- 1Children's Hospital Capital Institute of Pediatrics, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2Department of Cardiology, Children’s Hospital Capital Institute of Pediatrics, Beijing, China
- 3Center for Evidence-Based Medicine, Capital Institute of Pediatrics, Beijing, China
Objective: Fosinopril and amlodipine are commonly prescribed as first-line pharmacotherapeutic agents for pediatric hypertension, but there is a lack of comparative studies regarding the efficacy of these two drugs. We aimed to evaluate and compare the efficacy of fosinopril and amlodipine monotherapy in pediatric primary hypertension.
Methods: This was a single-center, bidirectional observational study. A total of 175 children and adolescents with primary hypertension receiving antihypertensive monotherapy from July 2020 to February 2023 were enrolled. According to antihypertensive drugs, they were divided into the fosinopril group (n = 96) and the amlodipine group (n = 79). Subgroup analysis was performed to compare the efficacy of the two groups in terms of blood pressure (BP) control rates and reductions following a 4-week treatment.
Results: After 4 weeks of treatment, both groups achieved significant reductions in systolic BP (SBP) and diastolic BP (DBP) by more than 18 mmHg and 6 mmHg, respectively, with BP control rates of 61.5% in the fosinopril group and 59.5% in the amlodipine group, revealing no significant differences in the antihypertensive efficacy between the two groups except for DBP control rate (FDR adjusted P > 0.05). Further subsequent subgroup analyses revealed that the reductions in SBP and DBP in the fosinopril group were significantly greater than those in the amlodipine group in patients of females and hypo-HDL-cholesterolemia (FDR adjusted P < 0.05), and there was a trend of difference, although not significant, in patients with central obesity and insulin resistance (IR) (FDR adjusted 0.05 < P ≤ 0.1). However, there were no significant differences in treatment efficacy in patients without these characteristics. Furthermore, hypertriglyceridemia did not exhibit a significant association with the difference in treatment efficacy between the two medications (FDR adjusted P > 0.05).
Conclusions: Fosinopril and amlodipine monotherapy were both effective in pediatric primary hypertension during a short-term follow-up. Fosinopril may be particularly effective in reducing BP in hypertensive patients of females, central obesity, IR, and hypo-HDL-cholesterolemia. These findings indicate that optimizing antihypertensive medication selection based on the individualized characteristics of children with hypertension may improve the efficacy of antihypertensive treatment.
1. Introduction
Increasing evidence showed that hypertension is becoming more common in children and adolescents, which may be related to the increased incidence of obesity (1, 2), and also due to the widespread use of blood pressure (BP) measurement (3). Pediatric hypertension follows a certain trajectory that increases the risk of cardiovascular disease and kidney disease in adulthood (4, 5). Therefore, timely diagnosis and effective treatment of hypertension in children are important.
Despite the increasing need for antihypertensive medication among children with primary hypertension (6), and the number of antihypertensive agents on the market has increased significantly (7), current studies on antihypertensive drugs are mostly focused on adults, and there is limited knowledge about antihypertensive drug choices in children and adolescents. Guidelines and scientific statements generally recommend Angiotensin-Converting Enzyme Inhibitors (ACEI), Calcium Channel Blockers (CCB), Angiotensin Receptor Blockers (ARB), and thiazide diuretics as initial treatment for hypertension in children and adolescents (8–10). However, the comparison of the efficacy of various antihypertensive drugs in children has not been fully documented by large-scale clinical study, and pediatricians usually choose drugs based on the experience of clinical practice in adults. In fact, the pharmacokinetics of children and adults are different, which means that the antihypertensive medication regimens for children cannot be completely referenced to that for adults.
Fosinopril is a long-acting agent of ACEI on the Food and Drug Administration (FDA) Pediatric Priority List (11), and is recommended for use in hypertensive children by the 2018 Chinese Guidelines on Hypertension Prevention and Treatment (12). Amlodipine is the only CCB antihypertensive drug recommended by FDA for hypertensive children (13), which has a long-lasting effect and is well tolerated. As the first-line treatment for hypertension in children, there is a lack of comparative studies of the two drugs. Thus, children and adolescents diagnosed with primary hypertension and treated with fosinopril or amlodipine monotherapy were enrolled in this study. BP levels were compared after 4 weeks in order to provide a reference for the selection of appropriate antihypertensive drugs in children with primary hypertension.
2. Methods
2.1. Study population
This is an ongoing single-center, bidirectional cohort study conducted at Capital Institute of Pediatrics. A total of 175 children and adolescents with primary hypertension receiving antihypertensive monotherapy from July 2020 to February 2023 were included in the final analysis population. Antihypertensive drug therapy is indicated for patients with at least one of the following: (a) symptomatic hypertension, (b) hypertensive target organ damage, (c) hypertension stage 2, or (d) hypertension stage 1 with inadequate response to 6-month lifestyle modification. The study population was divided into two groups: the fosinopril group (n = 96) and the amlodipine group (n = 79), with no specific drug intentionally prescribed for the purpose of this comparative study. All subjects completed necessary tests, as described in our previous study (14). Patients with secondary hypertension caused by renal disease, endocrine disease, central nervous system disease or medication, and patients with primary hypertension receiving nonpharmacologic treatment or other drugs treatment were excluded.
2.2. BP measurement and diagnostic criteria
BP was measured with mercury sphygmomanometer in all subjects and graded according to the 2018 Chinese Guidelines for Prevention and Treatment of Hypertension (12), and 24-h ambulatory blood pressure monitoring was used to exclude white coat hypertension (10, 15). Hypertension is diagnosed when systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) ≥95th percentile for age, sex, and height at least 3 separate occasions. Further, the stages are classified as stage 1 when blood pressure is ≥95th but <99th percentile + 5 mmHg for age, sex, and height, and stage 2 when SBP or DBP ≥99th percentile + 5 mmHg for age, sex, and height.
2.3. Clinical data collection
All data was collected from the electronic medical record system. General data included age, sex, family history, height, weight, waist circumference, and calculated body mass index (BMI). Obesity was diagnosed when BMI exceeded the 95th BMI in children of the same age and sex, and when waist circumference/height >0.48 in males or 0.46 in females, central obesity was determined (16). The laboratory parameters such as fasting blood glucose (FBG), fasting insulin, uric acid, creatinine, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol were recorded. Echocardiographic data were recorded and left ventricular mass index and relative wall thickness were calculated as described in our previous study (14). Urine microalbumin and urine creatinine were recorded, and albumin/creatinine quotient and estimated glomerular filtration rate (eGFR) were calculated as described elsewhere (17).
2.4. Treatment protocol and follow-up
An initial dosage of 10 mg daily fosinopril or 5 mg daily amlodipine was prescribed to all subjects. BP was evaluated weekly, and if necessary, the dosage was adjusted from the second week of treatment until the goal BP (95th percentile for age, sex, and height) was achieved or the maximum dose (40 mg daily for fosinopril and 10 mg daily for amlodipine) was reached. According to previous studies and our clinical observations, 4-week treatment of fosinopril or amlodipine can lead to a stable BP level (18, 19). For those patients who have reached the maximum dosage but not achieved the goal BP, alternative or supplementary other antihypertensive agents should be considered after 4 weeks of treatment. Hence, the last follow-up with BP measured was completed after 4-week monotherapy. We calculated BP control rate as the ratio of the number of subjects achieved goal BP to the total subjects in each group. The study flowchart is shown in Figure 1.
2.5. Subgroup analyses
Subgroup analyses were performed on efficacy parameters (BP control rate and BP reduction) to compare the differences between fosinopril and amlodipine for subgroups of males and females, as well as subgroups of patients with central obesity and those without. Furthermore, homeostasis model assessments of insulin resistance (HOMA-IR) score was calculated following the formula (HOMA-IR = fasting insulin (μU/ml) × FBG (mmol/L)/22.5), and subjects were classified into subgroups of IR (>3.16) and non-IR (≤3.16) in accordance with this score (20). Additionally, subjects were divided into subgroups of hypertriglyceridemia (yes/no), hypo-HDL-cholesterolemia (yes/no) according to 2022 expert consensus on diagnosis and management of dyslipidemia in children (21).
2.6. Statistical analyses
The Shapiro–Wilk test was utilized to assess the normality of the data. Mean ± standard deviation (SD) was used to express parametric continuous data, and independent t test and paired t test were employed for between- and within-group comparisons, respectively. Non-parametric data was presented as median (the interquartile range) and analyzed using the Mann–Whitney test. Categorical variables were compared using the Chi-squared test and Fisher exact test. P-values for treatment efficacy were adjusted by false discovery rate (FDR), which was calculated according to Benjamini-Hochberg, and all P < 0.05 were considered statistically significant. All statistical analyses were done using SPSS 25.0 and GraphPad Prism 8 software.
3. Results
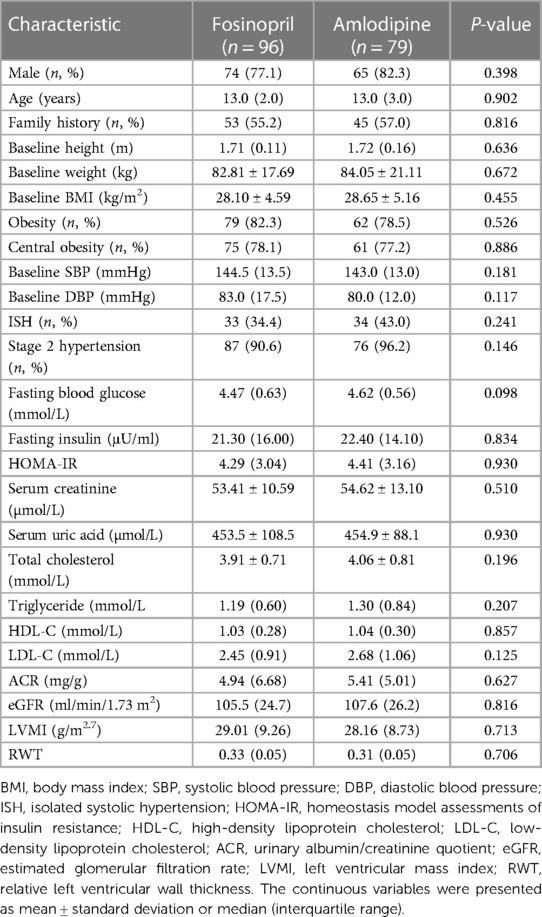
A total of 175 subjects were included in this study, including 139 males and 36 females, with age ranged 13.0 (2.0) years, of whom 136 (77.7%) were central obesity. The median initial dosages of fosinopril and amlodipine were 0.27 and 0.06 mg/kg, respectively. The baseline characteristics of the study participants are shown in Table 1, and there were no statistically significant differences in those between the fosinopril and amlodipine groups.
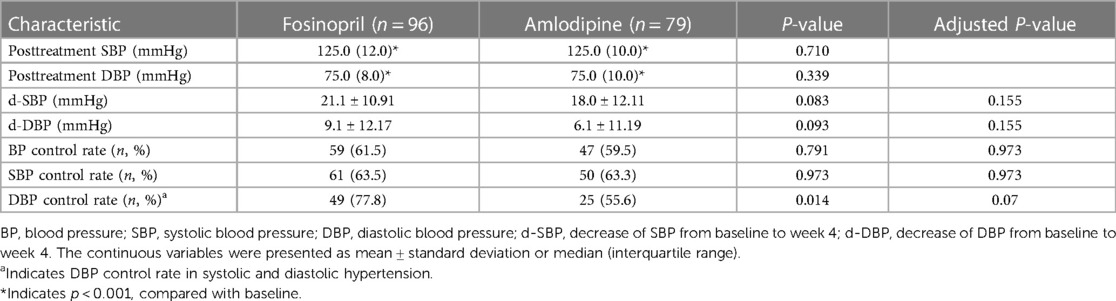
After 4 weeks of treatment, both the two groups achieved significant SBP and DBP reductions (P < 0.001) (Figure 2), with BP control rates of 61.5% in the fosinopril group and 59.5% in the amlodipine group. Furthermore, SBP decreased by 21.1 mmHg and 18.0 mmHg in the fosinopril and amlodipine groups, and DBP decreased by 9.1 mmHg and 6.1 mmHg, respectively, leading to a significant 10% difference in the extent of SBP and DBP reductions between the two groups (P = 0.083 and 0.093), but no differences in BP and SBP control rates, except a higher DBP control rate in the fosinopril group (Table 2). After adjustment, there was no significant difference in treatment efficacy between the two groups (FDR adjusted P > 0.05). At week 4, the median dosage of fosinopril was increased in 53 patients to 0.35 mg/kg, while increased in 30 patients to 0.09 mg/kg of amlodipine. Furthermore, considering the potential effect of fosinopril on renal function, we followed up 82 patients prescribed fosinopril and found no significant difference in eGFR levels between before and after treatment [108.3 (24.8) vs. 109.5 (27.7) ml/min/1.73 m2, P = 0.314].
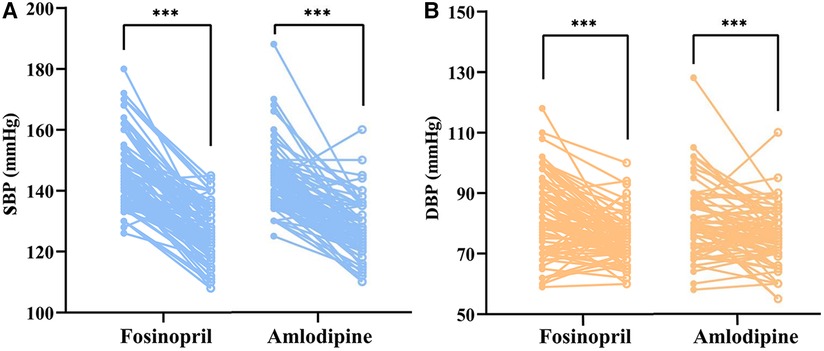
Figure 2. Paired dot plots compared changes in SBP (left) and DBP (right) before and after treatment with fosinopril and amlodipine. SBP, systolic blood pressure; DBP, diastolic blood pressure. *** indicates p < 0.001, compared with baseline.
Subgroup analysis showed that the fosinopril group had greater extent of BP reduction and higher BP and DBP control rates than the amlodipine group in female patients (FDR adjusted P < 0.05), with a borderline difference in SBP control rate (FDR adjusted P = 0.056), but these differences were not statistically significant in male patients. Additionally, in patients with central obesity, fosinopril group had a tendency to a greater reduction in SBP and DBP (FDR adjusted P = 0.07) (Table 3). To further illustrate these findings, a scatterplot comparing the decreases in SBP and DBP distributions between the fosinopril and amlodipine groups in gender and central obesity subgroups is presented in Figure 3. The fitted regression lines showed good discrimination in the subgroups of females and central obesity, suggesting that there are significant differences in the degree of SBP and DBP reductions between the fosinopril and amlodipine groups.
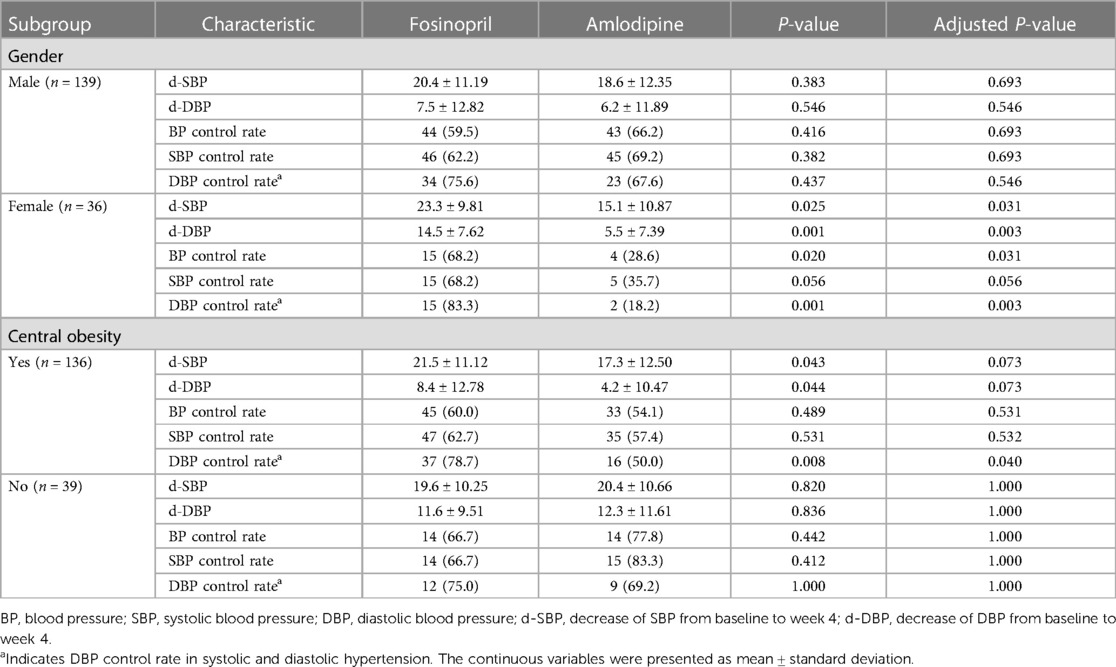
Table 3. Subgroup analyses for BP reductions (mmHg) and control rates (%) after treatment with fosinopril or amlodipine.
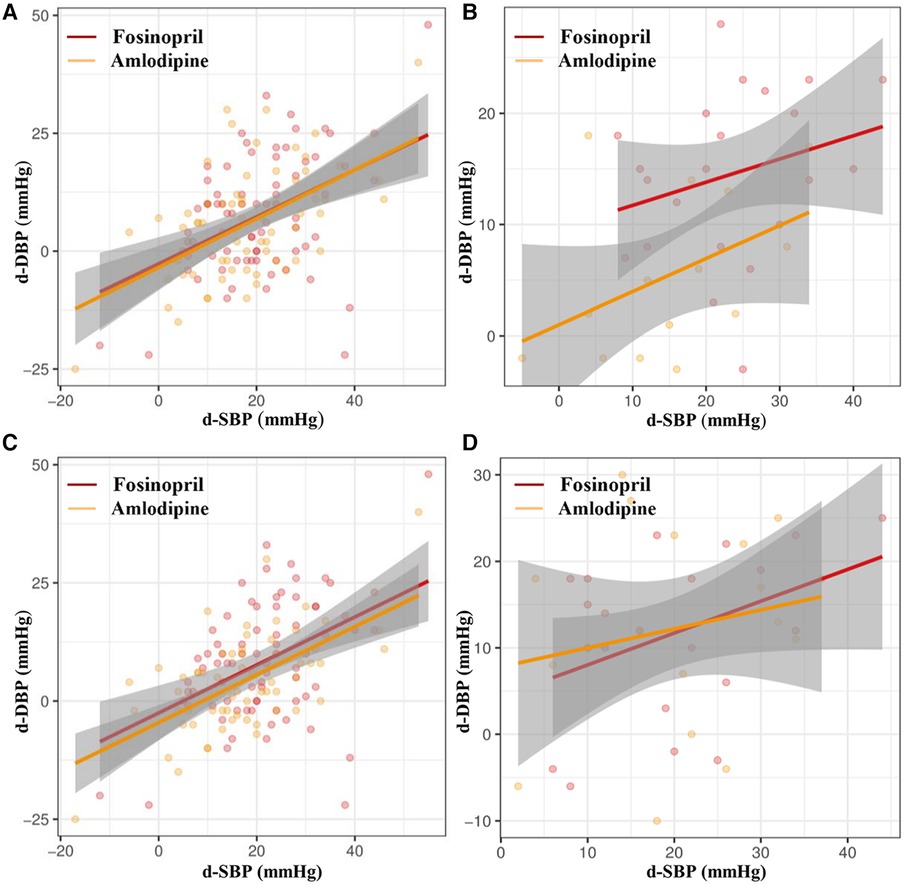
Figure 3. Scatter diagrams of correlation analysis of d-SBP and d-DBP of fosinopril and amlodipine in (A) male and (B) female subgroups, and (C) central obesity and (D) non-central obesity subgroups. d-SBP, decrease of SBP from baseline to week 4; d-DBP, decrease of DBP from baseline to week 4.
Furthermore, we observed that SBP and DBP reductions in the fosinopril group were significantly greater than those of the amlodipine group in patients with hypo-HDL-cholesterolemia (FDR adjusted P < 0.05). In patients with IR, the control rate of DBP was significantly higher in the fosinopril group than in the amlodipine group, and there was a trend, although not significant, difference in the reduction of SBP and DBP between the two groups (FDR adjusted P = 0.080 and 0.100). However, no significant differences on treatment efficacy were found in patients without these characteristics (FDR adjusted P > 0.05). In addition, hypertriglyceridemia did not appear to show a relationship with the difference in treatment efficacy between the two medications (FDR adjusted P > 0.05). Notably, there were no significant differences in baseline characteristics between the treatment arms in all subgroups (P > 0.05), except for the gender in patients without central obesity. All analyses are presented in Supplementary Table S1.
It is worth mentioning that there were no occurrences of hypotensive events or serious adverse events occurred throughout the period of treatment. 2 patients (2.4%) in the fosinopril group suffered transient dry cough with dosage of 20 mg once daily, and 1 patient (1.3%) from the amlodipine group complained of palpitation after receiving the initial dose of amlodipine with no abnormality detected on the electrocardiogram. The incidence of adverse events was not significantly different between the two groups (P > 0.05). All adverse events were resolved at the end of follow-up period. None of the other participants reported any adverse reactions.
4. Discussion
In our study, we evaluated and compared the efficacy of fosinopril or amlodipine monotherapy in children and adolescents with primary hypertension for the first time. We found significant reductions in SBP and DBP after 4 weeks of treatment with fosinopril or amlodipine, with BP control rates of 61.5% and 59.5%, respectively. Furthermore, the fosinopril group exhibited higher, at least trend, reductions in SBP and DBP, and DBP control rates among subgroups of females, patients with central obesity, IR, and hypo-HDL-cholesterolemia, in comparison to the amlodipine group.
Fosinopril has a rapid onset of action and is the first-line antihypertensive drug in clinical practice. Previous studies have shown that fosinopril can lower BP levels and is well tolerated in both adults and children (22, 23). Li et al. found that fosinopril significantly reduced SBP and DBP in hypertensive children, with BP control rate of 47% at 4 weeks of medium-dose (0.1–0.3 mg/kg) administration (23). In our study, the BP control rate of fosinopril were 61.5% after 4 weeks of treatment with median dosage of 0.27 mg/kg, higher than that in their study. Of note, 17.2% patients had high-normal BP with an associated clinical condition in their study, while our subjects were all hypertensive children, which may be speculated as the possible reason for the difference in BP control rate. Additionally, their study did not distinguish BP control rate in different groups of primary hypertension and renal etiology for hypertension, whereas our study is more reflective of the efficacy of fosinopril in pediatric primary hypertension.
Amlodipine is known to induce peripheral vasodilation and improve hypertension-related endothelial dysfunction (24, 25). A large-scale epidemiological survey in China showed that CCB were the most commonly used antihypertensive drugs in adults with hypertension, with BP control rate of 45.8% (26). Amlodipine is acknowledged as a first-line antihypertensive drug, but there are few reports on its efficacy in treating pediatric primary hypertension. The systematic review showed that amlodipine achieves significant decrease in both SBP and DBP in children with different disease states, but limited evidence suggested that the BP control rate is only 30%–50% (7). Flynn et al. found that treatment with amlodipine at a dose of 2.5 mg or 5 mg daily for 8 weeks significantly reduced SBP in hypertensive children, with BP, SBP and DBP control rates in children with primary hypertension of 8.3%, 33.3%, and 45%, respectively (19), while 59.5%, 63.3%, and 55.6% in our 4-week study. Our initial dosage was 5 mg daily and titrated gradually according to BP levels, which may be the reason for the higher control rates in our study.
To our knowledge, this is the first clinical report comparing the efficacy of fosinopril and amlodipine in the treatment of children with primary hypertension. PavlovićK et al. found no difference in BP control rates between fosinopril or amlodipine monotherapy for 3 months in patients older than 60 years with isolated systolic hypertension (76.6% vs. 79.9%) (27). Similar to their results, we found no difference in BP control rates between the two groups, but a 10% difference in the degree of SBP and DBP reductions, although this difference was no longer significant after adjustment. However, given that the small sample size may have limited the power to determine significant between-group differences, we considered the difference to be trending toward significance and may evolve as the sample size increased. Taken together, we believed that there should be some differences in certain populations, and in this regard, we conducted subgroup analyses based on demographic characteristics. Considering that most of our subjects were adolescents, we did not perform age-stratified subgroup analysis. The results showed that BP reductions were significantly greater with fosinopril in female and central obesity subgroups. Considering the mechanism of both drugs, we speculate that it may be the blockade of Renin-angiotensin System (RAS) that mediates this difference, at least partially.
RAS activation is an important mechanism for the development of hypertension in children. ACE, the main component of RAS, converts angiotensin I into angiotensin II (Ang II), which binds to its receptor to cause vasoconstriction, and release of aldosterone, resulting in a rise in BP. RAS is known to have sex differences, and endogenous sex hormones have been shown to interact with RAS, which can be upregulated by androgens and antagonized by estrogens (28, 29). However, there is limited data on the influence of sex on efficacy of antihypertensive drugs. A meta-analysis of clinical trials with sex-specific outcomes showed a slight increase in cardiovascular benefit of ACEI in male compared with female (30). However, that the majority of women in these trials were postmenopausal, and anti-RAS therapy may vary based on the reproductive status of female. Limited evidence suggests that female may exhibit a greater response to anti-RAS therapy than male in younger subjects. Miller et al. found more effective angiotensin receptor blockade in response to irbesartan administration in female than in male in a population of normotensive young participants (mean age in female, 28 ± 2 years) (31). In a trial of efficacy in young healthy volunteers, it was observed that the effectiveness of enalapril increased in females as plasma concentrations increased over time, with lower SBP levels and ACE activity at the time of maximal BP-lowering effects than in male (32). Thus, although we are not yet able to explain this sex-induced difference in efficacy, it cannot be denied that understanding this difference may lead to better treatment options for children.
It is estimated that at least 75% of the incidence of hypertension in children is directly related to obesity. Obese patients are prone to develop hypertension, which is mainly attributed to the excessive activation of RAAS. In obesity, adipose tissue produces large amounts of AngII, and plasma RAS levels are elevated, especially those with central obesity (33). In addition, obese children are often accompanied by metabolic abnormalities such as IR, elevated triglycerides, and decreased HDL-C (34–36). Studies have shown that IR and its concomitant hyperinsulinemia tend to enhance sympathetic tone, RAS activity, and sodium and water reabsorption at the renal tubular level, leading to a progressive increase in vascular stiffness that further raises BP (37). And hyperlipidemia contributes to the activation of intrarenal RAS but not circulating RAS, which further aggravates water and sodium reabsorption and renal injury (38). Our subsequent subgroup analysis also demonstrated a greater, at least trend, reduction in BP with fosinopril in patients with central obesity, IR and hypo-HDL-cholesterolemia. However, there have been no clinical trials to date comparing the efficacy of ACEI with CCBs in lean and obese patients, especially in pediatric primary hypertension. Our results may therefore have implications for the selection of antihypertensive agents for obesity-related hypertensive children and adolescents.
There are several limitations in this study, including that this is a hospital-based, single-center observational study that cannot completely avoid selection bias, and further multicenter, randomized large-scale trials are necessary. The small sample size also limited the power of subgroup analyses, and future studies with large sample sizes and predefined subgroup analyses are needed to confirm or refute our findings. The antihypertensive efficacy may be affected by individual gene polymorphisms, such as ACE1 and CYP3A5, and study on this aspect is currently ongoing. However, this study may help guide pediatricians to choose antihypertensive agents according to the different clinical characteristics of hypertensive children and was expected to improve hypertension treatment.
5. Conclusion
Fosinopril and amlodipine monotherapy were both effective in pediatric primary hypertension during a short-term follow-up. Fosinopril may be particularly effective in reducing BP in hypertensive patients of females, central obesity, insulin resistance, and hypo-HDL-cholesterolemia. These findings indicate that the optimization of antihypertensive medication selection in conjunction with the individualized characteristics of children with hypertension may improve the efficacy of antihypertensive treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Capital Institute of Pediatrics (No. SHERLL2022017). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HW collected and analyzed data, drafted the initial manuscript, and critically reviewed and revised the manuscript. LS conceptualized and designed the study, and critically reviewed and revised the manuscript. YL critically reviewed and revised the manuscript. YW and YL collected data, and critically reviewed the manuscript. WN performed statistical analysis of the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by The Special Fund of Beijing Municipal Science & Technology Commission (Z211100002921035), and Capital's Funds for Health Improvement and Research (CFH2022-3-2105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1247192/full#supplementary-material
References
1. Drozdz D, Alvarez-Pitti J, Wójcik M, Borghi C, Gabbianelli R, Mazur A, et al. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. (2021) 13:4176. doi: 10.3390/nu13114176
2. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. (2015) 116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697
3. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European society of hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. doi: 10.1097/HJH.0000000000001039
4. Chiolero A, Bovet P, Paradis G. Screening for elevated blood pressure in children and adolescents: a critical appraisal. JAMA pediatr. (2013) 167:266–73. doi: 10.1001/jamapediatrics.2013.438
5. Yan Y, Hou D, Liu J, Zhao X, Cheng H, Xi B, et al. Childhood body mass index and blood pressure in prediction of subclinical vascular damage in adulthood: Beijing blood pressure cohort. J Hypertens. (2017) 35:47–54. doi: 10.1097/HJH.0000000000001118
6. Samuel JP, Tyson JE, Green C, Bell CS, Pedroza C, Molony D, et al. Treating hypertension in children with n-of-1 trials. Pediatrics. (2019) 143:e20181818. doi: 10.1542/peds.2018-1818
7. Meyers RS, Siu A. Pharmacotherapy review of chronic pediatric hypertension. Clin Ther. (2011) 33:1331–56. doi: 10.1016/j.clinthera.2011.09.003
8. Halbach SM, Flynn J. Treatment of obesity-related hypertension in children and adolescents. Curr Hypertens Rep. (2013) 15:224–31. doi: 10.1007/s11906-013-0334-7
9. Bouhanick B, Sosner P, Brochard K, Mounier-Véhier C, Plu-Bureau G, Hascoet S, et al. Hypertension in children and adolescents: a position statement from a panel of multidisciplinary experts coordinated by the French society of hypertension. Front Pediatr. (2021) 9:680803. doi: 10.3389/fped.2021.680803
10. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140:e20171904. doi: 10.1542/peds.2017-1904
11. Snauwaert E, Vande Walle J, De Bruyne P. Therapeutic efficacy and safety of ACE inhibitors in the hypertensive paediatric population: a review. Arch Dis Child. (2017) 102:63–71. doi: 10.1136/archdischild-2016-310582
12. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. (2019)16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014
13. Welch WP, Yang W, Taylor-Zapata P, Flynn JT. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens (Greenwich). (2012) 14:388–95. doi: 10.1111/j.1751-7176.2012.00656.x
14. Lin Y, Shi L, Liu Y, Zhang H, Liu Y, Huang X, et al. Plasma fibroblast growth factor 23 is elevated in pediatric primary hypertension. Front Pediatr. (2019) 7:135. doi: 10.3389/fped.2019.00135
15. Bhatt GC, Pakhare AP, Gogia P, Jain S, Gupta N, Goel SK, et al. Predictive model for ambulatory hypertension based on office blood pressure in obese children. Front Pediatr. (2020) 8:232. doi: 10.3389/fped.2020.00232
16. The definition of metabolic syndrome and prophylaxis and treatment proposal in Chinese children and adolescents. Zhonghua Er Ke Za Zhi. (2012)50:420–2. doi: 10.3760/cma.j.issn.0578-1310.2012.06.005
17. de Simone G, Mancusi C, Hanssen H, Genovesi S, Lurbe E, Parati G, et al. Hypertension in children and adolescents. Eur Heart J. (2022) 43:3290–301. doi: 10.1093/eurheartj/ehac328
18. Lin Y, Cui Y, Yuan Y, Gao L, Li Q, Huang X, et al. Plasma fibroblast growth factor 23 as a predictor for fosinopril therapeutic efficacy in pediatric primary hypertension. J Am Heart Assoc. (2022) 11:e023182. doi: 10.1161/JAHA.121.023182
19. Flynn JT, Newburger JW, Daniels SR, Sanders SP, Portman RJ, Hogg RJ, et al. A randomized, placebo-controlled trial of amlodipine in children with hypertension. J Pediatr. (2004) 145:353–9. doi: 10.1016/j.jpeds.2004.04.009
20. Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. (2005) 115:e500–3. doi: 10.1542/peds.2004-1921
21. Expert consensus on diagnosis and management of dyslipidemia in children. Zhonghua Er Ke Za Zhi. (2022) 60:633–9. doi: 10.3760/cma.j.cn112140-20211108-00936
22. Anderson RJ, Duchin KL, Gore RD, Herman TS, Michaels RS, Nichola PS, et al. Once-daily fosinopril in the treatment of hypertension. Hypertension. (1991) 17:636–42. doi: 10.1161/01.hyp.17.5.636
23. Li JS, Berezny K, Kilaru R, Hazan L, Portman R, Hogg R, et al. Is the extrapolated adult dose of fosinopril safe and effective in treating hypertensive children? Hypertension. (2004) 44:289–93. doi: 10.1161/01.HYP.0000138069.68413.f0
24. Fuss D, Gondé H, Lamoureux F, Pereira T, Colnot M, Buchbinder N, et al. Stability study of a compounded oral solution of nicardipine for the treatment of hypertension in children. Eur J Pharm Sci. (2021) 160:105738. doi: 10.1016/j.ejps.2021.105738
25. Berkels R, Taubert D, Bartels H, Breitenbach T, Klaus W, Roesen R. Amlodipine increases endothelial nitric oxide by dual mechanisms. Pharmacology. (2004) 70:39–45. doi: 10.1159/000074241
26. Hu DY, Liu LS, Yu JM, Yao CH. National survey of blood pressure control rate in Chinese hypertensive outpatients-China STATUS. Zhonghua Xin Xue Guan Bing Za Zhi. (2010) 38:230–8. doi: 10.3760/cma.j.issn.0253-3758.2010.03.007
27. Pavlović K, Benc D, Kmezić-Grujin J, Stojiljković J, Cemerlić-Adić N, Miljković T, et al. Fosinopril and amlodipine in the treatment of isolated systolic hypertension. Med Pregl. (2004) 57:45–53. doi: 10.2298/mpns0402045p
28. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. (2015) 116:960–75. doi: 10.1161/CIRCRESAHA.116.303587
29. White MC, Fleeman R, Arnold AC. Sex differences in the metabolic effects of the renin-angiotensin system. Biol Sex Differ. (2019) 10:31. doi: 10.1186/s13293-019-0247-5
30. Rabi DM, Khan N, Vallee M, Hladunewich MA, Tobe SW, Pilote L. Reporting on sex-based analysis in clinical trials of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker efficacy. Can J Cardiol. (2008) 24:491–6. doi: 10.1016/s0828-282x(08)70624-x
31. Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, et al. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. (2006) 17:2554–60. doi: 10.1681/ASN.2005101095
32. Zapater P, Novalbos J, Gallego-Sandín S, Hernández FT, Abad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. (2004) 43:737–44. doi: 10.1097/00005344-200405000-00018
33. Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda). (2017) 32:197–209. doi: 10.1152/physiol.00037.2016
34. Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the The Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). (2013) 21:8–24. doi: 10.1002/oby.20181
35. Maffeis C, Morandi A. Body composition and insulin resistance in children. Eur J Clin Nutr. (2018) 72:1239–45. doi: 10.1038/s41430-018-0239-2
36. Zhu J, Zhang Y, Wu Y, Xiang Y, Tong X, Yu Y, et al. Obesity and dyslipidemia in Chinese adults: a cross-sectional study in Shanghai, China. Nutrients. (2022) 14:2321. doi: 10.3390/nu14112321
37. Strazzullo P, Barbato A, Galletti F, Barba G, Siani A, Iacone R, et al. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the olivetti heart study. J Hypertens. (2006) 24:1633–9. doi: 10.1097/01.hjh.0000239300.48130.07
Keywords: fosinopril, amlodipine, efficacy, primary hypertension, children
Citation: Wang H, Shi L, Lin Y, Wang Y, Niu W and Li Y (2023) Efficacy of fosinopril and amlodipine in pediatric primary hypertension: a single-center observational study. Front. Pediatr. 11:1247192. doi: 10.3389/fped.2023.1247192
Received: 25 June 2023; Accepted: 16 October 2023;
Published: 27 October 2023.
Edited by:
Daniel De Wolf, Ghent University Hospital, BelgiumReviewed by:
Aftab S. Chishti, University of Kentucky, United StatesKarolis Azukaitis, Vilnius University, Lithuania
© 2023 Wang, Shi, Lin, Wang, Niu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Shi c2hpbGluOTc4OUAxMjYuY29t
 Hui Wang
Hui Wang Lin Shi
Lin Shi Yao Lin
Yao Lin Yuting Wang
Yuting Wang Wenquan Niu
Wenquan Niu Yaqi Li
Yaqi Li