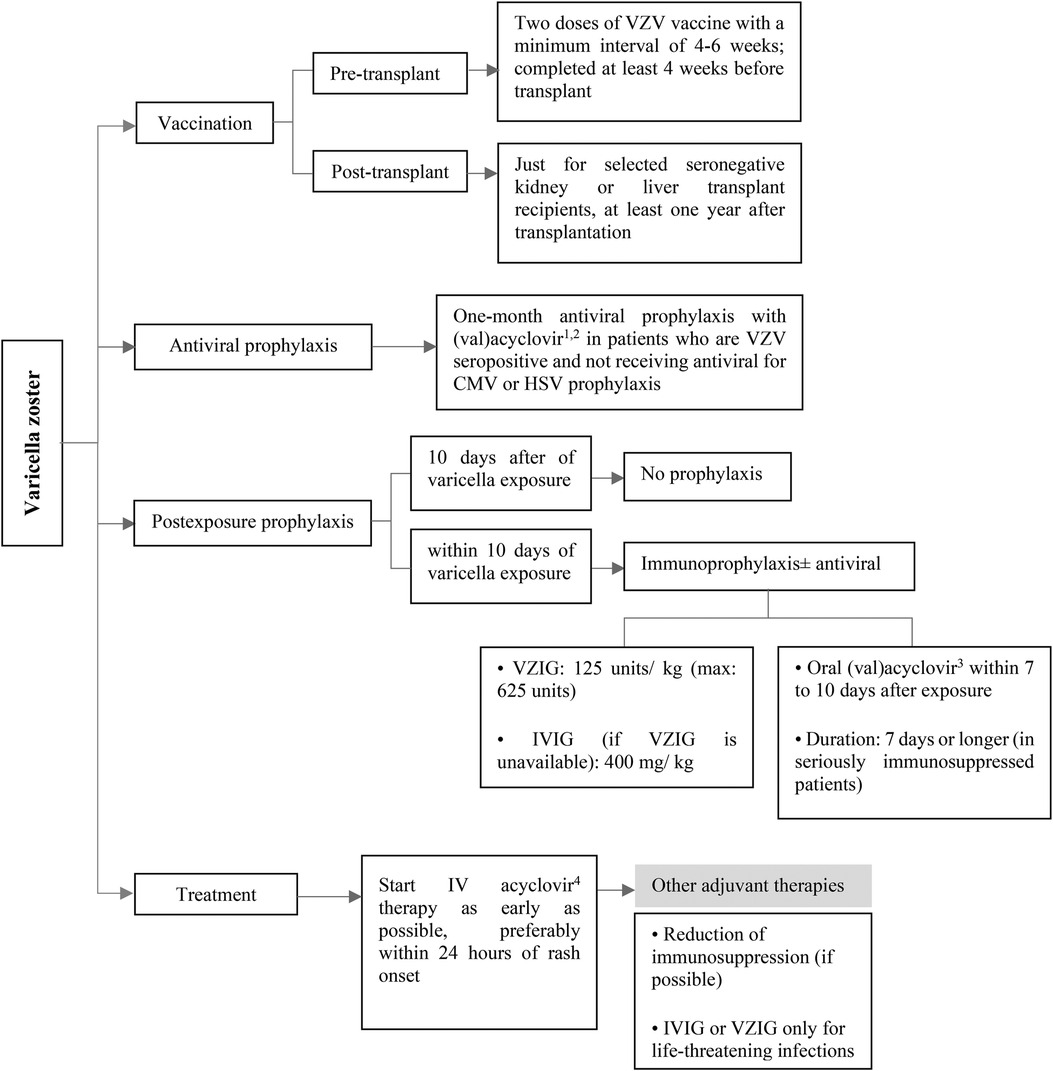
Figure 5. Prevention and treatment of VZV infection after pediatric SOT. CMV, cytomegalovirus; HSV, herpes simplex virus; intravenous; IV, intravenous; IVIG, intravenous immunoglobulin; VZIG, varicella zoster immune globulin; VZV, varicella zoster virus. 1Acyclovir: 200 mg/dose, 3–5 times/day in normal kidney function. 2Valacyclovir: <40 kg: 250 mg every 12 h, ≥40 kg: 500 mg every 12–24 h in normal kidney function. 3Acyclovir: 20 mg/kg/dose every 6 h and valacyclovir: 20 mg/kg/dose every 8 h in normal kidney function. 410 mg/kg/dose every 8 h in normal kidney function.
For those SOT recipients who are seropositive for VZV and not taking antiviral medication for CMV or HSV prophylaxis (which is a rare situation), it is recommended to consider administering (val)acyclovir for at least one month after the transplantation (Figure 5) (9, 40, 48).
Post-exposure prophylaxis should be considered in seronegative pediatric SOT recipients after a significant exposure. It is recommended to use immunoprophylaxis with Varicella-zoster immune globulin (VZIG) or non-specific IVIG (as an alternative if VZIG is not available) as early as possible, with a maximum lag of 10 days after exposure (40, 49–52). Antivirals can be considered as an adjunct in patients who are receiving immunoprophylaxis (40, 49). In addition, antiviral drugs should be administered to the patients who are unable to receive VZIG (40, 53). Preemptive therapy with (val)acyclovir should be started within 7–10 days after VZV exposure for a treatment duration of 7 days (40, 52). Extended duration to 28 may be considered in severely immunosuppressed patients (Figure 5) (40).
Post-transplant patients have a higher risk of developing severe and disseminated VZV disease, so early initiation of intravenous acyclovir therapy, particularly within 24 h of rash onset, is recommended to maximize treatment benefit (40, 52). The American Society of Transplantation recommends against the routine use of immune globulin preparations, including IVIG or VZIG, for the treatment of VZV infection. These treatments may only be considered anecdotally for life-threatening infections (40). Reduction of patient's immunosuppression may be considered (Figure 5). In children with varicella who are taking aspirin to prevent early thrombotic events after transplantation or other indications, aspirin should be stopped due to the risk of Reye syndrome (52).
Influenza is the most common vaccine-preventable infection affecting SOT recipients and results in considerable healthcare utilization (5). Influenza is linked with significant hospitalization rates, lower respiratory tract infections, mechanical ventilation requirements, and even mortality in SOT recipients. Annual influenza vaccination and prompt initiation of antiviral therapy improves the clinical outcomes of Influenza infected SOT patients (54).
Annual influenza vaccination is highly recommended for SOT patients age 6 months and older, as it serves as the primary method of preventing influenza and its associated complications. Close contacts of SOT recipients, especially those residing in the same household, should undergo yearly influenza immunization (55). Three types of influenza vaccines are available, including inactivated influenza vaccine [IIV], recombinant influenza vaccine [RIV], and live attenuated influenza vaccine (LAIV) (56). LAIV (intranasal vaccine) is not recommended post-transplantation due to the possible risk of developing vaccine-related viral disease (57). RIV is still not approved in pediatric population (58). The SOT pediatrics can receive any licensed, age-appropriate IIV at least one month after the transplantation (Figure 6). Alternative methods and formulations including high-dose or adjuvanted vaccines, and booster shots can enhance immunity without any major safety concerns in SOT populations (59), but limited studies have investigated these strategies specifically in pediatrics (57).

Figure 6. Prevention and treatment of influenza infection after pediatric SOT. yo, years old. 1Children who are 36 months of age or older should receive a 0.5-ml dose of IIV, while children aged between 6 and 35 months may receive either a 0.25-ml or 0.5-ml dose based on the manufacturer's recommendations.
Preexposure antiviral prophylaxis with oseltamivir may be considered for children age 3 months or older who have not received the influenza vaccine due to contraindications, unavailability, or expected low effectiveness (e.g., high immunosuppression due to acute rejection treatment or early months after transplantation) during the flu season or for a period of 12 weeks (60–62).
Postexposure antiviral prophylaxis with oseltamivir is suggested for SOT recipients aged more than 3 months who have close contacts with a patient with confirmed influenza (especially in situations of nosocomial influenza and in severely immunosuppressed patients). A period of 10 days has been suggested for postexposure chemoprophylaxis (55, 61).
All SOT children with confirmed or suspected influenza should take antiviral treatment as soon as possible after symptom onset, preferably within 48 h (61). Oral oseltamivir is the preferred medication for treatment of influenza due to more information on its effectiveness in pediatric transplant recipients (9). The usual duration of antiviral therapy with oseltamivir is 5 days; an extended duration may be needed in patients with long-term clinical symptoms (Figure 6) (61, 62). Alternative antiviral options include inhaled zanamivir for children 7 years or older, intravenous peramivir for children 2 years or older, and oral baloxavir for children 12 years or older weighing at least 40 kg (52). Experiences with zanamivir, peramivir, and baloxavir are lacking in pediatric transplant patients. Intravenous formulations (zanamivir or peramivir) may be used in severely ill patients or those unable to tolerate oral drugs (9). In addition, antiviral therapy should be considered for symptomatic individuals who reside with SOT recipients, particularly severely immunocompromised ones (52, 62).
Hepatitis B virus (HBV) infection significantly increases the risk of hepatic dysfunction in SOT recipients (63). Liver transplantation in the context of HBV-induced cirrhosis or hepatocellular carcinoma is rare in children (64). The importance of HBV in pediatric SOT is due to the risks associated with HBV reactivation or de novo infection (65). The risk of HBV reactivation or de novo infection in a SOT recipient who does not receive antiviral treatment or prophylaxis is determined by the HBV status of both the donor and the recipient (66). The routine screening for HBV using HBsAg, anti-HBs, and anti-HBc testing was previously recommended for all children at risk of HBV reactivation. However, according to the latest U.S. Public Health Service Guideline, pre-transplant HBV testing is no longer necessary for pediatric SOT candidates under the age of 12 who have already undergone infectious disease testing after birth (67).
The risk of HBV transmission through organ donation is higher in liver transplantation, and it is significantly lower in non-liver transplantation (68). Most pediatric liver transplant candidates have not been exposed to HBV at the time of transplantation, so they are at a greater risk for developing de novo infections if they receive organs from anti-HBc-positive donors (69). The use of antiviral agents reduces the rate of de novo HBV infection.
All pediatric recipients with a history of HBV infection receive prophylaxis or continue antiviral therapy if they have positive HBV DNA at the time of transplantation to prevent HBV recurrence. Algorithmic approaches to management of HBV in livre and non-liver transplant are shown in Figures 7–9 (63, 70, 71).
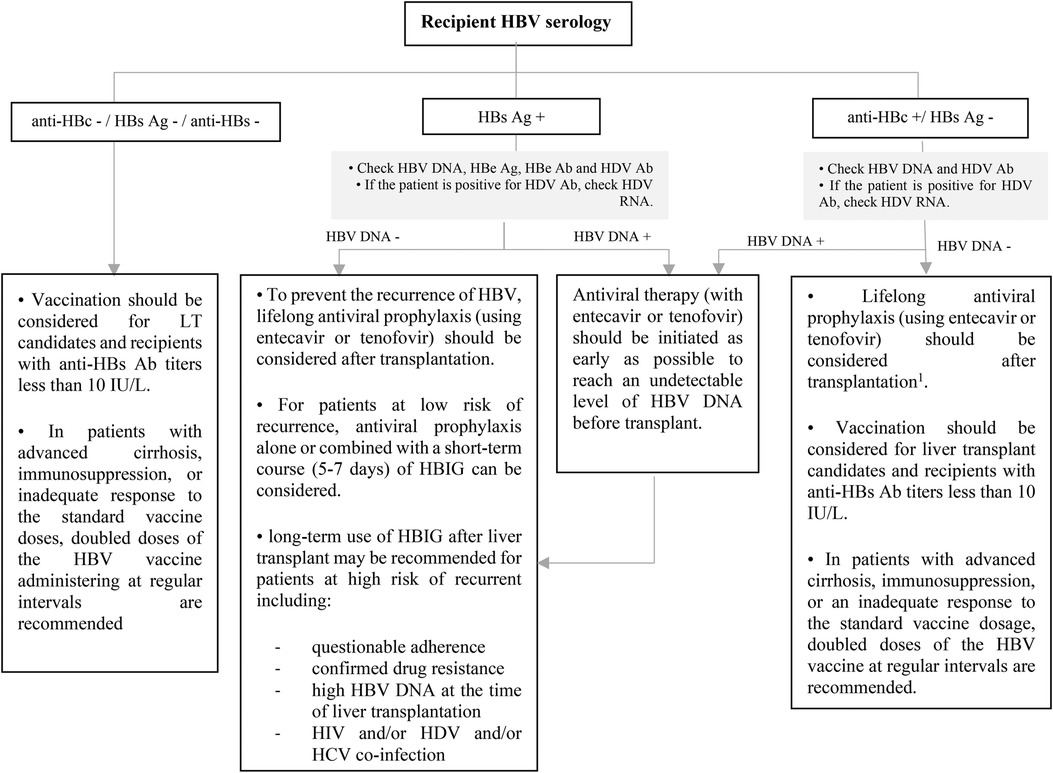
Figure 7. Approach to management of HBV in liver transplant candidates or recipients according to recipient HBV serology. Ab, antibody; Ag, antigen; DNA, deoxyribonucleic acid; HBe, hepatitis B e; HBc, hepatitis B core; HBs, hepatitis B surface; HBV, hepatitis B virus; HDV, hepatitis D virus; HBIG, hepatitis B immune globulin; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LT, liver transplantation; RNA, ribonucleic acid. 1This recommendation is based on ESPGHAN guideline, while the AST guideline does not recommend for the using of antiviral prophylaxis in this context unless for patients with intense immunosuppression such as patients who received lymphocyte depleting agents.
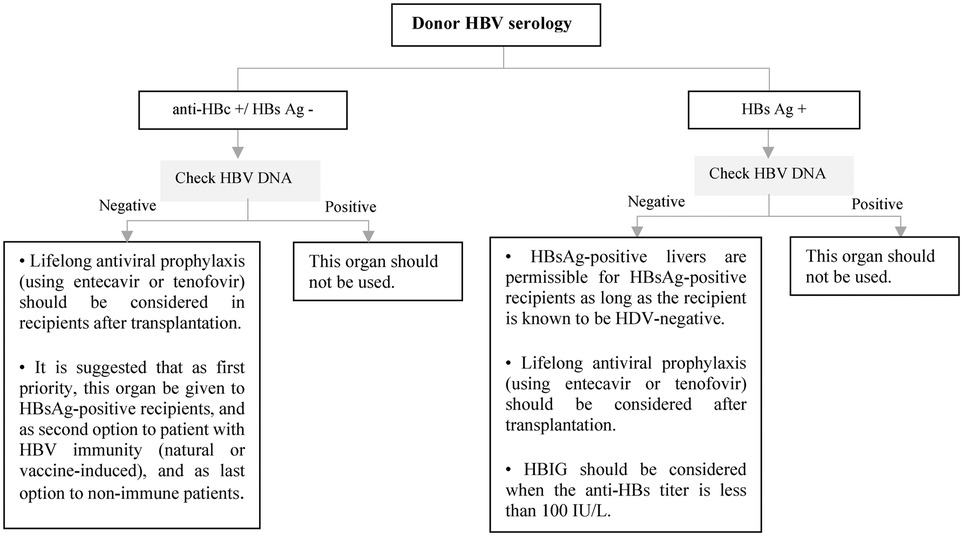
Figure 8. Approach to management of HBV in liver transplant candidates or recipients according to doner HBV serology. Ag, antigen; DNA, deoxyribonucleic acid; HBc, hepatitis B core; HBs, hepatitis B surface; HBV, hepatitis B virus; HDV, hepatitis D virus; HBIG, hepatitis B immune globulin.
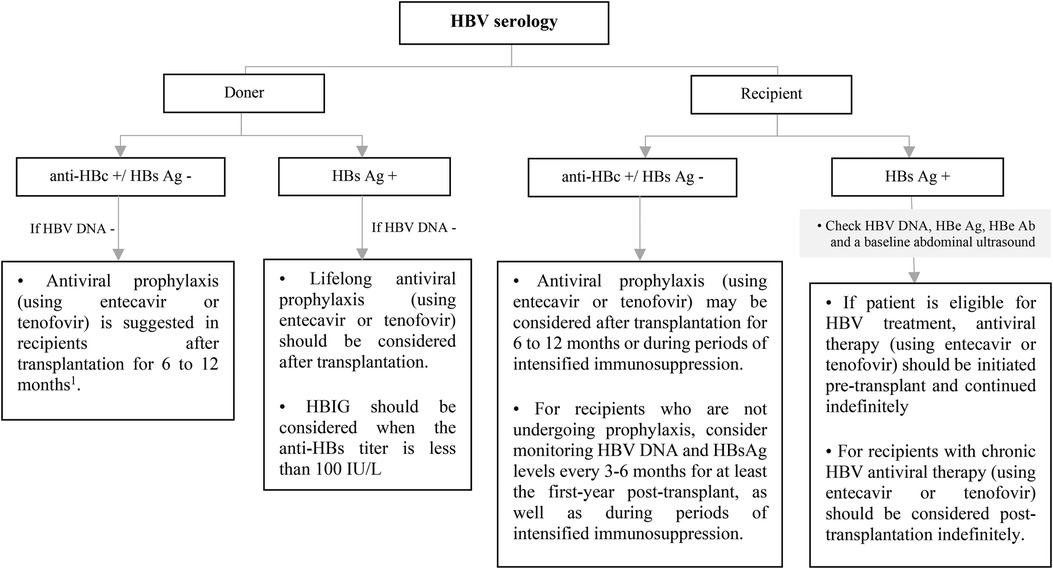
Figure 9. Approach to management of HBV in non-liver SOT transplant candidates or recipients according to recipient and doner HBV serology. Ab, antibody; Ag, antigen; DNA, deoxyribonucleic acid; HBe, hepatitis B e; HBc, hepatitis B core; HBs, hepatitis B surface; HBV, hepatitis B virus; HDV, hepatitis D virus; HBIG, hepatitis B immune globulin. *Vaccination should be considered for HBV-uninfected, non-immune SOT candidates or recipients. 1This recommendation is based on ESPGHAN guideline, while the AST guideline recommends antiviral prophylaxis is not necessary for in HBV immune recipients and it should be considered only in non-immune (anti-HBs -) recipients up to 1-year after transplantation.
The Hepatology Committee of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guideline suggests that antiviral drugs with a high barrier to resistance, such as entecavir or tenofovir, should be used instead of lamivudine for prophylaxis, pre-emptive treatment, and the treatment of HBV reactivation in pediatric patients (71). Two formulations of tenofovir are available, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). It seems that renal and bone toxicity is lower with TAF (72, 73). TAF is approved for use in patients 12 years of age or older with chronic HBV, while the use of TDF and entecavir are approved in children 2 years of age or older weighing at least 10 kg.
Unless there are any contraindications, all transplant recipients are eligible for coronavirus disease 2019 (COVID-19) vaccination. Administering vaccines has been shown to offer a degree of safeguarding against COVID-19 infections and could potentially also serve as a protective measure towards the emergence of post-COVID sequelae in children, such as immune-mediated multisystem inflammatory syndrome (MIS-C) and post-COVID syndrome, which is also known as “long COVID” (74). In comparison to the general population, adult SOT recipients exhibit reduced immunogenicity and efficacy of COVID-19 vaccines (75). Conversely, results from pediatric studies reveal a somewhat moderate increase in COVID-19 vaccine immunogenicity when compared to adult SOT recipients, but the observed level remains lower than that seen in healthy children (76–79).
Although monovalent vaccines have been used since the introduction of mRNA COVID-19 vaccines for patients, after April 2023 only bivalent vaccines including Pfizer-BioNTech and Moderna COVID-19 Vaccine are authorized for persons ≥6 months of age in the United States. Centers for Disease Control and Prevention (CDC) suggests administering three bivalent mRNA doses to unvaccinated individuals aged ≥6 months who have moderate or severe immunodeficiency, including SOT recipients. Individuals aged 6 months or older who previously received only monovalent doses are advised to receive either one or two bivalent mRNA vaccine doses based on their age and the vaccine product used. Those who have already received bivalent mRNA vaccine doses can opt to get one or more additional doses (80). To access more details about COVID-19 vaccination schedule in immunocompromised patients please refer to CDC guidance (80).
The optimal schedule for administering vaccines in the post-transplantation context remains uncertain. To ensure effectiveness, COVID-19 vaccines should be given at least 14 days before transplantation. If pre-transplantation vaccination is unfeasible, it is recommended to delay it by at least one month after the transplantation procedure. In individuals treated with T-cell depleting agents (such as anti-thymocyte globulin) or B-cell depleting agents (such as rituximab), vaccine administration should be postponed for a minimum of three months (81–83). Patients who are on continuous B-cell-depleting therapies should receive COVID-19 vaccines around four weeks before their next scheduled therapy (80).
Current research provides insufficient evidence regarding the optimal management of COVID-19 in pediatric transplant recipients. The optimal therapeutic approach for COVID-19 in this particular population remains unknown. Consequently, the standard procedures for evaluating and managing COVID-19 in nontransplant patients may be applicable for transplant recipients (81). Figure 10 shows an overview of the treatment of COVID-19 in the pediatric population based on the Infectious Diseases Society of America (IDSA) and National Institutes of Health (NIH) guidelines (81, 84). It's important to note that certain antiviral drugs, specifically Nirmatrelvir-ritonavir and Janus kinase (JAK) inhibitors, exhibit substantial drug-drug interactions with immunosuppressive drugs which are frequently administered to SOT recipients. If these immunosuppressive agents persist during COVID-19 infection, it is essential to conduct frequent monitoring of their blood levels and consider adjusting the dose to maintain optimal immunosuppression (85, 86).
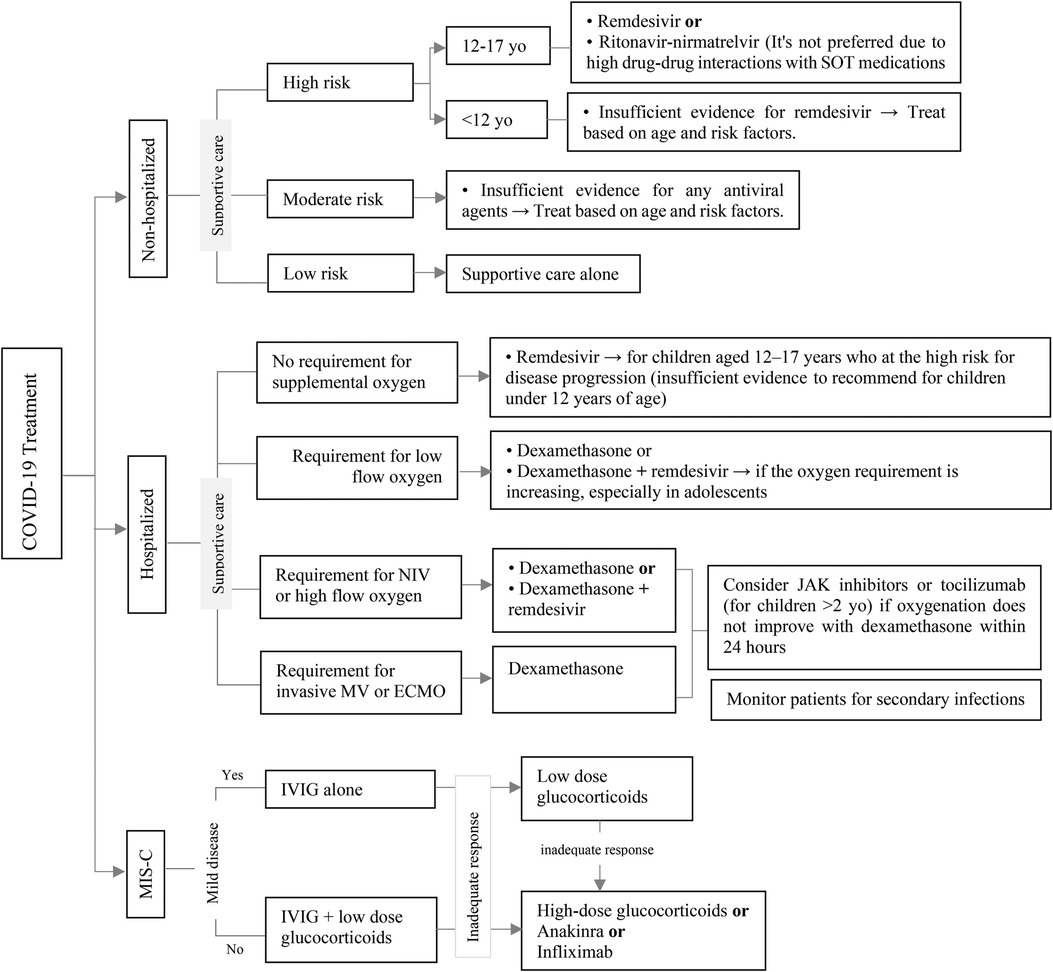
Figure 10. Approach to management of COVI-19 infection in pediatric SOT recipients. ECMO, extracorporeal membrane oxygenation; JAK, Janus kinase; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; MV, mechanical ventilation; NIV, non-invasive ventilation; SOT, solid organ transplantation.
According to the data collected from pediatric patients who underwent SOT, immunosuppression did not pose a supplementary risk for severe or complicated COVID-19 cases. The majority of patients demonstrated mild to moderate levels of disease severity, which is similar to that observed in the general population (87). A systematic review and meta-analysis suggests that continuing immune suppressive therapy may be safe for SOT recipients with moderate or severe COVID-19 (88), as it can potentially alleviate the cytokine storm (89). The decision to adjust the patient's immunosuppressive regimen should be approached individually, with careful consideration given to factors such as disease severity, the specific immunosuppressants administered, the type of transplant, the time since transplantation, the concentration of the drug, and the likelihood of graft rejection and superinfection (81). Some experts and transplant centers suggest to decrease or withhold the use of antimetabolites in patients with lymphopenia who have developed moderate to severe COVID-19, while continuing to administer CNIs (90).
Using antiviral drugs as a preventive measure for adenovirus infection is not recommended due to the lack of evidence regarding their effectiveness. Therefore, prophylaxis is not advised at this time. Routine screening and preemptive strategies are not recommended for adenovirus infection (91).
Patients who do not exhibit any symptoms are not advised to receive treatment, whereas those with adenovirus disease (i.e., symptomatic patients) are eligible for treatment. The therapeutic approach involves providing supportive care, reducing immunosuppression, and administering antiviral therapy (91, 92). Supportive care may include the replacement of fluids and electrolytes in dehydrated patients, as well as the use of appropriate medications to manage diarrhea, nausea, and vomiting (92). There is currently no agreement on which immunosuppressive drug should be stopped or reduced, or when it should be resumed (91). Antiviral medications can be initiated either at the same time as adjusting immunosuppression or afterwards. Cidofovir is the most prescribed antiviral in transplant centers as the standard treatment for severe, progressive, or disseminated adenovirus disease in SOT recipients (91). There are no clinical trials supporting the use of cidofovir in pediatric patients, and there are only a few retrospective studies with controversial results (93–96). In a more recent study about the use of cidofovir for the treatment of adenovirus infection in hematopoietic and solid organ pediatric transplant recipients, the benefits of cidofovir could not be established (96). Cidofovir can be administered in two ways: 1 mg/kg three times per week for 2 weeks or 5 mg/kg per week for 2 weeks, followed by maintenance therapy with 5 mg/kg every other week (91, 97). Maintenance therapy should continue until all symptoms have disappeared, and three consecutive negative samples, taken one week apart from the initially positive sites, have been documented (91). The most significant adverse effects of cidofovir are nephrotoxicity and neutropenia. To minimize nephrotoxicity, it is recommended to administer cidofovir alongside oral probenecid and intravenous normal saline hydration (91). Brincidofovir is a lipid-conjugated prodrug of cidofovir that has been shown to have a lower risk of nephrotoxicity compared to cidofovir and to have more potent in vitro activity against adenoviruses, but it is not currently available commercially (92).
BK polyomavirus (BKPyV) is primarily responsible for polyomavirus-associated nephropathy (PyVAN) following kidney transplantation. However, BKPyV-related complications are infrequent in other types of solid organ transplantations (98). Children face a higher risk of BKPyV-related complications due to a higher rate of BKPyV-seronegativity at the time of transplantation (99, 100).
The preferred screening test for BKPyV is quantitative plasma PCR due to its high sensitivity, specificity, and positive predictive value (98, 101). The use of urine BKPyV PCR as a screening test is not generally recommended due to concerns about specificity and cost. If a positive result is obtained from the urine test, confirmation with plasma PCR is necessary (102). Furthermore, monitoring the response to therapy through urine BKPyV PCR is less effective compared to plasma because the decrease in immunosuppression shows a delay in changes in urine viral load compared to plasma viral load (103). AST guideline recommended to screen on a monthly basis for the first 9 months, and then every 3 months for the following 2 years (Figure 11) (98). In pediatric recipients, extending screening every three months until the third-year post-transplant may be useful (100).
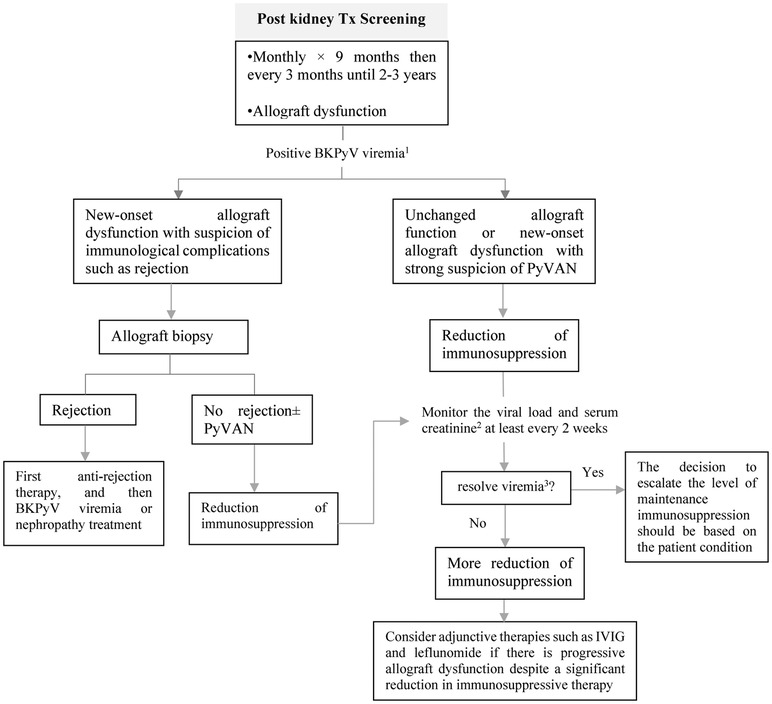
Figure 11. Approach to screening and treating BKPyV in pediatric kidney transplant recipients. PyVAN, polyomavirus-associated nephropathy; IVIG, intravenous immunoglobulin. 1To confirm BKPyV viremia, it is essential to have either sustained viral load above 1,000 copies/ml in two consecutive measurements within a 3-week period or an increase to over 10,000 copies/ml in one of two measurements. 2If the serum creatinine level rises by 25% or more from its initial level during immunosuppression reduction, acute rejection should be evaluated. 3Two consecutive undetectable plasma viral load tests, performed at least one week apart.
Biopsy is considered the gold standard for the diagnosis of BKPyV-associated nephropathy as it enables the detection of any underlying conditions or complications. According to the AST guideline, biopsy should primarily be considered for patients with BKPyV viremia who are experiencing new-onset kidney dysfunction or who have markers indicating an elevated risk of immunological complications (98).
Treatment should be considered for patients with BKPyV-associated nephropathy or those with sustained BKPyV viremia. The primary approach is to reduce immunosuppression therapy (98, 101). This can be achieved by gradually reducing or discontinuing antiproliferative or calcineurin inhibitors. However, there is currently no standardized approach to reducing immunosuppression, as transplant centers typically have their own unique protocols (104). In cases where patients continue to experience progressive allograft dysfunction despite a significant reduction in immunosuppressive therapy over several weeks to months, the addition of IVIG may be considered (105, 106). In such situations, other adjunctive therapies such as leflunomide, cidofovir, and quinolone antibiotics could also be explored (Figure 11) (98).
In conclusion, viral infections remain a significant factor affecting the outcomes of pediatric organ transplantation. Some viral infections, such as CMV and EBV, are more important in children than adults due to their higher rate of seronegativity. Prevention and treatment of viral infections can reduce morbidity and mortality associated with these infections and improve the long-term outcomes for pediatric organ recipients. preventative measures including vaccination, antiviral prophylaxis and preemptive therapy have been shown to be effective in reducing the incidence of viral diseases. Close monitoring and prompt treatment of viral infections are crucial for successful outcomes. To further improve prevention and treatment strategies for viral infections in this population, ongoing research efforts are necessary. By identifying the most effective approaches to preventing and treating viral infections in pediatric organ recipients, we can continue to improve their overall health and well-being.
MM: contributed in idea development, literature review, algorithms development, manuscript drafting and finalization. HE and HA: contributed in literature review, algorithms development and manuscript finalization. SD-K: contributed in idea development, literature review, algorithms’ development, manuscript finalization. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malik S, Kassaï B, Cochat P. Overview of pediatric organ transplantation: current opinion and future perspectives on immunosuppression. Curr Opin Organ Transplant. (2015) 20(5):527–35. doi: 10.1097/MOT.0000000000000224
2. Van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. (2020) 71(7):e159–69. doi: 10.1093/cid/ciz1113
3. Roberts MB, Fishman JA. Immunosuppressive agents and infectious risk in transplantation: managing the “net state of immunosuppression”. Clin Infect Dis. (2021) 73(7):e1302–17. doi: 10.1093/cid/ciaa1189
4. Fishman J. Infection in organ transplantation. Am J Transplant. (2017) 17(4):856–79. doi: 10.1111/ajt.14208
5. Walti LN, Mugglin C, Mombelli M, Manuel O, Hirsch HH, Khanna N, et al. Vaccine-preventable infections among solid organ transplant recipients in Switzerland. JAMA Netw Open. (2023) 6(4):e2310687-e. doi: 10.1001/jamanetworkopen.2023.10687
6. Höcker B, Zencke S, Krupka K, Fichtner A, Pape L, Strologo LD, et al. Cytomegalovirus infection in pediatric renal transplantation and the impact of chemoprophylaxis with (val-) ganciclovir. Transplantation. (2016) 100(4):862–70. doi: 10.1097/TP.0000000000000888
7. Allen UD, Preiksaitis JK, Practice AIDCo. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13652. doi: 10.1111/ctr.13652
8. Timsit J-F, Sonneville R, Kalil AC, Bassetti M, Ferrer R, Jaber S, et al. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intensive Care Med. (2019) 45:573–91. doi: 10.1007/s00134-019-05597-y
9. Otto WR, Green A. Antiviral therapeutics in pediatric transplant recipients. Clin Infect Dis. (2022) 36(1):125–46. doi: 10.1016/j.idc.2021.11.004
10. Katz DT, Torres NS, Chatani B, Gonzalez IA, Chandar J, Miloh T, et al. Care of pediatric solid organ transplant recipients: an overview for primary care providers. Pediatrics. (2020) 146(6):e20200696. doi: 10.1542/peds.2020-0696
11. Abdullatif H, Dhawan A, Verma A. Epidemiology and risk factors for viral infections in pediatric liver transplant recipients and impact on outcome. Viruses. (2023) 15(5):1059. doi: 10.3390/v15051059
12. Green M. Introduction: infections in solid organ transplantation. Am J Transplant. (2013) 13:3–8. doi: 10.1111/ajt.12093
13. Bowman JS III, Green M, Scantlebury VP, Todo S, Tzakis A, Iwatsuki S, et al. OKT3 And viral disease in pediatric liver transplant recipients. Clin Transplant. (1991) 5(4):294. PMID: 21170278.21170278
14. Nicastro E, Giovannozzi S, Stroppa P, Casotti V, Callegaro AP, Tebaldi A, et al. Effectiveness of preemptive therapy for cytomegalovirus disease in pediatric liver transplantation. Transplantation. (2017) 101(4):804. doi: 10.1097/TP.0000000000001531
15. Madan RP, Campbell AL, Shust GF, Kahn AR, Wistinghausen B, Posada R, et al. A hybrid strategy for the prevention of cytomegalovirus-related complications in pediatric liver transplantation recipients. Transplantation. (2009) 87(9):1318–24. doi: 10.1097/TP.0b013e3181a19cda
16. Gerna G, Lilleri D, Callegaro A, Goglio A, Cortese S, Stroppa P, et al. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation. (2008) 86(1):163–6. doi: 10.1097/TP.0b013e31817889e4
17. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients—guidelines of the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13512. doi: 10.1111/ctr.13512
18. Lin A, Worley S, Brubaker J, Boyle G, Nasman C, Sabella C, et al. Assessment of cytomegalovirus hybrid preventative strategy in pediatric heart transplant patients. J Pediatric Infect Dis Soc. (2012) 1(4):278–83. doi: 10.1093/jpids/pis056
19. Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. (2018) 102(6):900–31. doi: 10.1097/TP.0000000000002191
20. Hadzic N, Baumann U, Mclin V. Pediatric liver transplantation: A clinical guide. Elsevier Health Sciences (2020).
21. UK Guideline on prevention and management of cytomegalovirus (CMV) infection and disease following solid organ transplantation. British Transplantation Society. (2022). Available at: https://bts.org.uk/uk-guideline-on-prevention-and-management-of-cytomegalovirus-cmv-infection-and-disease-following-solid-organ-transplantation/
22. Onpoaree N, Sanpavat A, Sintusek P. Cytomegalovirus infection in liver-transplanted children. World J Hepatol. (2022) 14(2):338. doi: 10.4254/wjh.v14.i2.338
23. Vaudrya W, Ettengerb R, Jarac P, Varela-Fascinetto G, Bouw M, Ives J, et al. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am J Transplant. (2009) 9(3):636–43. doi: 10.1111/j.1600-6143.2008.02528.x
24. Balani SS, Sadiq S, Jensen CJ, Kizilbash SJ. Prevention and management of CMV infection in pediatric solid organ transplant recipients. Front Pediatr. (2023) 11:1098434. doi: 10.3389/fped.2023.1098434
25. Walti LN, Mugglin C, Sidler D, Mombelli M, Manuel O, Hirsch HH, et al. Association of antiviral prophylaxis and rituximab use with posttransplant lymphoproliferative disorders (PTLDs): a nationwide cohort study. Am J Transplant. (2021) 21(7):2532–42. doi: 10.1111/ajt.16423
26. Cheyssac E, Savadogo H, Lagoutte N, Baudouin V, Charbit M, Novo R, et al. Valganciclovir is not associated with decreased EBV infection rate in pediatric kidney transplantation. Front Pediatr. (2022) 10:1085101. doi: 10.3389/fped.2022.1085101
27. Albatati S, Sharma A, Haubrich K, Wright A, Gantt S, Blydt-Hansen TD. Valganciclovir prophylaxis delays onset of EBV viremia in high-risk pediatric solid organ transplant recipients. Pediatr Res. (2020) 87(5):892–6. doi: 10.1038/s41390-019-0523-4
28. Cameron BM, Kennedy SE, Rawlinson WD, Mackie FE. The efficacy of valganciclovir for prevention of infections with cytomegalovirus and Epstein-Barr virus after kidney transplant in children. Pediatr Transplant. (2017) 21(1):e12816. doi: 10.1111/petr.12816
29. Höcker B, Böhm S, Fickenscher H, Küsters U, Schnitzler P, Pohl M, et al. (Val-) ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transpl Int. (2012) 25(7):723–31. doi: 10.1111/j.1432-2277.2012.01485.x
30. Ville S, Imbert-Marcille BM, Coste-Burel M, Garandeau C, Meurette A, Cantarovitch D, et al. Impact of antiviral prophylaxis in adults Epstein–Barr Virus-seronegative kidney recipients on early and late post-transplantation lymphoproliferative disorder onset: a retrospective cohort study. Transpl Int. (2018) 31(5):484–94. doi: 10.1111/tri.13085
31. AlDabbagh M, Gitman M, Kumar D, Humar A, Rotstein C, Husain S. The role of antiviral prophylaxis for the prevention of epstein–barr virus–associated posttransplant lymphoproliferative disease in solid organ transplant recipients: a systematic review. A J Transplant. (2017) 17(3):770–81. doi: 10.1111/ajt.14020
32. Green M, Squires JE, Chinnock RE, Comoli P, Danziger–Isakov L, Dulek DE, et al. The IPTA Nashville consensus conference on post-transplant lymphoproliferative disorders after solid organ transplantation in children: II—consensus guidelines for prevention. Pediatr Transplant. (2022):e14350. doi: 10.1111/petr.14350
33. Fulchiero R, Amaral S. Post-transplant lymphoproliferative disease after pediatric kidney transplant. Front Pediatr. (2022) 10:1087864. doi: 10.3389/fped.2022.1087864
34. Krams SM, Martinez OM. Epstein–Barr virus, rapamycin, and host immune responses. Curr Opin Organ Transplant. (2008) 13(6):563–8. doi: 10.1097/MOT.0b013e3283186ba9
35. Stocker N, Labopin M, Boussen I, Paccoud O, Bonnin A, Malard F, et al. Pre-emptive rituximab treatment for Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes. Bone Marrow Transplant. (2020) 55(3):586–94. doi: 10.1038/s41409-019-0699-6
36. Rouce RH, Louis CU, Heslop HE. EBV Lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol. (2014) 21(6):476. doi: 10.1097/MOH.0000000000000083
37. Kiskaddon AL, Landmesser K, Carapellucci J, Wisotzkey B, Asante-Korang A. Expanded utilization of rituximab in paediatric cardiac transplant patients. J Clin Pharm Ther. (2021) 46(3):762–6. doi: 10.1111/jcpt.13346
38. Choquet S, Varnous S, Deback C, Golmard J, Leblond V. Adapted treatment of Epstein–Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. A J Transplant. (2014) 14(4):857–66. doi: 10.1111/ajt.12640
39. Lee DH, Zuckerman RA, Practice AIDCo. Herpes simplex virus infections in solid organ transplantation: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13526. doi: 10.1111/ctr.13526
40. Pergam SA, Limaye AP, Practice AIDCo. Varicella zoster virus in solid organ transplantation: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13622. doi: 10.1111/ctr.13622
41. Danziger-Isakov L, Kumar D, Practice AICo. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13563. doi: 10.1111/ctr.13563
42. Levin MJ. Varicella vaccination of immunocompromised children. J Infect Dis. (2008) 197(Suppl. 2):S197–206. doi: 10.1086/522133
43. Kelgeri C, Kelly DF, Brant A, Patel M, Gupte GL. Principles of immunisation in children with solid organ transplant. Arch Dis Child. (2021) 106(3):219–23. doi: 10.1136/archdischild-2020-319822
44. Suresh S, Upton J, Green M, Pham-Huy A, Posfay-Barbe KM, Michaels MG, et al. Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Pediatr Transplant. (2019) 23(7):e13571. doi: 10.1111/petr.13571
45. Laue T, Oms E, Ohlendorf J, Baumann U. Long-term varicella zoster virus immunity in paediatric liver transplant patients can be achieved by booster vaccinations—a single-centre, retrospective, observational analysis. Children. (2022) 9(2):130. doi: 10.3390/children9020130
46. Yoeli JK, Yoeli D, Miloh TA, Rana A, Goss JA, Munoz-Rivas F. Measles, mumps, rubella (vaccine) and varicella vaccines in pediatric liver transplant: an initial analysis of post-transplant immunity. Pediatr Transplant. (2019) 23(5):e13490. doi: 10.1111/petr.13490
47. Varicella. In: Epidemiology and Prevention of Vaccine-Preventable Diseases. Available at: https://www.cdc.gov/chickenpox/hcp/index.html
48. Bomgaars L, Thompson P, Berg S, Serabe B, Aleksic A, Blaney S. Valacyclovir and Acyclovir pharmacokinetics in immunocompromised children. Pediatr Blood Cancer. (2008) 51(4):504–8. doi: 10.1002/pbc.21638
49. Goldstein S, Somers M, Lande M, Brewer E, Jabs K. Acyclovir Prophylaxis of varicella in children with renal disease receiving steroids. Pediatr Nephrol. (2000) 14:305–8. doi: 10.1007/s004670050764
50. Orenstein WA, Heymann DL, Ellis RJ, Rosenberg RL, Nakano J, Halsey NA, et al. Prophylaxis of varicella in high-risk children: dose-response effect of zoster immune globulin. J Pediatr. (1981) 98(3):368–73. doi: 10.1016/S0022-3476(81)80697-X
51. Zaia J, Levin M, Preblud S, Leszczynski J, Wright G, Ellis R, et al. Evaluation of varicella-zoster immune globulin: protection of immunosuppressed children after household exposure to varicella. J Infect Dis. (1983) 147(4):737–43. doi: 10.1093/infdis/147.4.737
52. Kimberlin DW. Red Book: 2018-2021 report of the committee on infectious diseases. American Academy of Pediatrics (2018).
53. Suga S, Yoshikawa T, Ozaki T, Asano Y. Effect of oral Acyclovir against primary and secondary viraemia in incubation period of varicella. Arch Dis Child. (1993) 69(6):639–3. doi: 10.1136/adc.69.6.639
54. Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. (2018) 67(9):1322–9. doi: 10.1093/cid/ciy294
55. Mombelli M, Kampouri E, Manuel O. Influenza in solid organ transplant recipients: epidemiology, management, and outcomes. Expert Rev Anti Infect Ther. (2020) 18(2):103–12. doi: 10.1080/14787210.2020.1713098
56. Nypaver C, Dehlinger C, Carter C. Influenza and influenza vaccine: a review. J Midwifery Womens Health. (2021) 66(1):45–53. doi: 10.1111/jmwh.13203
57. Dunkle LM, Izikson R, Patriarca PA, Goldenthal KL, Cox M, Treanor JJ. Safety and immunogenicity of a recombinant influenza vaccine: a randomized trial. Pediatrics. (2018) 141(5):e20173021. doi: 10.1542/peds.2017-3021
58. Mameli C, Cocchi I, Fumagalli M, Zuccotti G. Influenza vaccination: effectiveness, indications, and limits in the pediatric population. Front Pediatr. (2019) 7:317. doi: 10.3389/fped.2019.00317
59. Haddadin Z, Krueger K, Thomas LD, Overton ET, Ison M, Halasa N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am J Transplant. (2021) 21(3):938–49. doi: 10.1111/ajt.16295
60. López-Medrano F, Cordero E, Gavaldá J, Cruzado JM, Marcos MÁ, Pérez-Romero P, et al. Management of influenza infection in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the spanish society of infectious diseases and clinical microbiology (SEIMC) and the spanish network for research in infectious diseases (REIPI). Enferm Infecc Microbiol Clin. (2013) 31(8):526.e1–e20. doi: 10.1016/j.eimc.2013.01.013
61. Manuel O, Estabrook M, Practice ASoTIDCo. RNA Respiratory viral infections in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13511. doi: 10.1111/ctr.13511
62. Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM Jr, Fry AM, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. (2019) 68(6):e1–47. doi: 10.1093/cid/ciy866
63. Ayoub WS, Martin P, Bhamidimarri KR. Hepatitis B virus infection and organ transplantation. Gastroenterol Hepatol (NY). (2018) 14(1):33. PMID: 29491759.
64. Zhao P, Lu Y, Wang C, Wang L, Li J, Li M. Clinical, pathological and genetic characteristics of pediatric hepatocellular carcinoma associated with hepatitis B virus infection. J Hepatocell Carcinoma. (2021) 8:361–7. doi: 10.2147/JHC.S306963
65. Patel SS, Leung DH. Hepatitis B immunity in the pediatric liver transplant population. Curr Opin Pediatr. (2016) 28(5):653–8. doi: 10.1097/MOP.0000000000000391
66. Guidelines for Hepatitis B & Solid Organ Transplantation. British Transplantation Society. (2018). Available at: https://bts.org.uk/wp-content/uploads/2018/03/BTS_HepB_Guidelines_FINAL_09.03.18.pdf
67. Free RJ, Levi ME, Bowman JS, Bixler D, Brooks JT, Buchacz K, et al. Updated US public health service guideline for testing of transplant candidates aged 12 years for infection with HIV, hepatitis B virus, and hepatitis C virus—united States. Am J Transplant. (2022) 22(9):2269–72. doi: 10.15585/mmwr.mm7126a2
68. Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery R, et al. Solid organ transplantation from hepatitis B virus–positive donors: consensus guidelines for recipient management. Am J Transplant. (2015) 15(5):1162–72. doi: 10.1111/ajt.13187
69. Xi ZF, Xia Q, Zhang JJ, Chen XS, Han LZ, Zhu JJ, et al. D e novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. J Dig Dis. (2013) 14(8):439–45. doi: 10.1111/1751-2980.12066
70. Te H, Doucette K. Viral hepatitis: guidelines by the American society of transplantation infectious disease community of practice. Clin Transplant. (2019) 33(9):e13514. doi: 10.1111/ctr.13514
71. Indolfi G, Abdel-Hady M, Bansal S, Debray D, Smets F, Czubkowski P, et al. Management of hepatitis B virus infection and prevention of hepatitis B virus reactivation in children with acquired immunodeficiencies or undergoing immune suppressive, cytotoxic, or biological modifier therapies. J Pediatr Gastroenterol Nutr. (2020) 70(4):527–38. doi: 10.1097/MPG.0000000000002628
72. Tao X, Lu Y, Zhou Y, Zhang L, Chen Y. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infect Dis. (2020) 93:108–17. doi: 10.1016/j.ijid.2020.01.035
73. Wassner C, Bradley N, Lee Y. A review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. JIAPAC. (2020) 19:2325958220919231. doi: 10.1177/2325958220919231
74. Dulek DE, Ardura MI, Green M, Michaels MG, Chaudhuri A, Vasquez L, et al. Update on COVID-19 vaccination in pediatric solid organ transplant recipients. Pediatr Transplant. (2022) 26(5):e14235. doi: 10.1111/petr.14235
75. Li J, Ayada I, Wang Y, den Hoed CM, Kamar N, Peppelenbosch MP, et al. Factors associated with COVID-19 vaccine response in transplant recipients: a systematic review and meta-analysis. Transplantation. (2022) 106(10):2068–75. doi: 10.1097/TP.0000000000004256
76. Ziv N, Gimelraikh Y, Ashkenazi-Hoffnung L, Alfandary H, Borovitz Y, Dagan A, et al. Serologic response to COVID-19 infection or vaccination in pediatric kidney transplant recipients compared to healthy children. Transpl Immunol. (2023) 78:101839. doi: 10.1016/j.trim.2023.101839
77. Nel I, Parmentier C, Dehoux L, Minier M, Duneton C, Charbit M, et al. Optimizing COVID-19 vaccination strategy in pediatric kidney transplant recipients: humoral and cellular response to SARS-CoV-2 mRNA vaccination. Transpl Int. (2023) 36:11153. doi: 10.3389/ti.2023.11153
78. Crane C, Phebus E, Ingulli E. Immunologic response of mRNA SARS-CoV-2 vaccination in adolescent kidney transplant recipients. Pediatr Nephrol. (2022) 37(2):449–54. doi: 10.1007/s00467-021-05256-9
79. Kermond RF, Ozimek-Kulik JE, Kim S, Alexander SI, Hahn D, Kesson A, et al. Immunologic response to SARS-CoV-2 mRNA vaccination in pediatric kidney transplant recipients. Pediatr Nephrol. (2023) 38(3):859–66. doi: 10.1007/s00467-022-05679-y
80. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
81. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://covid19treatmentguidelines.nih.gov/ (Accessed August 8).
82. SARS-CoV-2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID-19 Task Force. Available at: https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_3-15-2021.pdf (Accessed March 19, 2021).
83. American Society of Transplantation COVID-19 Vaccination Guidance. Available at: http://www.myast.org/sites/default/files/AST-COVID-PtVaccine-3.18.21-final.pdf (Accessed March 21, 2021).
84. America IDSo. IDSA Guidelines on the treatment and management of patients with COVID-19. Arlington, VA: Infectious Diseases Society of America. (2021) (Updated 2023 May 15).
85. Avery RK. Update on COVID-19 therapeutics for solid organ transplant recipients, including the omicron surge. Transplantation. (2022) 106(8):1528. doi: 10.1097/TP.0000000000004200
86. Tang Y, Li Y, Song T. Optimizing the use of nirmatrelvir/ritonavir in solid organ transplant recipients with COVID-19: a review of immunosuppressant adjustment strategies. Front Immunol. (2023) 14:1150341. doi: 10.3389/fimmu.2023.1150341
87. Kwak BO, Eun BW. COVID-19 in immunocompromised children and adolescents. Clin Exp Pediatr. (2023) 66(5):182. doi: 10.3345/cep.2022.00878
88. Karruli A, Spiezia S, Boccia F, Gagliardi M, Patauner F, Salemme A, et al. Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis. Transpl Infect Dis. (2021) 23(4):e13595. doi: 10.1111/tid.13595
89. Kakos CD, Ziogas IA, Tsoulfas G. Pediatric transplantation during the COVID-19 pandemic. World J Transplant. (2022) 12(5):88. doi: 10.5500/wjt.v12.i5.88
90. COVID-19: Issues related to solid organ transplantation: UpToDate. (2023). Available at: www.uptodate.com
91. Florescu DF, Schaenman JM, Practice AIDCo. Adenovirus in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13527. doi: 10.1111/ctr.13527
92. Florescu DF, Stohs EJ. Approach to infection and disease due to adenoviruses in solid organ transplantation. Curr Opin Infect Dis. (2019) 32(4):300–6. doi: 10.1097/QCO.0000000000000558
93. Neant N, Klifa R, Bouazza N, Moshous D, Neven B, Leruez-Ville M, et al. Model of population pharmacokinetics of cidofovir in immunocompromised children with cytomegalovirus and adenovirus infection. J Antimicrob Chemother. (2018) 73(9):2422–9. doi: 10.1093/jac/dky192
94. Fisher BT, Boge CL, Petersen H, Seif AE, Bryan M, Hodinka RL, et al. Outcomes of human adenovirus infection and disease in a retrospective cohort of pediatric hematopoietic cell transplant recipients. Pediatric Infect Dis Soc. (2019) 8(4):317–24. doi: 10.1093/jpids/piy049
95. Boge CL, Fisher BT, Petersen H, Seif AE, Purdy DR, Galetaki DM, et al. Outcomes of human adenovirus infection and disease in a retrospective cohort of pediatric solid organ transplant recipients. Pediatr Transplant. (2019) 23(6):e13510. doi: 10.1111/petr.13510
96. Grasa C, Monteagudo-Vilavedra E, Pérez-Arenas E, Falces-Romero I. Mozo del Castillo Y, Schüffelmann-Gutiérrez C, et al. Adenovirus infection in hematopoietic and solid organ paediatric transplant recipients: treatment, outcomes, and use of cidofovir. Microorganisms. (2023) 11(7):1750. doi: 10.3390/microorganisms11071750
97. Riggsbee DL, Alali M, Kussin ML. Cidofovir for viral infections in immunocompromised children: guidance on dosing, safety, efficacy, and a review of the literature. Ann Pharmacother. (2023):10600280231176135. doi: 10.1177/10600280231176135
98. Hirsch HH, Randhawa PS, Practice AIDCo. BK Polyomavirus in solid organ transplantation—guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33(9):e13528. doi: 10.1111/ctr.13528
99. McCaffrey J, Bhute VJ, Shenoy M. BK Virus infection and outcome following kidney transplantation in childhood. Sci Rep. (2021) 11(1):2468. doi: 10.1038/s41598-021-82160-0
100. Hoecker B, Schneble L, Murer L, Carraro A, Pape L, Kranz B, et al. Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: an international CERTAIN registry study. Transplantation. (2019) 103(6):1224–33. doi: 10.1097/TP.0000000000002414
101. Eckardt K-U, Kasiske BL, Zeier MG. KDIGO Clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
102. Laskin B, Goebel J. Cost-efficient screening for BK virus in pediatric kidney transplantation: a single-center experience and review of the literature. Pediatr Transplant. (2010) 14(5):589–95. doi: 10.1111/j.1399-3046.2010.01318.x
103. Chakrabarti U, Chaturvedy M, Bajpai NK, Goswami J, Garsa RK, Jhorawat R. BK virus infection and its management in renal transplantation: an update. OBM Transplant. (2023) 7(3):1–23. doi: 10.21926/obm.transplant.2303192
104. Cohen-Bucay A, Ramirez-Andrade SE, Gordon CE, Francis JM, Chitalia VC. Advances in BK virus complications in organ transplantation and beyond. Kidney Medicine. (2020) 2(6):771–86. doi: 10.1016/j.xkme.2020.06.015
105. Kable K, Davies CD, O’connell PJ, Chapman JR, Nankivell BJ. Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct. (2017) 3(4):e142. doi: 10.1097/TXD.0000000000000641
Keywords: pediatrics, solid organ, transplantation, viral infections, antiviral
Citation: Moghadamnia M, Eshaghi H, Alimadadi H and Dashti-Khavidaki S (2023) A quick algorithmic review on management of viral infectious diseases in pediatric solid organ transplant recipients. Front. Pediatr. 11:1252495. doi: 10.3389/fped.2023.1252495
Received: 3 July 2023; Accepted: 21 August 2023;
Published: 4 September 2023.
Edited by:
Carsten Heilmann, Rigshospitalet, DenmarkReviewed by:
Jon Jin Kim, University of Cambridge, United Kingdom© 2023 Moghadamnia, Eshaghi, Alimadadi and Dashti-Khavidaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simin Dashti-Khavidaki ZGFzaHRpc0BzaW5hLnR1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.