- 1Department of Neonatology, Motol University Hospital, Prague, Czechia
- 2Department of Biomedical Technology, Faculty of Biomedical Engineering, Czech Technical University in Prague, Kladno, Czechia
Objective: Changes in oximeter averaging times have been noted to affect alarm settings. Automated algorithms (A-FiO2) assess FiO2 faster than oximeter averaging, potentially impacting their effectiveness.
Methods: In a single NICU routinely using 15 fabian-PRICO A-FiO2 systems, neonates were randomly exposed to SpO2 averaging time settings switched every 12 h among short (2–4 s), medium (10 s), and long (16 s) oximeter averaging times for the entire duration of their A-FiO2 exposure. Primary endpoints were the percent time in the set SpO2 target range (dependent on PMA), SpO2 < 80%, and SpO2 > 98%, excluding FiO2 = 0.21.
Results: Ten VLBW neonates were enrolled over 11 months. At entry, they were 17 days old (IQR: 14–19), with an adjusted gestational age of 29 weeks (IQR: 27–30). The study included data from 272 days of A-FiO2 control (34% short, 32% medium, and 34% long). Respiratory support was predominantly non-invasive (53% NCPAP, 40% HFNC, and 6% NIPPV). The aggregate SpO2 exposure levels were 67% (IQR: 55–82) in the target range, 5.4% (IQR: 2.0–10) with SpO2 < 80%, and 1.2% (IQR: 0.4–3.1) with SpO2 > 98%. There were no differences in the target range time between the SpO2 averaging time settings. There were differences at the SpO2 extremes (p ≤ 0.001). The medium and long averaging were both lower than the short, with the difference larger than predicted. Multivariate analysis revealed that these findings were independent of subject, ventilation mode, target range, and overall stability.
Conclusions: This A-FiO2 algorithm is effective regardless of the SpO2 averaging time setting. There is an advantage to the longer settings, which suggest an interaction with the controller.
Background
Continuous monitoring of oxygenation (SpO2) by pulse oximetry is the standard of care for preterm infants receiving supplemental oxygen. Relatively modest changes in oxygen saturation exposure are associated with a marked impact on outcomes (1–3). Neonatal oxygenation is unstable, and nurses struggle to manage SpO2 within prescribed target ranges. Compliance is routinely only 50%, and excessive hyperoxemia and hypoxemia are prevalent in routine care (4–6). An essential part of oxygenation management is temporarily increasing inspired oxygen (FiO2) to address intermittent hypoxemic episodes. Importantly, once the instability has resolved, a prompt return to baseline FiO2 is needed to reduce potential hyperoxemia.
Following decades of development, newer neonatal ventilators offer closed-loop titration of FiO2 based on the continuous monitoring of SpO2 (A-FiO2). A-FiO2 systems have been consistently shown to be effective (7). Nevertheless, while there are dozens of positive studies, the evaluative populations are narrow. Most of the studies include a few systems, and the control algorithms are quite different (8). Further, nearly all of the studies have a short physiological cross-over design, which is not necessarily reflective of the routine practice of weeks of supplemental oxygen. There are a few studies of the extended use of a few systems (9–12) and the subtleties of A-FiO2 settings in routine care (13, 14).
Most commercial oximeters used in neonatal care offer clinicians the option of adjusting the averaging time to mitigate physiologic and measurement noise. Even the shortest averaging times, however, reflect multiple peripheral pulses. While other internal oximeter software attempts to reduce the impact of artifacts, it remains a common problem. In contrast, fidelity is clearly lost with averaging, and analyses confirm that averaging time affects the monitored depth and duration of reported exposure (15, 16). In all A-FiO2 systems, the SpO2 averaging time is much slower than the frequency of SpO2 assessment and much slower than the rate of FiO2 adjustment in some systems. Nevertheless, there are no thorough evaluations of the interaction of the set SpO2 averaging time setting with the performance of A-FiO2.
Motol University Hospital has a large tertiary neonatal care center, and 15 A-FiO2 systems have been used routinely since January 2019. Although it is known that shorter averaging times are more accurate, the subjective impressions of the staff regarding an optimal approach to setting the SpO2 averaging time have been inconsistent. The aim of this study was to explore the impact of averaging time on SpO2 control during A-FiO2.
Methods
This is a single-site randomized cross-over study in which the SpO2 averaging time setting switched every 12 h over the course of routine care. The study was approved by the institution's Bioresearch Ethics Committee (Reference number EK-1548/21, 1 December 2021, Ethics Committee of the University Hospital Motol and Second Faculty of Medicine, Charles University, Prague). Written parental informed consent was required and received before enrollment. The study was prospectively registered (ClinicalTrials.gov, NCT05274386).
Only fabian-PRICO A-FiO2 systems (Vyaire Medical, Mettawa, USA) were used in this study. In these systems, A-FiO2 control is available for all ventilation modes (HFOV, CMV, NIPPV, NCPAP, and HFNC). The PRICO A-FiO2 (PRedictive Intelligent Control of Oxygenation) system monitors SpO2 every second using an integrated Masimo pulse oximeter. Based on the weighted average of these data, an adjustment in FiO2 is made every 30 s if warranted. Within the set target range, this adjustment is ±1% toward the midpoint. Outside the target range, the adjustment varies by ±1%–10%. The amount of adjustment is based on a proprietary algorithm that takes into account the depth and trajectory of the predicted response to changes in oxygen. In addition, when SpO2 moves outside the target range, an initial adjustment is made and the 30-s period is reinitiated. Under certain conditions (SpO2 dropout, exceeding operator-set parameters), the system falls back to manual control at a FiO2 level previously specified by the clinician. The system returns to A-FiO2 control when the condition resolves or with operator reactivation.
Written Case Report Forms (CRF) for each subject captured the demographic and baseline information, as well as the exact time and average-time setting, relevant study events, and reason for exit from the study. Ventilator system data were collected concurrently from a bedside PC using purpose-coded Matlab software (MathWorks, Natick, USA). These ventilator data were captured every 2 s and included the measured SpO2, set FiO2, set SpO2 control range, and set ventilation mode. CRFs and digital data were concurrently reviewed by the investigators, and potentially spurious information was evaluated. These data were merged with the averaging settings and gestational age from the CRFs into an analytical database.
The SpO2 averaging time setting was changed every 12 h (10 am, 10 pm). Subjects were alternated between three averaging times (2–4, 10, and 16 s, or short, medium, and long, respectively). The sequence was assigned a predetermined random order, different for each subject, and integrated into the subject-specific CRF. The assigned sequence was composed of balanced blocks, so every subject was exposed to all three average settings twice every 3 days. All other aspects of care were standard according to the unit policy and clinical judgment. The unit policy included tiered target ranges/alarms based on postmenstrual age (<29 weeks: 88%–92%, 29–33 weeks: 90%–94%, 34–36 weeks: 92%–96%, >36 weeks: 95%–98%, with alarms set at 1% outside the target range).
With informed consent, infants were eligible for enrollment if their birth weight was <1,500 g, they had no congenital anomalies, and they required respiratory support and supplemental oxygen at 2 weeks of age. The latter is consistent with unit policy, as A-FiO2 is not routinely used in the acute phase following birth. Subjects were excluded from the study if they were weaned from supplemental oxygen, transferred, or completed 30 days of intervention.
All endpoints were prospectively defined for the study, except as indicated. The primary endpoints were compliance (percentage of time in the intended SpO2 target range) and safety (SpO2 < 80% and SpO2 > 98%). Secondary endpoints included time above and below the target range. Periods with SpO2 higher than the target range and FiO2 = 0.21 were included in the target range compliance and excluded from time above the target range. Other descriptive parameters were also collected. Sensitivity analyses were prescribed to evaluate the impact of covariables. These covariables were set: target range, ventilation mode, and, added on a post hoc basis, stability. Stability was assessed based on the mean time in the SpO2 target range in each 3-day block. It was categorized as stable if greater than the median time (67%) for all subjects in the study or less stable if lower.
The power analysis was based on the safety endpoints and specified differences that were small but considered potentially clinically relevant. Based on other trials, we nominally expected a mean SpO2 < 80% of 2% ± 3% and a mean SpO2 > 98% of 4% ± 6%. We determined that a difference for SpO2 < 80% of 1.5% and a difference for SpO2 > 98% of 3.0% would be detected with more than 80% power, an alpha of 0.05, and a total of 50 measurements in each averaging group. These differences were larger than expected based on the change in fidelity from averaging SpO2 (13). The projected rate of enrollment, considering the likely unit census, two data collection systems, and staff resources, was one subject per month. A minimum of 10 neonates was considered an acceptable sample size. With 10 subjects, 50 measurements would be achieved with an average of 2 weeks of intervention. Thus, the study was designed to continue until at least 10 subjects were enrolled with at least 50 SpO2 paired averaging time measurements.
To address potential carry-over between averaging epochs, the first and last 10 min of each epoch were excluded from all analyses. A general linear model (ANOVA) was used for each of the three independent primary endpoints, with independent (explanatory) covariables. These included SpO2 set-average as a fixed variable and four random control covariables (target range, mode of ventilation, stability, and subject). If needed, the dependent variables were to be log-transformed to address a lack of normality (Shapiro–Wilk), which was the case. The effect size was determined with the shortest averaging time as the baseline. For consistency, descriptive data were presented as median and IQR, regardless of normality. p < 0.05 was considered statistically significant. Covariables needed to be cross-tabulated to explore potential clinical significance if they were relevant to the significance of the SpO2 averaging time setting. All statistical comparisons utilized XLSTAT 11.5 (Lumivero, NY, USA).
Results
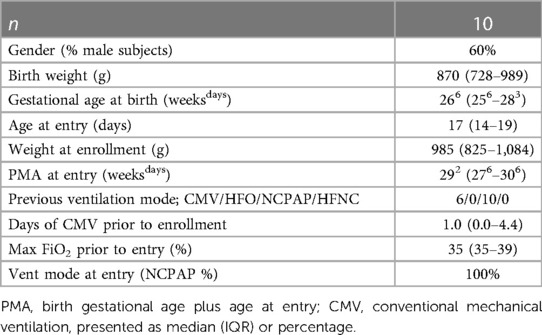
The study began in February 2022 and ended in December 2022, when a minimum of 10 subjects had completed the study. During this period, 11 infants met the enrollment criteria. In total, the parents of 10 infants were approached for informed consent when data collection systems were available. Most subjects were ELBW infants aged less than 3 weeks at enrollment. Most had been intubated before entry, but all were being supported on NCPAP at enrollment. Further details of the subjects are listed in Table 1.
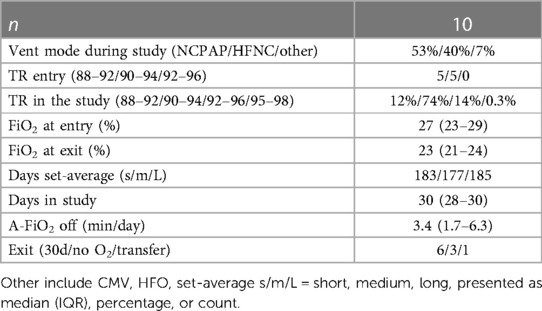
Details of the subjects’ progress during the study period are provided in Table 2. Most were studied for 30 days, and the data reflect a total of 272 days (545 measurements) of A-FiO2 control. Respiratory support was predominantly provided by either NCPAP or HFNC at low FiO2 levels, with the target range set at 90%–94% SpO2. The duration of exposure to the three set SpO2 averaging times was similar, with only 6 out of 545 periods set incorrectly. The balance of the difference was due to terminating enrollment in the middle of the 3-day balanced block. The periods during the study when A-FiO2 was disabled were quite short [3 min/12 h (IQR: 2–6)]. However, it was approximately a minute longer during the short averaging periods than during the medium and long averaging periods (p < 0.001). No adverse effects/events related to A-FiO2 were reported.
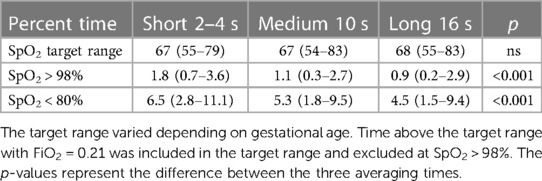
The primary endpoints of SpO2 exposure are listed in Table 3. The subjects spent two-thirds of the time in the target range, which was nearly identical to the three averaging times. However, the set SpO2 averaging time significantly affected the time at the two SpO2 extremes. SpO2 was higher during the short averaging time than during the longer averaging time. During the short averaging periods, the mean time with SpO2 > 98% was 1.8%, but it was about half that during the other two average-time settings (41% lower, p = 0.001 medium averaging, and 51% lower, p < 0.001 during long averaging). During the short average periods, the mean time with SpO2 < 80% was 6.5%. It was 18% lower during medium averaging (p = 0.08) and 31% lower (p = 0.003) during long averaging. The percent times below the target range were nearly identical [short: 17% (IQR: 11–26), medium: 17% (9–25), and long: 17% (10–26)]. There was a small difference in the percent times above the target range [short: 13% (IQR: 7–20), medium: 11% (6–19), and long: 12% (7–20), p = 0.025].
The analysis controlled for differences between subjects, ventilation modes, target ranges, and stabilities. Nevertheless, the set SpO2 averaging time in the multivariate analysis was independently significantly related to differences in SpO2 extremes. Not surprisingly, all of these control parameters were also significantly related to target range compliance and exposure to severe hypoxemia and hyperoxemia. The effectiveness of A-FiO2 in the 3-day stable and less stable cohorts offers some insight into its performance. For the two stable cohorts, time in the assigned target range was 82% (73–90) vs. 55% (48–62), time with SpO2 < 80% was 2.2% (1.0–4.2) vs. 9.7% (6.6–14), and time with SpO2 > 98% was 0.9% (0.3–2.2) vs. 1.8% (0.5–3.8), p < 0.001 for all three settings.
Discussion
Using a cross-over design, we evaluated the impact of the set SpO2 averaging time during 39 weeks of routine use in 10 preterm infants. While the differences were small, we found that the shortest of the three averaging times resulted in an increase in time at SpO2 extremes but no difference in the times within the intended SpO2 target range. This difference was independent of subject, target range, mode of ventilatory support, and stability.
The reported differences suggest that the set SpO2 averaging time affects the FiO2 control algorithm. Based on the arithmetic of averaging, one would expect that a shorter averaging time would detect more episodes of fluctuations away from the target range with more extreme nadirs but of shorter duration. How this effect might impact the time above or below specific SpO2 thresholds is less obvious. The impact of these averaging effects on SpO2 monitoring in infants has been studied. In two reports, Vagedes et al. evaluated healthy infants with apnea and children being assessed for sleep apnea (16, 17). They documented the predictability of the effect of a set SpO2 averaging time on desaturation. They also reported that longer averaging times result in an inaccurate decrease in the burden of hypoxemia (area under the curve). McClure and colleagues reprocessed 24 days of 2–4-s SpO2 data to reflect SpO2 averaging of 8 and 16 s (15). They aimed to determine the trade-off between averaging, alarm delay, and alarm settings in the NICU. They reported that the percent time decreased by 4% and 7% (8 and 16 s average, respectively) for hypoxemic events (SpO2 < 70%). For hyperoxemic events (SpO2 > 98%), they reported that the percent time increased by 1% for 8 s and decreased by 8% for 16 s. In contrast, we found that the impact of averaging time settings was 5–10 times greater. This suggests that the differences we reported are primarily the result of an interaction with the A-FiO2 control algorithm. Importantly, the effect we reported was independent of infant stability, ventilation mode, and target range, suggesting consistency across clinical conditions. We can only speculate as to the cause. We ruled out the impact of SpO2 dropouts in our study because the time for A-FiO2 fallback was very short, although it may be relevant in other situations. This suggests that the set averaging time affects the processing of the second-to-second data by the proprietary predictive equation. However, we cannot exclude a lack of comparability of the McClure data, as the decrease in the area under the curve predicted by Vagedes, albeit in a less comparable population of healthy infants without respiratory or oxygen supplementation, is similar to our findings for hypoxemia.
Short-term cross-over studies may have a selection bias. Our study did not evaluate A-FiO2 performance compared to manual control. However, this device has been evaluated by others in 24-h cross-over studies (18, 19). These two investigations reported time in the target range and time >98% during A-FiO2, which were comparable to our findings. However, they reported that the exposure to SpO2 < 80% was less than 2%, similar to what we found in our stable cohort but markedly less than our less-stable cohort. Although small, a difference of 1% time with SpO2 < 80% is definitely of potential clinical relevance and thus important (2). The difference in target range between studies is certainly a factor in the increased exposure to hypoxemia in our study. We also suggest that the timing of the selection of subjects for short-term cross-over studies is also highly relevant. The infants in these reports were studied on A-FiO2 for only one day and generally later in life. They were also selected considering their stability for a 48 h cross-over period. We do not discount the importance of structured manual vs. automated short cross-over studies in evaluating the potential of A-FiO2. However, we believe that assessing the treatment across the continuum of care is not only more reflective of what can be expected with routine use but also essential to considering the potential impact on neonatal outcomes.
As with all medical devices, it is also important that they are used optimally. The performance of A-FiO2 systems has been consistent in numerous studies across a range of different devices (7). New systems, or system enhancements, must be thoroughly evaluated before being routinely used. Determining optimal practices is particularly relevant when introducing new technologies such as A-FiO2. Such studies have thoroughly evaluated different target ranges (20–22). Explorations of other subtleties are limited. One study evaluated different alarm strategies (13) and another evaluated different FiO2 adjustment rates (14). Further variation in clinical approaches to use, often seen in pragmatic studies, may mitigate potential benefits or risks.
This study has some limitations. While it reflects routine use in our unit, there is insufficient experience with intubated infants (conventional and HFOV) and infants with BPD. Thus, these findings should be applied with caution to those modes of support that are more common in the first 2 weeks of life. There are seven averaging time settings available with the Masimo oximeter, and we only tested three. However, these cover the range of the seven settings, and our results showed that longer is better. Consistent with our subjective impression, the system works well with these three set SpO2 averaging time settings, and the differences we found were small and perhaps not clinically relevant. Further, while the consensus is that the short averaging periods are most reflective of clinical exposure, this may not be the case (14). They typically reflect only three peripheral pulses and may be less clinically relevant than a more prolonged average when considering measurement noise and duration. Finally, our findings apply to only one A-FiO2 device.
Conclusion
This A-FiO2 algorithm is effective regardless of the SpO2 averaging time setting. There appears to be an advantage of a small decrease in exposure to SpO2 extremes associated with longer settings. This suggests there is an interaction between the averaging setting and the controller.
Our unit changed the default setting to 10 s, with an option for the attending physician to adjust if necessary. More research is needed to explore the optimal use of A-FiO2 systems, whether in the context of CQI or controlled studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved under the reference number EK-1548/21, 1 December 2021, by the Ethics Committee of the University Hospital Motol and the Second Faculty of Medicine, Charles University, Prague. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JJ, TB, and JR conceptualized and organized the study. JJ and JR directed the project. TB and VR-H analyzed the data. VR-H and JR developed the data collection system. MR oversaw all faculty activities. JJ, JD, VK, and RB provided all site activities. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Czech Technical University, Prague (grant nos. SGS22/202/OHK4/3T/17 and SGS23/198/OHK4/3T/17).
Conflict of interest
TB receiving consulting fees for Vyaire Medical, none associated with this project.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA. (2018) 319(21):2190–201. doi: 10.1001/jama.2018.5725
2. Poets C, Roberts R, Schmidt B, Whyte R, Asztolos E, Bader D, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. (2015) 314(6):595–603. doi: 10.1001/jama.2015.8841
3. Schmidt B, Whyte RK, Shah PS, Abbasi S, Bairam A, Harrold J, et al. Effects of targeting higher or lower oxygen saturations in centers with more versus less separation between median saturations. J Pediatr. (2016) 178:288–91. doi: 10.1016/j.jpeds.2016.08.002
4. Hagadorn JJ, Sink DW, Buus-Frank ME, Edwards E, Marrow L, Hobar J, et al. Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 collaborative. J Perinatol. (2017) 37:270–6. doi: 10.1038/jp.2016.219
5. van Zanten HA, Tan RN, van den Hoogen A, Laporiore E, tePas AB. Compliance in oxygen saturation targeting in preterm infants: a systematic review. Eur J Pediatr. (2015) 174(12):1561–72. doi: 10.1007/s00431-015-2643-0
6. van Zanten HA, Tan RN, Thio M, de Man-van Ginkel JM, van Zwet EW, Lopriore E, et al. The risk for hyperoxaemia after apnoea, bradycardia and hypoxaemia in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2014) 99(4):F269–73. doi: 10.1136/archdischild-2013-305745
7. Abdo M, Hanbal A, Asla MM, Ishqair A, Alfar M, Elnaiem W, et al. Automated versus manual oxygen control in preterm infants receiving respiratory support: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2022) 35(25):6069–76. doi: 10.1080/14767058.2021.1904875
8. Salverda HH, Cramer SJE, Witlox RSGM, Dargaville PA, Te Pas AB. Automated oxygen control in preterm infants, how does it work and what to expect: a narrative review. Arch Dis Child Fetal Neonatal Ed. (2021) 106(2):215–21. doi: 10.1136/archdischild-2020-318918
9. Van Zanten HA, Kuypers KLAM, Stenson BJ, Bachman TE, Pauws SC, Te Pas AB. The effect of implementing an automated oxygen control on oxygen saturation in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2017) 102(5):F395–9. doi: 10.1136/archdischild-2016-312172
10. Nair V, Kannan Loganathan P, Lal MK, Pringleton H, Bachman TE, Brodlie M, et al. Automatic oxygen control for reducing extremes of oxygen saturation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2023) 108(2):136–41. doi: 10.1136/archdischild-2022-324160
11. Wilinska M, Bachman T, Piwowarczyk P, Kostuch M, Tousty J, Berła K, et al. Routine use of automated FiO2 control in Poland: an observational report. Presented at Montreux-EPNV May 2023.
12. Salverda HH, Dekker J, Lopriore E, Dargaville PA, Pauws SC, Te Pas AB. Comparison of two automated oxygen controllers in oxygen targeting in preterm infants during admission: an observational study. Arch Dis Child Fetal Neonatal Ed. (2023) 108(1):26–30. doi: 10.1136/archdischild-2021-323690
13. Warakomska M, Bachman TE, Wilinska M. Evaluation of two SpO2 alarm strategies during automated FiO2 control in the NICU: a randomized cross-over study. BMC Pediatr. (2019) 6:19. doi: 10.1186/s12887-019-1496-5
14. Schwarz CE, Kreutzer KB, Langanky L, Wolf NS, Braun W, O’Sullivan MP, et al. Randomised cross-over trial comparing algorithms and averaging times for automatic oxygen control in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2022) 107(4):425–30. doi: 10.1136/archdischild-2021-322096
15. McClure C, Jang SY, Fairchild K. Alarms, oxygen saturations, and SpO2 averaging time in the NICU. J Neonatal Perinatal Med. (2016) 9(4):357–62. doi: 10.3233/NPM-16162
16. Vagedes J, Dietz K, Poets CF. Observational study on the influence of averaging time on oximetry results in infants and children. Acta Paediatr. (2019) 108(12):2246–52. doi: 10.1111/apa.14914
17. Vagedes J, Bialkowski A, Wiechers C, Poets CF, Dietz K. A conversion formula for comparing pulse oximeter desaturation rates obtained with different averaging times. PLoS One. (2014) 9(1):e87280. doi: 10.1371/journal.pone.0087280
18. Dijkman KP, Goos TG, Dieleman JP, Mohns T, van Pul C, Andriessen P, et al. Predictive intelligent control of oxygenation in preterm infants: a two-center feasibility study. Neonatology. (2023) 120(2):235–41. doi: 10.1159/000527539
19. Dijkman KP, Mohns T, Dieleman JP, van Pul C, Goos TG, Reiss IK, et al. Predictive intelligent control of oxygenation (PRICO) in preterm infants on high flow nasal cannula support: a randomised cross-over study. Arch Dis Child Fetal Neonatal Ed. (2021) 106(6):621–6. doi: 10.1136/archdischild-2020-320728
20. van Kaam AH, Humler H, Wilinska M, Swietlinsi J, Lal M, tePas A, et al. Automated versus manual oxygen control with different saturation targets and modes of respiratory support in preterm infants. J Pediatr. (2015) 167(3):545–50.e1-2. doi: 10.1016/j.jpeds.2015.06.012
21. Wilinska M, Bachman TE, Swietlinski J, Kostro A, Twardoch-Drozd M. Automated FiO2-SpO2 control system in neonates requiring respiratory support: a comparison of a standard to a narrow SpO2 control range. BMC Pediatr. (2014) 14:130. doi: 10.1186/1471-2431-14-130
Keywords: oxygen control, automated oxygen control, pulse oximetry, neonatal, SpO2 targeting
Citation: Janota J, Dornakova J, Karadyova V, Brabec R, Rafl-Huttova V, Bachman T, Rozanek M and Rafl J (2023) Evaluation of the impact of oximeter averaging times on automated FiO2 control in routine NICU care: a randomized cross-over study. Front. Pediatr. 11:1240363. doi: 10.3389/fped.2023.1240363
Received: 14 June 2023; Accepted: 28 August 2023;
Published: 22 September 2023.
Edited by:
Suksham Jain, Government Medical College and Hospital, IndiaReviewed by:
Noa Ofek-Shlomai, Hadassah Medical Center, IsraelDeepak Chawla, Government Medical College and Hospital, India
© 2023 Janota, Dornakova, Karadyova, Brabec, Rafl-Huttova, Bachman, Rozanek and Rafl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Janota amFuLmphbm90YUBmbm1vdG9sLmN6
 J. Janota1*
J. Janota1* V. Rafl-Huttova
V. Rafl-Huttova T. Bachman
T. Bachman M. Rozanek
M. Rozanek J. Rafl
J. Rafl