
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 02 November 2023
Sec. Pediatric Immunology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1239365
 Enza D’Auria1
Enza D’Auria1 Martina Minutoli1
Martina Minutoli1 Alessandra Colombo1
Alessandra Colombo1 Marco Ugo Andrea Sartorio2*
Marco Ugo Andrea Sartorio2* Fiammetta Zunica1
Fiammetta Zunica1 Gianvincenzo Zuccotti1,3
Gianvincenzo Zuccotti1,3 Vassilios Lougaris4
Vassilios Lougaris4
In last decades a simultaneous increase in the prevalence of atopic and autoimmune disorders in pediatric population has been observed. Despite the Th1-Th2 paradigm, supporting the polarization of the immune system with Th1 response involved in autoimmune diseases and Th2 response leading to hypersensitivity reactions, recent evidence suggests a possible coexistence of common pathogenic pathways as result of shared immune dysregulation. Similar genes and other mechanisms such as epithelial barrier damage, gut microbiota dysbiosis and reduced number of T regs and IL-10 contribute to the onset of allergy and autoimmunity. IgA deficiency is also hypothesized to be the crosslink between celiac disease and allergy by lowering gut mucous membrane protection from antigens and allergens. The present narrative review aims to give an overview of the co-occurrence of allergic and autoimmune disorders (celiac disease, inflammatory bowel diseases, type 1 diabetes mellitus, thyroid disease, juvenile idiopathic arthritis) in pediatric population, based on the available evidence. We also highlighted the common pathogenic pathways that may underpin both. Our findings confirm that allergic and autoimmune diseases are commonly associated, and clinicians should therefore be aware of the possible coexistence of these conditions in order to ameliorate disease management and patient care. Particular attention should be paid to the association between atopic dermatitis or asthma and celiac disease or type 1 diabetes and vice versa, for therapeutic interventions. Further studies are needed to better clarify mechanisms involved in the pathogenesis and eventually identify new therapeutic strategies.
For more than two decades the Th1-Th2 paradigm supported the polarization of the immune system: according to this theory, internal and external factors act together to induce either a Th1 or a Th2-mediated response defining the balance between these two different inflammatory patterns (1).
The Th1-response is mediated by the release of proinflammatory cytokines such as interferon-γ, IL-2 and lymphotoxin-α, which leads to B-cell production of IgG antibodies, macrophageactivation, cell cytotoxicity, and induction of cellular immunity; an abnormal activation of this pathway may lead to autoimmune phenomena. On the other hand, TH2 cells produce cytokines such as IL-4, IL-5, IL-13, promote eosinophils’ activation, induce antibody class-switching to IgE and are implicated in allergic-type hypersensitivity reactions (2).
However, other cells, such as macrophages and epithelial cells have been shown to have a similar cytokine production pattern, thus making even more complex the pathogenetic mechanism of allergic diseases (3).
A recently identified cell population, the ILCs (Helper innate lymphoid cells), play a fundamental role in the early immune response and therefore in the homeostasis of the immune system. They are a source of cytokines whose phenotype is matched by the adaptive system. In particular, ILC2s are involved in allergic diseases of the respiratory system as well as TH2 cells (4) and promote the expansion of IL10-producing T-regs (5). ILC2s are activated in allergic conditions such as asthma, allergic rhinosinusitis and atopic dermatitis (5). on the other hand, INF-gamma-producing ILC1s, via the ILC1/alarm axis, seem to play a central role in the pathogenesis of inflammation in autoimmune diseases, including celiac disease (6).
Thus, understanding their functions is necessary to deeply understand the pathogenetic basis of allergic manifestations and autoimmune diseases.
In recent decades there has been a significant increase in the incidence of allergic disorders, reaching almost 20% of high-income populations (7–9), In parallel, an increasing trend of autoimmune disorders has been recorded in children living in developed countries (10–13) with an estimated prevalence ranging between 7.6 and 9.4% worldwide (14).
This simultaneous increase in the prevalence of atopic and autoimmune disorders has supported the hypothesis of a common etiopathogenetic background, although the exact pathogenetic pathways are far to be understood (15).
The major hypothesis to support this theory is currently the hygiene hypothesis, proposed in 1989 by Strachan (16). It suggests that the increased incidence of allergic and autoimmune diseases has been a result of a reduced infectious pressure given to the modern Western lifestyle: the exposure to certain infectious agents early in life seems to play a protective role against inflammatory diseases and its significant progressive reduction over the last decades, due to hygiene measures, has resulted in the immune system's dysregulation and in the development of allergy and autoimmunity.
Recently, it has been postulated that epithelial barrier damage (the so called “barrier hypothesis”) caused by different environmental factors, such as agents of industrial and urban environment, may drive the transepithelial translocation of pathogenic opportunistic microbes, promoting inflammatory processes and eliciting immune dysregulation (17).
For instance, substances as laundry detergents and household cleaning agents have widely been shown a directly disruptive effects on the tight junctions barrier integrity of epithelial cells (18), as well prolonged exposures to cigarettes smoke and air pollutants have been demonstrated to result not only in lung epithelium damage, but also in immune system impairment through release of proinflammatory cytokines and chemokines, enhanced recruitment of macrophages and neutrophils and increased Th2-dependent responses (19).
Of note, epithelial barrier leakiness has been reported in several autoimmune diseases, e.g., celiac disease, IBD, eosinophilic esophagitis, diabetes mellitus type 1, rheumatoid arthritis, systemic lupus erythematous and atopic disorders (AR, AA, AD and FA) (20–22).
Recent studies have also highlighted the effect of diet on gut microbiota composition and the connection to immunological pathways. The insufficient intake of ‘‘healthy foods” in Western dietary habits adversely affects the production of bacterial metabolites which are crucial for regulation of inflammatory response (23). Furthermore, the consumption of processed food containing additives and preservatives increases intestinal permeability to allergens and pathogens (24).
In recent years, the B regs have assumed an important role in the understanding of immune mechanisms: they perform regulatory functions in inflammatory conditions with an immunosuppressive effect and maintaining tolerance, re-establishing the immune homeostasis. Recent studies have shown that in chronic inflammatory conditions such as autoimmune and allergic diseases the number of circulating B regs and the production of IL10 are reduced. IL-10 has a protective role in allergic inflammation and can inhibit TH2 polarization and Th17 mediated responses (25, 26).
The complex mechanism of tolerance is maintained thanks to a complex network between several cell types, such as T and B lymphocytes, dendritic cells (DC) and others. Alterations in DC migration can lead to their abnormal activation, resulting in imbalance of immune responses that may eventually contribute to the onset of autoimmune manifestations, infectious and allergic diseases, as well as cancers (27). Furthermore, the chronic inflammatory state may induce the formation of neo-epitopes that escape central tolerance and promote the formation of autoantigens with massive activation of self-reactive T lymphocytes (28). The loss of certain microbial species in gut microbiota, in the presence of reduced microbiota diversity and dysbiosis characteristic of different chronic non communicable diseases, plays a crucial role in tolerance disruption (29, 30).
These environmental factors add up to a genetic predisposition for immunity impairment. Studies on the human genome have revealed a large number of genetic factors implicated in the origin of atopic and autoimmune conditions, such as HLA haplotypes, genes encoding cytokines or their receptors (31).
The review’s purpose is to present an overview of the existing evidence supporting the co-occurrence of allergy and autoimmunity in pediatric population.
A comprehensive search on Medline via PubMed and EMBASE (from January 1, 2000, through June 30, 2022), restricted to pediatric age by using the medical subject heading terms referring to atopic disease (“atopy”, “allergy”, “food allergy”, “asthma”, “allergic rhinitis”, “atopic dermatitis”, “urticaria”) each one combined with terms referring to autoimmune disease (“celiac disease”, “inflammatory bowel disease”, “Crohn’s disease”, “ulcerative colitis”, “diabetes mellitus”, “type-1 diabetes mellitus”, “thyroid autoimmune disease”,“Hashimoto disease”, “Juvenile Idiopathic Arthritis”) building search strings with Boolean operators “AND”.
We restricted our search to English-language publications. We did not restrict for type or study design. Duplicates found between searches were identified and removed. Studies were excluded if the information was not specific to the topic of this review.
Celiac disease (CD) is a rather common chronic autoimmune disease involving the gastrointestinal tract in genetically predisposed subjects, affecting about 1% of the general population (32), and up to 3% of some Western pediatric populations (33).
Several studies have investigated the association between CD and allergic disorders, both in adults and children.
A higher incidence of CD in subjects suffering from allergic diseases (e.g., asthma, AD, allergic rhinitis/conjunctivitis) has been observed in most observational studies (33–38).
Narla et al.'s study found that AD was correlated to several autoimmune disorders in children, particularly alopecia areata, vitiligo, scleroderma, and chronic urticaria; however, when examining specifically the correlation between AD and CD in pediatric populations, this correlation did not result significant (39).
The meta-analysis by Lu et al. showed a significant association of atopic dermatitis with multiple autoimmune diseases, including celiac disease (40).
In parallel, the frequency of AD has been also found to be significantly higher in children with CD respect to general population, pointing to a bidirectional link between these diseases (35, 37).
Concerning food allergy (FA), existing data are still controversial.
IgE-mediated FA was found to be more common in children affected by CD, and CD prevalence resulted to be higher in the occurrence of severe FA compared to the general pediatric population and subjects with mild forms of allergy (37, 41). Conversely, Lanzarin et al. did not find a significant increase in sensitization to wheat, rye, barley, and malt in children with CD (42).
The relationship between CD and asthma has also been investigated (35, 43–47) in pediatric populations.
Kero et al. found a significantly higher incidence of asthma in children with CD than in controls (43) and this correlation seemed to be present both before and after CD diagnosis (45).
A Swedish study also showed an increased incidence of celiac disease in asthmatic patients, but only in young age (44).
Similarly, Canova et al. found asthmatic children to have an increased risk of developing CD (46). Of note, a retrospective study conducted in the USA confirmed the association between asthma and CD only in a subgroup of children who also had a positive family history for asthma (47).
Table 1 summarizes the studies concerning the association between CD and allergic disorders.
The exact mechanisms linking CD to allergic disorders are still unknown. A reduced microbial exposure and altered intestinal microbiota, caused by genetic and multiple environmental factors underpin common immune dysregulation, both antibodies-mediated and tissue-mediated (43). The crucial role of gut dysbiosis is supported by the fact that children exposed to antibiotics in early life seem to be at increased risk of developing childhood-onset asthma, allergic rhinitis, AD, and CD. Furthermore, multiple prescriptions appear to predispose to the coexistence of multiple conditions (48).
A poor vitamin D status has also been considered as a possible factor contributing to the development of both allergic and autoimmune diseases, as more than half of CD patients present reduced levels of 25-(OH) vitamin D several years after being diagnosed with this disorder (49).
Furthermore, a vitamin D deficiency is inversely proportional to the increase in ILCP cells involved in tissue inflammation at the level of the duodenal mucosa (50) Vitamin D has anti-inflammatory properties by reducing the release of IFN-gamma from (51). Low levels of vitamin D reduce the effect of regulatory T cells (T-regs), causing ineffective control of T-cell mediated responses, which could lead to the development of asthma. On top of this, shared genetic factors and excessive oxidative stress might also be possible mechanisms linking CD and asthma (45).
In regard to the co-occurrence of CD and FA, a damaged epithelial barrier and an impaired intestinal permeability might represent a possible causative link (37, 41).
ILC1-induced inflammation, mediated by IFN-gamma, contributes to barrier damage which in turn induces the release of alarmins, which cause loss of tolerance of gluten-derived peptides (6).
Some authors speculate whether elimination of foods from diet, as gluten-free diet, could result in loss of tolerance, favoring IgE-mediated reactions (52–54). Furthermore, chronic up-regulation of IL-15 in the intestinal mucosa, a typical finding in CD, might be responsible for the dysregulation of several immune mechanisms, resulting in Th1- and Th2-related disorders (55, 56). Finally, dysfunction of the epidermal barrier, altered microbiota, and immune dysregulation might play a role the mechanisms linking CD and AD (37, 40).
IgA deficiency (IgAD) deserves a specific attention. It is the most common primary immunodeficiency strongly associated with an increased predisposition to develop allergic and autoimmune disorders such as celiac disease with a risk of about 10–20 times. Although the pathogenetic factors of the aforementioned association are not yet fully known, many studies have described it in the pertinent literature (57).
Janzi et al. measured the serum IgA levels in 2423 children of 52 months of age, 14 with IgAD and 2,409 without IgAD, observing that subjects with low levels of IgA have a major prevalence non-IgE mediated food allergy (58).
Aghamohammadi et al. studied a sample of 37 patients with IgA deficiency, aged between 4 and 32 years, in which the onset of disease was represented by allergic conditions. Interestingly, the 25% of IgAD have been diagnosed during an allergologic assessment. Likewise, autoimmune diseases were found in 10 patients of which 4 affected by autoimmune thyroiditis (59).
Odineal et al. suggest to pay extra attention to patients with IgAD considering its strong association with the autoimmune diseases—e.g., SLE, dysthyroidism, JIA, vitiligo (60).
Aytekin et al. confirmed the aforementioned results, demonstrating that allergic diseases often represent the second, clinical manifestation in patients with IgAD in 43.2% of cases; likewise, the prevalence of autoimmune conditions is 3%–5% with a diagnosis of celiac disease in 4 patients (61).
A single-center study evaluated the clinical features of 184 patients with IgA deficiency demonstrating a high occurrence of allergic manifestations and celiac disease, respectively 39% and 14% (62). A retrospective cohort study, including both adult and pediatric populations, showed a prevalence of 2.06% and 1.89% of celiac disease in patients with selective IgA deficiency and partial IgA deficiency respectively, without significant differences sex and age related (63).
Recent studies support the association between IgA deficiency and allergy with good prognosis, although the possible association with autoimmune features and recurrent respiratory infections may have different clinical outcomes (64).
Cinicola et al. showed that patients with IgA deficiency and allergies do not have a complex immune defect, except for transient mild lymphopenia and low count CD19 + at diagnosis vs. follow-up (65% vs. 1.5%, p < 0.0001% and 57% vs. 11%, p < 0.0001, respectively) (65).
The salivary IgA seems to play a pivotal role in the protection of the mucous membranes by hindering the entry of allergens (66). In this sense, the authors speculate that the IgAD may be involved in the development of sensitization (66). Of note, the association between the IgAD and autoimmune diseases may also be related to a common genetic background—e.g., the TNFRSF13B gene encodings TACI (67). Lastly, it has been demonstrated that the HLA 8.1 haplotype is a genetic marker characterizing both autoimmune diseases and IgA deficiency (68).
To date, it is not possible to point out a clear association between celiac disease and allergies, but there is increasing evidence on emerging role of ILC at the core of inflammatory pathways both in autoimmune and allergic diseases, which mayexplain the coexistence in the same patient of these disorders. Furthermore, many studies, although a heterogeneity of the prevalence estimates, confirm the direct association existing between IgA deficiency and allergic diseases.
Ulcerative colitis and Crohn disease are chronic inflammatory disorders of the gastrointestinal tract with a rising incidence in pediatric populations (about 25% of patients with IBD receive diagnosis before the age of 20 years). IBDs are classically defined as chronic inflammatory immune-mediated disorders (69). Many genetics and external factors, such as intestinal bacterial antigens, can indeed alter host's mucosal barrier function and trigger inappropriate and ongoing activation of both humoral and cell-mediated mucosal immune system (70, 71). However, autoimmune phenomena also take place in IBD pathogenesis, resulting in antibodies and autoantibodies production, such as anti-neutrophil cytoplasm antibodies (ANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA), although their pathogenic role has not been fully clarified (69).
The disruption of the balance between Th17 and Treg underlies the pathogenesis of IBD. Th17 cells infiltrate the intestinal mucosa and release a greater amount of IL17 with a proinflammatory action. At the same time, Tregs decrease and lose their inhibitory action (72). It was also shown that genetic susceptibility plays an important role in pediatric, but not adult, onset of IBD (73).
Most studies found a positive correlation between AD and autoimmune disease of gut mucosa, as Crohn's disease and Ulcerative colitis (40, 74–76). In addition, there is also evidence, both in pediatric and adult population, of a bidirectional association between atopic dermatitis and inflammatory bowel diseases, which could be explained from a common genetic background: in fact, impaired expression of genes such as ILR6, IL1RL1, IL18R1, IL18RAP was detected in AD as well as in IBD patients (40, 77).
Specific IgE to food allergens were detected in serum of children with IBD (78), although this phenomenon could be related to IL-5 release from overactivated mast cells in inflamed mucosa (78, 79). Nevertheless, there is evidence of clinical coexistence of food allergy and IBD. A significantly increased risk of developing pediatric-onset IBD was observed in children with CMA in infancy (8.2% among patients with CD, 6.4% in UC, 4.0% among the controls). Besides, children with a diagnosis of CMA seemed to contract PIBD at a younger age than the respective non-CMA group (80, 81).
Furthermore, an increased prevalence of asthma, eczema, and allergic rhinitis was found in children who suffered from IBD (82). A population-based study confirmed an increased risk of developing IBD in asthma-affected patients, without any influence of the age at diagnosis for Crohn's disease. Of note, a significant association of asthma with UC was observed only in individuals diagnosed with asthma before age 17 (83). The risk of asthma seems also to diminish with the increase of IBD onset age: VEO-IBD children accounts for the highest risks of developing asthma compared to a progressively lower risk in EO-IBD and pediatric IBD (84, 85).
The possible underlying etiopathogenetic mechanism linking atopy to IBD is still under discussion. One of the IBD hallmarks is a defective mucosal barrier with decreased or altered tight junctions (86). Microbiota alterations play a key role, since it has been demonstrated that intestinal Th17 cells increases and induced colonic inflammation in germ-free mice by transferring gut microbes from IBD mice (87–89). Gut microbes promote naive CD4+ T cells differentiation into Th17 cells by metabolites and direct induction; the upregulation of TH 17 contributes to the recall of cells and production of inflammatory cytokines, e.g IL-6, IL-1b and IL-23. IL-6, IL-22 (90).
Existing evidence has suggested that the gut microbiota can induce Th17 cell differentiation either directly through contact with immune cells or through metabolites indirectly.
In recent years, more data was published regarding the pathogenetic role of IL-17 in some subgroups of allergic diseases, although the involvement of the Th17 cytokine in the pathophysiological pathway is not conclusively understood (91, 92).
In clinical practice, an increasing occurrence of AD, food allergy and asthma in children with inflammatory bowel diseases has been observed, probably due to alteration of the common alteration of the barrier function and pivotal role of IL17.
Type 1 diabetes mellitus is an autoimmune disorder typically affecting children. The incidence of type 1 diabetes mellitus in childhood and adolescence is steadily rising and now stands at 22.9 new cases per year per 100,000 persons up to age 15 (93).
In the last two decades several studies have investigated the association between diabetes mellitus type 1 and atopic diseases in children, with controversial results.
There is some evidence of a decreased risk of atopy in subjects with T1DM compared to non-diabetic subjects, supporting the Th1-Th2 hypothesis (94–99).
In the multicenter case control study EURODIAB study 2 Study Group, a negative association in children was proved with AD, asthma and rhino-conjunctivitis, respectively (100).
Studies are however contrasting (101–103). Although previous results seem to prove a protective role of atopy from the development of T1DM, other authors suggested a positive association between T1DM and allergic diseases (Table 2).
Notably, the strongest associations have been seen with atopic dermatitis and asthma (43, 104): a data analysis from 31 countries in the 13- to 14-year-old age group seemed to demonstrate a common predisposition from environmental and/or genetic factors to type 1 diabetes, wheezing and atopic eczema (105).
On the contrary, no correlation has been observed between rhinitis/rhino-conjunctivitis and T1DM (105).
Few data are available regarding food allergy and T1DM. Still Villanova et al. found low prevalence of sensitization to food allergens in T1DM population (106). An increased risk of developing T1DM was demonstrated in patients who were previously diagnosed with CMA at a mean age of 7.2 months (107). A possible relationship between CMA and DM type 1 could be suggested also from other studies, widely revealing high levels of antibodies against betalactoglobuline and bovine serum albumin in diabetic children (108, 109).
Among different atopic manifestations, these studies mainly focused on the presence of asthma in T1D population (Table 3). Recently Zeng et al.'s metanalysis (110) and Sgrazzutti et al.'s review (111) found a higher risk ratio of developing T1DM in patients who were previously diagnosed with asthma, but not vice versa. In addition, the occurrence of wheezing during the first year of life, which has been considered as a risk factor for later development of asthma, seems to be strongly associated with the risk of β-cell autoimmunity (112).
The co-occurrence of both diseases could be explained by defects in immune system response, driven by both peculiar genetic and environmental factors. A promising hypothesis may be the overstimulation of Treg cells: patients with both T1DM and allergy show higher levels of inflammatory cytokines compared to children with only one disease, which also persist despite hypersecretion of anti-inflammatory IL-10, suggesting a functional exhaustion of Tregs (113). Moreover, microbiota and gut barrier's dysfunction seem to play a role in triggering β-cells autoimmunity (114). The concomitant presence of T1DM and asthma seems to have implication also for therapy management, with higher insulin doses needed to keep glycemic level in range (115); on the other hand, an increased use of asthma medication in children and adolescent with T1DM in the first year after the diabetes onset than gender- and age-matched diabetes-free controls has been reported (116).
Summing up, in a patient with T1DM, we need to think about the possible coexistence of cow's milk allergy, atopic dermatitis and asthma. In the latter case there is a therapeutic implication as the drugs used in asthma can lead to an increase in insulin doses required to maintain euglycemia.
Thyroid autoimmune diseases (TAD) are among the most common autoimmune disorders, with a prevalence in the general population of around 5% (118).
TA is known to be associated with chronic urticaria in adult population (119) and in more recent years this correlation has been observed also in children. Urticaria is a skin condition characterized by the appearance of transient (<24 h) red and itchy wheals, of different size, number, and distribution (120); it may present isolated or in association with angioedema. Urticaria is defined chronic (CU) when acute episodes occur (almost) daily for at least 6 consecutive weeks (121). Between 0.5% and 3% of the general population experience CU, with a peak incidence in middle-aged females (122). Concerning childhood, CU is detected at a median age of 6–11 years old, but cases in younger children have also reported (120).
Two types of chronic spontaneous urticaria (CSU) are recognized: autoallergy (also called type I autoimmunity) with IgE autoantibody involvement, and type IIb autoimmunity with IgG autoantibody involvement (123).
Co-occurrence of TAD and CU in children and adolescents has been reported to be lower than in adults, ranging between 4.3 and 26.9%, according to different studies (120, 124, 125).
On the basis of these studies, European Guidelines include screening of TAD in the diagnostic work-up of CU (126). It is also important to periodically screen children diagnosed with CU for TAD over time (120, 124, 125).
Thyroid autoimmune diseases seems to be also associated with atopic dermatitis (AD) (Table 4). Pedullà et al. conducted a study on a pediatric population affected by AD and found that the prevalence of TA was higher than controls, especially in case of IgE-mediated (vs. non-IgE-mediated) AD (127).
Concerning the link between TAD and other allergic diseases, as asthma and allergic rhinitis, up to now, there is no evidence of a possible association between these disorders (128).
The mechanism whereby CU and TAD are associated is not clear yet. It can be hypothesized that the action of the thyroid stimulating hormone on the thyroid gland, when excessive, could lead to inflammation. This may result in disruption of the normal architecture of the gland and subsequent release of antigens, recognized as non-self. A low-grade autoimmune response is then established and immune complexes can activate the complement pathway, leading to C3a and C5a production, and finally mast cell degranulation (129). Another possible explanation is the presence of a specific type of antibodies [IgG autoantibody against the alpha chain of the high affinity IgE receptor (FceRIα) on mast cells]able to induce basophildegranulation, found in patients with both CU and Hashimoto thyroiditis (130). In a study by Greaves et al., seven children were tested and three of them presented functional anti- FceRIα antibodies (131).
The key message is the one-to-one correlation between autoimmune thyroiditis and chronic urticaria, meaning that the presence of one of the two conditions must lead to screen for the other.
Juvenile idiopathic arthritis (JIA) is the most frequent chronic childhood rheumatic disease. Recently, several molecular mechanisms involved in the pathogenesis of the disease have been better defined (132). JIA can be considered an immune-mediated pathology - multifactorial in nature—with simultaneous involvement of both environmental and genetic factors.
Schubert et al. (133) performed a genetic analysis to define a risk profile for asthmatic disease and JIA by studying several genetic polymorphisms on 231 asthmatic children, 86 children with JIA, and 270 controls. They demonstrated an association of IL-4, CTLA4 and TNF-alpha polymorphisms related to asthmatic pathology and/or JIA, with an inverse distribution. The aforementioned genetic data do not allow to define a clear identification of a genetic risk profile, but they strongly suggest that asthma and JIA share the same genetic background and potentially a similar cytokine pattern as well.
Heinzmann et al. (134) recognized the pivotal role played by IL-18 in the regulation of the immunological response as the immune system polarization toward a Th1 and/or Th2 phenotype largely depends on the aforementioned cytokine. Likewise, to our best knowledge, full understanding of IL-18 effects on asthma and JIA is lacking.
Guo et al. (135) suggested that in patients with JIA, atopy coexistence worsens the outcome of arthritis with enthesitis. Indeed, patients with enthesitis-related arthritis (ERA) show involvement of multiple anatomical districts and a higher disease-activity score. The results indicate that atopic patients with ERA requiring biological therapy show a less satisfactory response when compared to the ones without atopy.
Of note, multiple molecular mechanisms are shared between ERA and allergy. In patients with ERA, high levels of IL-17 have been identified, which induces class E antibody recombination. Moreover, in the same patients, increased expression of TLR2 and TLR4—which provide powerful proinflammatory signals—has been recognized. Finally, allergic rhinitis predicts the increased expression of TLR4.
On the other hand, Ozge Avar-Avyn et al. (136) suggest that patients with Th1-driven chronic inflammatory diseases have a reduced frequency of atopic diseases and no effect on disease activity. Similarly, patients with chronic inflammatory diseases on immunosuppressive therapies undergo resolution of allergic symptoms, where present.
Table 5 shows the main studies investigating the association between JIA and atopic disorders.
Ramirez-Bello et al. (137) have stated that the FCLR3 3C, 5C, and 6A alleles are protective factors for the onset of JIA and asthma. FCLR3 has been particularly studied as protective or modifying factor for some diseases, as RA and SLE. This study is not conclusive and other genes and cohorts require genotyping in order to highlight the different effects of FCRL3 variants on immune diseases.
Karsch et al. (138) reported that the cumulative incidence of asthma is significantly correlated with the concomitant presence of CD and rheumatoid arthritis. According to Bach et al, the observed increase in allergic and autoimmune diseases is due to a reduction in infectious diseases which, by means of IL10 and TGF-beta, can inhibit Th1 and Th2 responses. Jimenez-Morales et al. (139) have confirmed the association between a specific TNF 308 A allele and pediatric inflammatory and immune diseases in the Latin American population.
Heinzmann et al. (134) have focused on the same gene variant in populations with different chronic inflammatory diseases such as bronchial asthma, atopy, and JIA. They showed that the Arg110Gln variant is much more common in asthmatic patients. This genetic variant is responsible for an increased production of IL-13 which is a potent inducer of the Th2 response. In this sense, immunological phenotypes with high serum IL-13 levels may have a greater risk of developing allergic diseases and a lower risk of Th1-related immune diseases.
Chi-Wu et al. (140) highlighted the link between AD and SLE (Systemic lupus erythematosus) sharing an immune dysregulation characterized by functional/quantitative reduction of CD4 + cd25 + Foxp3 + cells. They stated that female patients with AD under the age of 18 have a high risk of developing SLE. This association is also confirmed by the retrospective cohort study of Wei et al. (141) that revealed a significantly increased incidence of SLE in children with AD, regardless of sex.
To date, an exhaustive analysis of the pathogenetic factors underlying JIA is not yet known. Barrier defects and the breakdown of immunological tolerance could be involved in the pathophysiology.
An explanatory model may be represented by the formation of anti-citrulline antibodies in JIA: the citrullinated proteins produced in the inflamed synovium bypass the mechanism of thymic selection and induce the abnormal activation of self-reactive T lymphocytes (142).
The evidence regarding the link between JIA and atopy is weak and inconsistent. What is known is that the two conditions share the same cytokine pattern and genetic background;, by a clinical point of view, the strongest association is that patients affected by ERA in biological therapy have a less satisfactory response if they are atopic too.
Our review presents some limitations. First, the study has considered associations between allergy and most common autoimmune disease where data are more abundant. Data from other autoimmune disease such as autoimmune hepatitis, Addison's disease, juvenile dermatomyositis, lupus erythematosus, scleroderma, have not been included in the review, since the data are more scarce. Second, the search strategy of the review only has focused on scientific research since 2,000 and restricted to the pediatric age. Furthermore, many studies are retrospective cohort studies and the timing of occurrence of the disease is not well understood; both of them limit the quality of the evidence and hamper to draw conclusions.
Further powered-prospective studies are warranted to investigate the association among autoimmune and allergic diseases both in children and adults.
Allergic diseases and autoimmune disorders are often associated in children, proving that Th1 and Th2 responses may coexist, as result of shared immune dysregulation (Figure 1).
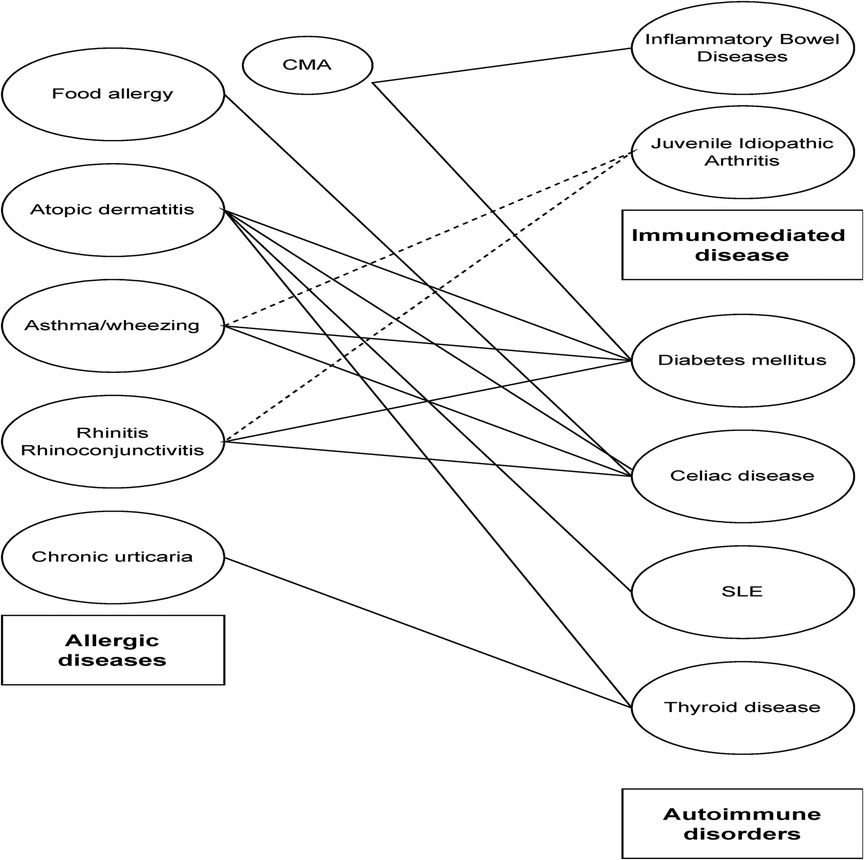
Figure 1. Association between allergic, autoimmune and immune-mediated disorders. Solid lines refer to association between diseases, while dotted lines suggest weak association as highlighted in the main text.
B reg and T reg cells play an essential role, given their role in modulating the inflammation's inhibition. Their imbalance leads to the persistence of an inflammatory stimulus, which in turn causes dysregulation of both innate and antibody immunity.
As a consequence, clinicians should pay particular attention to the presence or the development of autoimmune diseases in patients with allergic disorders, particularly atopic dermatitis and asthma, celiac disease and type 1 diabetes and vice versa.
Further studies are warranted to better clarify the common pathogenetic mechanisms underlying these disorders and their temporal association, in order to ameliorate affected patients’ management, quality of life and prognosis.
AC and MM wrote the manuscript with support from ED, MS, FZ, GZ, and VL helped supervise the project. ED and FZ conceived the original idea. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SR declared a past co-authorship with the author VL to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. (2000) 85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X
2. Dong C, Flavell RA. Th1 and Th2 cells. Curr Opin Hematol. (2001) 8(1):47–51. doi: 10.1097/00062752-200101000-00009
3. Noel JC, Berin MC. Role of innate immunity and myeloid cells in susceptibility to allergic disease. Ann N Y Acad Sci. (2021) 1499(1):42–53. doi: 10.1111/nyas.14654
4. Trabanelli S, Gomez-Cadena A, Salomé B, Michaud K, Mavilio D, Landis BN, et al. Human innate lymphoid cells (ILCs): toward a uniform immune-phenotyping. Cytometry B Clin Cytom. (2018) 94(3):392–9. doi: 10.1002/cyto.b.21614
5. Ebihara T. Dichotomous regulation of acquired immunity by innate lymphoid cells. Cells. (2020) 9(5):1193. doi: 10.3390/cells9051193
6. Rizzi A, Di Gioacchino M, Gammeri L, Inchingolo R, Chini R, Santilli F, et al. The emerging role of innate lymphoid cells (ILCs) and alarmins in celiac disease: an update on pathophysiological insights, potential use as disease biomarkers, and therapeutic implications. Cells. (2023) 12(14):1910. doi: 10.3390/cells12141910
7. Loh W, Tang MLK. The epidemiology of food allergy in the global context. Int J Environ Res Public Health. (2018) 15(9):E2043. doi: 10.3390/ijerph15092043
8. Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) phase three. Allergy. (2009) 64(1):123–48. doi: 10.1111/j.1398-9995.2008.01884.x
9. Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J. (2020) 56(6):2002094. doi: 10.1183/13993003.02094-2020
10. Sahin Y. Celiac disease in children: a review of the literature. World J Clin Pediatr. (2021) 10(4):53–71. doi: 10.5409/wjcp.v10.i4.53
11. Ziegler R, Neu A. Diabetes in Childhood and Adolescence. Dtsch Ärztebl Int 2018 Mar 2 Available at: https://www.aerzteblatt.de/10.3238/arztebl.2018.0146 (Cited June 3, 2022).
12. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. (2014) 81(2):112–7. doi: 10.1016/j.jbspin.2013.09.003
13. Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. (2018) 24(25):2741–63. doi: 10.3748/wjg.v24.i25.2741
14. Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. (2009) 33(3–4):197–207. doi: 10.1016/j.jaut.2009.09.008
15. Arefieva AS, Smoldovskaya OV, Tikhonov AA, Rubina AY. [Allergy and autoimmunity: molecular diagnostics, therapy, and presumable pathogenesis]. Mol Biol (Mosk. (2017) 51(2):227–39. doi: 10.1134/S0026893317020030
16. Strachan DP. Hay fever, hygiene, and household size. Br Med J. (1989) 299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259
17. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. (2021) 21(11):739–51. doi: 10.1038/s41577-021-00538-7
18. Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. (2019) 143(5):1892–903. doi: 10.1016/j.jaci.2018.11.016
19. Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. (2018) 15(5):1033. doi: 10.3390/ijerph15051033
20. Viggiano D, Ianiro G, Vanella G, Bibbò S, Bruno G, Simeone G, et al. Gut barrier in health and disease: focus on childhood – PubMed Available at: https://pubmed.ncbi.nlm.nih.gov/25855935/ (Cited June 13, 2022).
21. Ghezzi M, Pozzi E, Abbattista L, Lonoce L, Zuccotti GV, D’Auria E. Barrier impairment and type 2 inflammation in allergic diseases: the pediatric perspective. Children. (2021) 8(12):1165. doi: 10.3390/children8121165
22. Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. (2021) 12(673708):673–708. doi: 10.3389/fimmu.2021.673708
23. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. (2014) 40(6):833–42. doi: 10.1016/j.immuni.2014.05.014
24. Aguayo-Patrón SV, Calderón de la Barca AM. Old fashioned vs. ultra-processed-based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods Basel Switz. (2017) 6(11):100. doi: 10.3390/foods6110100
25. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. (2021) 76(9):2699–715. doi: 10.1111/all.14763
26. Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
27. Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. (2021) 18(11):2461–71. doi: 10.1038/s41423-021-00726-4
28. Harbige J, Eichmann M, Peakman M. New insights into non-conventional epitopes as T cell targets: the missing link for breaking immune tolerance in autoimmune disease? J Autoimmun. (2017) 84:12–20. doi: 10.1016/j.jaut.2017.08.001
29. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation - PubMed Available at: https://pubmed.ncbi.nlm.nih.gov/24679531/ (Cited June 13, 2022).
30. Penders J, Gerhold K, Thijs C, Zimmermann K, Wahn U, Lau S, et al. New insights into the hygiene hypothesis in allergic diseases: mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes. (2014) 5(2):239–44. doi: 10.4161/gmic.27905
31. Bartůnková J, Kayserová J, Shoenfeld Y. Allergy and autoimmunity: parallels and dissimilarity: the yin and yang of immunopathology. Autoimmun Rev. (2009) 8(4):302–8. doi: 10.1016/j.autrev.2008.09.004
32. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet Lond Engl. (2018) 391(10115):70–81. doi: 10.1016/S0140-6736(17)31796-8
33. Ress K, Annus T, Putnik U, Luts K, Uibo R, Uibo O. Celiac disease in children with atopic dermatitis. Pediatr Dermatol. (2014) 31(4):483–8. doi: 10.1111/pde.12372
34. Krishna MT, Subramanian A, Adderley NJ, Zemedikun DT, Gkoutos GV, Nirantharakumar K. Allergic diseases and long-term risk of autoimmune disorders: longitudinal cohort study and cluster analysis. Eur Respir J. (2019) 54(5):1900476. doi: 10.1183/13993003.00476-2019
35. Yavuzyilmaz F, Ozdogan S, Urganci N, Usta MK. Frequency of asthma and atopic diseases in inflammatory bowel disease and celiac disease. J Coll Physicians Surg–Pak JCPSP. (2019) 29(5):435–9. doi: 10.29271/jcpsp.2019.05.435
36. Kauppi S, Jokelainen J, Timonen M, Tasanen K, Huilaja L. Atopic dermatitis is associated with dermatitis herpetiformis and celiac disease in children. J Invest Dermatol. (2021) 141(1):191–193.e2. doi: 10.1016/j.jid.2020.05.091
37. Cudowska B, Lebensztejn DM. Immunogloboulin E-mediated food sensitization in children with celiac disease: a single-center experience. Pediatr Gastroenterol Hepatol Nutr. (2021) 24(5):492–9. doi: 10.5223/pghn.2021.24.5.492
38. Shalom G, Kridin K, Raviv KO, Freud T, Comaneshter D, Friedland R, et al. Atopic dermatitis and celiac disease: a cross-sectional study of 116,816 patients. Am J Clin Dermatol. (2020) 21(1):133–8. doi: 10.1007/s40257-019-00474-2
39. Narla S, Silverberg JI. Association between atopic dermatitis and autoimmune disorders in US adults and children: a cross-sectional study. J Am Acad Dermatol. (2019) 80(2):382–9. doi: 10.1016/j.jaad.2018.09.025
40. Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q, et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. (2021) 17(1):96. doi: 10.1186/s13223-021-00597-4
41. Pillon R, Ziberna F, Badina L, Ventura A, Longo G, Quaglia S, et al. Prevalence of celiac disease in patients with severe food allergy. Allergy. (2015) 70(10):1346–9. doi: 10.1111/all.12692
42. de Valois Lanzarin CM, de Oliveira E Silva N, Venturieri MO, Solé D, Oliveira RP, Sdepanian VL. Celiac disease and sensitization to wheat, rye, and barley: should we be concerned? Int Arch Allergy Immunol. (2021) 182(5):440–6. doi: 10.1159/000512108
43. Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. (2001) 108(5):781–3. doi: 10.1067/mai.2001.119557
44. Hemminki K, Li X, Sundquist J, Sundquist K. Subsequent autoimmune or related disease in asthma patients: clustering of diseases or medical care? Ann Epidemiol. (2010) 20(3):217–22. doi: 10.1016/j.annepidem.2009.11.007
45. Ludvigsson JF, Hemminki K, Wahlström J, Almqvist C. Celiac disease confers a 1.6-fold increased risk of asthma: a nationwide population-based cohort study. J Allergy Clin Immunol. (2011) 127(4):1071–3. doi: 10.1016/j.jaci.2010.12.1076
46. Canova C, Pitter G, Ludvigsson JF, Romor P, Zanier L, Zanotti R, et al. Coeliac disease and asthma association in children: the role of antibiotic consumption. Eur Respir J. (2015) 46(1):115–22. doi: 10.1183/09031936.00185714
47. Patel B, Wi CI, Hasassri ME, Divekar R, Absah I, Almallouhi E, et al. Heterogeneity of asthma and the risk of celiac disease in children. Allergy Asthma Proc. (2018) 39(1):51–8. doi: 10.2500/aap.2018.39.4100
48. Aversa Z, Atkinson EJ, Schafer MJ, Theiler RN, Rocca WA, Blaser MJ, et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin Proc. (2021) 96(1):66–77. doi: 10.1016/j.mayocp.2020.07.019
49. Hallert C, Grant C, Grehn S, Grännö C, Hultén S, Midhagen G, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. (2002) 16(7):1333–9. doi: 10.1046/j.1365-2036.2002.01283.x
50. Ercolano G, Moretti A, Falquet M, Wyss T, Tran NL, Senoner I, et al. Gliadin-reactive vitamin D-sensitive proinflammatory ILCPs are enriched in celiac patients. Cell Rep. (2022) 39(11):110956. doi: 10.1016/j.celrep.2022.110956
51. Cyprian F, Lefkou E, Varoudi K, Girardi G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front Immunol. (2019) 10:2739. doi: 10.3389/fimmu.2019.02739
52. Micozzi S, Infante S, Fuentes-Aparicio V, Álvarez-Perea A, Zapatero L. Celiac disease and wheat allergy: a growing association? Int Arch Allergy Immunol. (2018) 176(3–4):280–2. doi: 10.1159/000489305
53. Borghini R, Donato G, Marino M, Casale R, Tola MD, Picarelli A. In extremis diagnosis of celiac disease and concomitant wheat allergy. Turk J Gastroenterol Off J Turk Soc Gastroenterol. (2018) 29(4):515–7. doi: 10.5152/tjg.2018.17889
54. Barbi E, Berti I, Longo G. Food allergy: from the of loss of tolerance induced by exclusion diets to specific oral tolerance induction. Recent Pat Inflamm Allergy Drug Discov. (2008) 2(3):212–4. doi: 10.2174/187221308786241875
55. Bierbaum S, Nickel R, Zitnik S, Ahlert I, Lau S, Deichmann KA, et al. Confirmation of association of IL-15 with pediatric asthma and comparison of different controls. Allergy. (2006) 61(5):576–80. doi: 10.1111/j.1398-9995.2006.01059.x
56. Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunol Rev. (2014) 260(1):221–34. doi: 10.1111/imr.12191
57. Meini A, Pillan NM, Ugazio AG, Villanacci V, Monafo V, Plebani A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol. (1996) 77(4):333–6. doi: 10.1016/S1081-1206(10)63329-7
58. Janzi M, Kull I, Sjöberg R, Wan J, Melén E, Bayat N, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol Orlando Fla. (2009) 133(1):78–85. doi: 10.1016/j.clim.2009.05.014
59. Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, et al. Iga deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol. (2009) 29(1):130–6. doi: 10.1007/s10875-008-9229-9
60. Odineal DD, Gershwin ME. The epidemiology and clinical manifestations of autoimmunity in selective IgA deficiency. Clin Rev Allergy Immunol. (2020) 58(1):107–33. doi: 10.1007/s12016-019-08756-7
61. Aytekin C, Tuygun N, Gokce S, Dogu F, Ikinciogullari A. Selective IgA deficiency: clinical and laboratory features of 118 children in Turkey. J Clin Immunol. (2012) 32(5):961–6. doi: 10.1007/s10875-012-9702-3
62. Lougaris V, Sorlini A, Monfredini C, Ingrasciotta G, Caravaggio A, Lorenzini T, et al. Clinical and laboratory features of 184 Italian pediatric patients affected with selective IgA deficiency (SIgAD): a longitudinal single-center study. J Clin Immunol. (2019) 39(5):470–5. doi: 10.1007/s10875-019-00647-y
63. Chow MA, Lebwohl B, Reilly NR, Green PHR. Immunoglobulin A deficiency in celiac disease. J Clin Gastroenterol. (2012) 46(10):850–4. doi: 10.1097/MCG.0b013e31824b2277
64. Cinicola BL, Pulvirenti F, Capponi M, Bonetti M, Brindisi G, Gori A, et al. Selective IgA deficiency and allergy: a fresh Look to an old story. Med Kaunas Lith. (2022) 58(1):129. doi: 10.3390/medicina58010129
65. Cinicola BL, Brindisi G, Capponi M, Gori A, Loffredo L, De Castro G, et al. The allergic phenotype of children and adolescents with selective IgA deficiency: a longitudinal monocentric study. J Clin Med. (2022) 11(19):5705. doi: 10.3390/jcm11195705
66. Gleeson M, Cripps AW, Clancy RL, Hensley MJ, Henry RJ, Wlodarczyk JH. The significance of transient mucosal IgA deficiency on the development of asthma and atopy in children. Adv Exp Med Biol. (1995) 371B:861–4.7502913
67. Zhang J, van Oostrom D, Li J, Savelkoul HFJ. Innate mechanisms in selective IgA deficiency. Front Immunol. (2021) 12:649112. doi: 10.3389/fimmu.2021.649112
68. Price P, Witt C, Allcock R, Sayer D, Garlepp M, Kok CC, et al. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol Rev. (1999) 167:257–74. doi: 10.1111/j.1600-065X.1999.tb01398.x
69. Cassinotti A, Sarzi-Puttini P, Fichera M, Shoenfeld Y, de Franchis R, Ardizzone S. Immunity, autoimmunity and inflammatory bowel disease. Autoimmun Rev. (2014) 13(1):1–2. doi: 10.1016/j.autrev.2013.06.007
70. Podolsky DK. Inflammatory bowel disease. N Engl J Med. (2002) 347(6):417–29. doi: 10.1056/NEJMra020831
71. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. (2018) 15(1):39–49. doi: 10.1038/nrgastro.2017.136
72. Yan JB, Luo MM, Chen ZY, He BH. The function and role of the Th17/treg cell balance in inflammatory bowel disease. J Immunol Res. (2020) 2020:8813558. doi: 10.1155/2020/8813558
73. de Ridder L, Weersma RK, Dijkstra G, van der Steege G, Benninga MA, Nolte IM, et al. Genetic susceptibility has a more important role in pediatric-onset crohn’s disease than in adult-onset crohn’s disease. Inflamm Bowel Dis. (2007) 13(9):1083–92. doi: 10.1002/ibd.20171
74. Augustin M, Radtke MA, Glaeske G, Reich K, Christophers E, Schaefer I, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatol Basel Switz. (2015) 231(1):35–40. doi: 10.1159/000381913
75. Schmitt J, Schwarz K, Baurecht H, Hotze M, Fölster-Holst R, Rodríguez E, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. (2016) 137(1):130–6. doi: 10.1016/j.jaci.2015.06.029
76. Cipriani F, Marzatico A, Ricci G. Autoimmune diseases involving skin and intestinal mucosa are more frequent in adolescents and young adults suffering from atopic dermatitis. J Dermatol. (2017) 44(12):1341–8. doi: 10.1111/1346-8138.14031
77. Lee H, Lee JH, Koh SJ, Park H. Bidirectional relationship between atopic dermatitis and inflammatory bowel disease: a systematic review and meta-analysis. J Am Acad Dermatol. (2020) 83(5):1385–94. doi: 10.1016/j.jaad.2020.05.130
78. Grzybowska-Chlebowczyk U, Woś H, Sieroń AL, Wiecek S, Auguściak-Duma A, Koryciak-Komarska H, et al. Serologic investigations in children with inflammatory bowel disease and food allergy. Mediators Inflamm. (2009) 2009:512695. doi: 10.1155/2009/512695
79. Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. (1999) 29(5):1496–503. doi: 10.1002/(SICI)1521-4141(199905)29:05%3C1496::AID-IMMU1496%3E3.0.CO;2-5
80. Virta LJ, Ashorn M, Kolho KL. Cow’s milk allergy, asthma, and pediatric IBD. J Pediatr Gastroenterol Nutr. (2013) 56(6):649–51. doi: 10.1097/MPG.0b013e318285e9d8
81. Virta LJ, Kautiainen H, Kolho KL. Symptoms suggestive of cow’s milk allergy in infancy and pediatric inflammatory bowel disease. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. (2016) 27(4):361–7. doi: 10.1111/pai.12551
82. Kappelman MD, Galanko JA, Porter CQ, Sandler RS. Association of paediatric inflammatory bowel disease with other immune-mediated diseases. Arch Dis Child. (2011) 96(11):1042–6. doi: 10.1136/archdischild-2011-300633
83. Kuenzig ME, Barnabe C, Seow CH, Eksteen B, Negron ME, Rezaie A, et al. Asthma is associated with subsequent development of inflammatory bowel disease: a population-based case-control study. Clin Gastroenterol Hepatol. (2017) 15(9):1405–1412.e3. doi: 10.1016/j.cgh.2017.02.042
84. Amidei C B, Zingone F, Zanier L, Canova C. Risk of prevalent asthma among children affected by inflammatory bowel disease: a population-based birth cohort study. Int J Environ Res Public Health. (2020) 17(12):E4255. doi: 10.3390/ijerph17124255
85. Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, et al. Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur Respir J. (2015) 45(4):962–8. doi: 10.1183/09031936.00079414
86. Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi A, et al. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. (2010) 16(34):4264–71. doi: 10.3748/wjg.v16.i34.4264
87. Stange EF, Schroeder BO. Microbiota and mucosal defense in IBD: an update. Expert Rev Gastroenterol Hepatol. (2019) 13(10):963–76. doi: 10.1080/17474124.2019.1671822
88. Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. (2019) 31(3):418–25. doi: 10.1097/MOP.0000000000000766
89. Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, et al. Microbiotas from humans with inflammatory bowel disease Alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. (2019) 50(1):212–224.e4. doi: 10.1016/j.immuni.2018.12.015
90. Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. (2015) 2015:628157. doi: 10.1155/2015/628157
91. Soh H, Lee HJ, Han K, Park S, Hong SW, Moon JM, et al. Atopic diseases are associated with development of inflammatory bowel diseases in Korea: a nationwide population-based study. Clin Gastroenterol Hepatol. (2021) 19(10):2072–2081.e6. doi: 10.1016/j.cgh.2020.07.049
92. Hofmann MA, Fluhr JW, Ruwwe-Glösenkamp C, Stevanovic K, Bergmann KC, Zuberbier T. Role of IL-17 in atopy-A systematic review. Clin Transl Allergy. (2021) 11(6):e12047. doi: 10.1002/clt2.12047
93. Gillespie KM. Type 1 diabetes: pathogenesis and prevention. Can Med Assoc J. (2006) 175(2):165–70. doi: 10.1503/cmaj.060244
94. Stene LC, Joner G. Norwegian Childhood diabetes study group. Atopic disorders and risk of childhood-onset type 1 diabetes in individuals. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2004) 34(2):201–6. doi: 10.1111/j.1365-2222.2004.01864.x
95. Stene LC, Rønningen KS, Bjørnvold M, Undlien DE, Joner G. An inverse association between history of childhood eczema and subsequent risk of type 1 diabetes that is not likely to be explained by HLA-DQ, PTPN22, or CTLA4 polymorphisms. Pediatr Diabetes. (2010) 11(6):386–93. doi: 10.1111/j.1399-5448.2009.00605.x
96. Rosenbauer J, Herzig P, Giani G. Atopic eczema in early childhood could be protective against type 1 diabetes. Diabetologia. (2003) 46(6):784–8. doi: 10.1007/s00125-003-1108-6
97. Thomsen SF, Duffy DL, Kyvik KO, Skytthe A, Backer V. Relationship between type 1 diabetes and atopic diseases in a twin population. Allergy. (2011) 66(5):645–7. doi: 10.1111/j.1398-9995.2010.02517.x
98. Mattila PS, Tarkkanen J, Saxen H, Pitkäniemi J, Karvonen M, Tuomilehto J. Predisposition to atopic symptoms to inhaled antigens may protect from childhood type 1 diabetes. Diabetes Care. (2002) 25(5):865–8. doi: 10.2337/diacare.25.5.865
99. Meerwaldt R, Odink RJ, Landaeta R, Aarts F, Brunekreef B, Gerritsen J, et al. A lower prevalence of atopy symptoms in children with type 1 diabetes mellitus. Clin Exp Allergy. (2002) 32(2):254–5. doi: 10.1046/j.1365-2222.2002.01311.x
100. Decreased prevalence of atopic diseases in children with diabetes. The EURODIAB substudy 2 study group. J Pediatr. 2000;137(4):470–4. doi: 10.1067/mpd.2000.109109
101. Jasser-Nitsche H, Varga EM, Borkenstein HM, Höntzsch J, Suppan E, Weinhandl G, et al. Type 1 diabetes in children and adolescents is not associated with a reduced prevalence of atopy and allergic diseases. Pediatr Diabetes. (2017) 18(8):890–4. doi: 10.1111/pedi.12504
102. Karavanaki K, Tsoka E, Karayianni C, Petrou V, Pippidou E, Brisimitzi M, et al. Prevalence of allergic symptoms among children with diabetes mellitus type 1 of different socioeconomic status. Pediatr Diabetes. (2008) 9(4 Pt 2):407–16. doi: 10.1111/j.1399-5448.2008.00444.x
103. Dales R, Chen Y, Lin M, Karsh J. The association between allergy and diabetes in the Canadian population: implications for the Th1-Th2 hypothesis. Eur J Epidemiol. (2005) 20(8):713–7. doi: 10.1007/s10654-005-7920-1
104. Lin CH, Wei CC, Lin CL, Lin WC, Kao CH. Childhood type 1 diabetes may increase the risk of atopic dermatitis. Br J Dermatol. (2016) 174(1):88–94. doi: 10.1111/bjd.14166
105. Fsadni P, Fsadni C, Fava S, Montefort S. Correlation of worldwide incidence of type 1 diabetes (DiaMond) with prevalence of asthma and atopic eczema (ISAAC). Clin Respir J. (2012) 6:18–25. doi: 10.1111/j.1752-699X.2011.00239.x
106. Villa-Nova H, Spinola-Castro AM, Garcia FE, Solé D. Prevalence of allergic diseases and/or allergic sensitisation in children and adolescents with type 1 diabetes mellitus. Allergol Immunopathol (Madr). (2015) 43(2):157–61. doi: 10.1016/j.aller.2013.11.009
107. Lamminsalo A, Lundqvist A, Virta LJ, Gissler M, Kaila M, Metsälä J, et al. Cow’s milk allergy in infancy and later development of type 1 diabetes-nationwide case-cohort study. Pediatr Diabetes. (2021) 22(3):400–6. doi: 10.1111/pedi.13181
108. Luopajärvi K, Savilahti E, Virtanen SM, Ilonen J, Knip M, Akerblom HK, et al. Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr Diabetes. (2008) 9(5):434–41. doi: 10.1111/j.1399-5448.2008.00413.x
109. Hilger C, Grigioni F, De Beaufort C, Michel G, Freilinger J, Hentges F. Differential binding of IgG and IgA antibodies to antigenic determinants of bovine serum albumin. Clin Exp Immunol. (2001) 123(3):387–94. doi: 10.1046/j.1365-2249.2001.01451.x
110. Zeng R, Wang Z, Zhang J, Liang Z, Xu C, Wang J, et al. Type 1 diabetes and asthma: a systematic review and meta-analysis of observational studies. Endocrine. (2022) 75(3):709–17. doi: 10.1007/s12020-021-02973-x
111. Sgrazzutti L, Sansone F, Attanasi M, Di Pillo S, Chiarelli F. Coaggregation of asthma and type 1 diabetes in children: a narrative review. Int J Mol Sci. (2021) 22(11):5757. doi: 10.3390/ijms22115757
112. Wahlberg J, Vaarala O, Ludvigsson J, Study Group ABIS. Asthma and allergic symptoms and type 1 diabetes-related autoantibodies in 2.5-yr-old children. Pediatr Diabetes. (2011) 12(7):604–10. doi: 10.1111/j.1399-5448.2011.00758.x
113. Kainonen E, Rautava S, Korkeamäki M, Isolauri E. Unique cytokine secretion profile in children with both type I diabetes and asthma distinct from that of solely diabetic or asthmatic children. Cytokine. (2006) 34(3–4):198–205. doi: 10.1016/j.cyto.2006.04.015
114. Durazzo M, Ferro A, Gruden G. Gastrointestinal Microbiota and type 1 diabetes Mellitus: the state of art. J Clin Med. (2019 Nov 2) 8(11):E1843. doi: 10.3390/jcm8111843
115. Hörtenhuber T, Kiess W, Fröhlich-Reiterer E, Raile K, Stachow R, Bollow E, et al. Asthma in children and adolescents with type 1 diabetes in Germany and Austria: frequency and metabolic control. Pediatr Diabetes. (2018) 19(4):727–32. doi: 10.1111/pedi.12618
116. Ahmadizar F, Souverein PC, Arets HGM, de Boer A, Maitland-van der Zee AH. Asthma related medication use and exacerbations in children and adolescents with type 1 diabetes. Pediatr Pulmonol. (2016) 51(11):1113–21. doi: 10.1002/ppul.23428
117. Klamt S, Vogel M, Kapellen TM, Hiemisch A, Prenzel F, Zachariae S, et al. Association between IgE-mediated allergies and diabetes mellitus type 1 in children and adolescents. Pediatr Diabetes. (2015) 16(7):493–503. doi: 10.1111/pedi.12298
118. Pan XF, Gu JQ, Shan ZY. The prevalence of thyroid autoimmunity in patients with urticaria: a systematic review and meta-analysis. Endocrine. (2015) 48(3):804–10. doi: 10.1007/s12020-014-0367-y
119. Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. (1983) 119(8):636–40. doi: 10.1001/archderm.1983.01650320010007
120. Kilic G, Guler N, Suleyman A, Tamay Z. Chronic urticaria and autoimmunity in children. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. (2010) 21(5):837–42. doi: 10.1111/j.1399-3038.2010.00986.x
121. Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. (1999) 140(3):446–52. doi: 10.1046/j.1365-2133.1999.02707.x
122. Bagnasco M, Minciullo PL, Schiavo M, Saraceno G, Gangemi S, Benvenga S. Urticaria and thyroid autoimmunity. Thyroid. (2011) 21(4):401–10. doi: 10.1089/thy.2010.0103
123. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. (2018) 142(3):876–82. doi: 10.1016/j.jaci.2017.10.035
124. Levy Y, Segal N, Weintrob N, Danon YL. Chronic urticaria: association with thyroid autoimmunity. Arch Dis Child. (2003) 88(6):517–9. doi: 10.1136/adc.88.6.517
125. Pedullà M, Fierro V, Marzuillo P, Capuano F. Miraglia Del giudice E. Ruocco E. Skin Disease and Thyroid Autoimmunity in Atopic South Italian Children. World J Clin Pediatr. (2016) 5(3):288–92. doi: 10.5409/wjcp.v5.i3.288
126. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. (2022) 77(3):734–66. doi: 10.1111/all.15090
127. Pedullá M, Fierro V, Papacciuolo V, Alfano R, Ruocco E. Atopy as a risk factor for thyroid autoimmunity in children affected with atopic dermatitis. J Eur Acad Dermatol Venereol JEADV. (2014) 28(8):1057–60. doi: 10.1111/jdv.12281
128. Tural D A, Ucakturk SA, Guvenir H, Kocabas CN, Dibek Misirlioglu E. Evaluation of allergic disease prevalence and related risk factors in children with autoimmune thyroiditis. Int Arch Allergy Immunol. (2021) 182(10):932–40. doi: 10.1159/000515499
129. Schocket AL. Chronic urticaria: pathophysiology and etiology, or the what and why. Allergy Asthma Proc. (2006) 27(2):90–5. 16724623.16724623
130. Kandeel AA, Zeid M, Helm T, Lillie MA, Donahue E, Ambrus JL. Evaluation of chronic urticaria in patients with hashimoto thyroiditis. J Clin Immunol. (2001) 21(5):335–47. doi: 10.1023/A:1012288602962
131. Greaves MW. Chronic urticaria in childhood. Allergy. (2000) 55(4):309–20. doi: 10.1034/j.1398-9995.2000.00116.x
132. Glass DN, Giannini EH. Juvenile rheumatoid arthritis as a complex genetic trait. Arthritis Rheum. (1999 Nov) 42(11):2261–8. doi: 10.1002/1529-0131(199911)42:11%3C2261::AID-ANR1%3E3.0.CO;2-P
133. Schubert K, von Bonnsdorf H, Burke M, Ahlert I, Braun S, Berner R, et al. A comprehensive candidate gene study on bronchial asthma and juvenile idiopathic arthritis. Dis Markers. (2006) 22(3):127–32. doi: 10.1155/2006/373620
134. Heinzmann A, Gerhold K, Ganter K, Kurz T, Schuchmann L, Keitzer R, et al. Association study of polymorphisms within interleukin-18 in juvenile idiopathic arthritis and bronchial asthma. Allergy. (2004) 59(8):845–9. doi: 10.1111/j.1398-9995.2004.00538.x
135. Guo R, Cao L, Kong X, Xue H, Li X, Shen L. Atopy in children with the enthesitis-related arthritis (ERA) subtype of juvenile idiopathic arthritis is associated with a worse outcome. Eur J Pediatr. (2015) 174(11):1441–50. doi: 10.1007/s00431-015-2553-1
136. Avar-Aydin PO, Nepesov S, Barut K, Sahin S, Adrovic A, Cokugras HC, et al. Decreased frequency of allergy in juvenile idiopathic arthritis: results of a case-control study. Mod Rheumatol. (2021) 31(3):697–703. doi: 10.1080/14397595.2020.1812820
137. Ramírez-Bello J, Jiménez-Morales S, Espinosa-Rosales F, Gómez-Vera J, Gutiérrez A, Velázquez Cruz R, et al. Juvenile rheumatoid arthritis and asthma, but not childhood-onset systemic lupus erythematosus are associated with FCRL3 polymorphisms in Mexicans. Mol Immunol. (2013) 53(4):374–8. doi: 10.1016/j.molimm.2012.09.004
138. Karsh J, Chen Y, Lin M, Dales R. The association between allergy and rheumatoid arthritis in the Canadian population. Eur J Epidemiol. (2005) 20(9):783–7. doi: 10.1007/s10654-005-0704-9
139. Jiménez-Morales S, Velázquez-Cruz R, Ramírez-Bello J, Bonilla-González E, Romero-Hidalgo S, Escamilla-Guerrero G, et al. Tumor necrosis factor-alpha is a common genetic risk factor for asthma, juvenile rheumatoid arthritis, and systemic lupus erythematosus in a Mexican pediatric population. Hum Immunol. (2009) 70(4):251–6. doi: 10.1016/j.humimm.2009.01.027
140. Wu LC, Hwang CY, Chung PI, Hua TC, Chen YD, Chu SY, et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. (2014) 25(6):586–92. doi: 10.1111/pai.12274
141. Wei CC, Lin CL, Tsai JD, Shen TC, Sung FC. Increased incidence of juvenile onset systemic lupus erythematosus in children with atopic dermatitis. Lupus. (2014) 23(14):1494–9. doi: 10.1177/0961203314543920
Keywords: pediatrics, children, allergy, autoimmunity, atopic dermatitis, asthma, celiac disease, type 1 diabetes mellitus
Citation: D’Auria E, Minutoli M, Colombo A, Sartorio MUA, Zunica F, Zuccotti G and Lougaris V (2023) Allergy and autoimmunity in children: non-mutually exclusive diseases. A narrative review. Front. Pediatr. 11:1239365. doi: 10.3389/fped.2023.1239365
Received: 13 June 2023; Accepted: 4 October 2023;
Published: 2 November 2023.
Edited by:
Viviana Moschese, University of Rome Tor Vergata, ItalyReviewed by:
Silvia Ricci, Ospedale Universitario Meyer, Università di Firenze, Italy© 2023 D'Auria, Minutoli, Colombo, Sartorio, Zunica, Zuccotti and Lougaris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Ugo Andrea Sartorio bWFyY28uc2FydG9yaW9AYXNzdC1mYmYtc2FjY28uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.