
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 09 October 2023
Sec. Pediatric Critical Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1225684
 Ofran Almossawi1,2,*
Ofran Almossawi1,2,* Amanda Friend3
Amanda Friend3 Luigi Palla4,5
Luigi Palla4,5 Richard G. Feltbower6
Richard G. Feltbower6 Sofia Sardo-Infiri7
Sofia Sardo-Infiri7 Scott O’Brien8
Scott O’Brien8 Katie Harron2
Katie Harron2 Simon Nadel9
Simon Nadel9 Paul Saunders10
Paul Saunders10 Bianca De Stavola2
Bianca De Stavola2
Introduction: Mortality rates in infancy and childhood are lower in females than males. However, for children admitted to Paediatric Intensive Care Units (PICU), mortality has been reported to be lower in males, although males have higher admission rates. This female mortality excess for the subgroup of children admitted in intensive care is not well understood. To address this, we carried out a systematic literature review to summarise the available evidence. Our review studies the differences in mortality between males and females aged 0 to <18 years, while in a PICU, to examine whether there was a clear difference (in either direction) in PICU mortality between the two sexes, and, if present, to describe the magnitude and direction of this difference.
Methods: Any studies that directly or indirectly reported the rates of mortality in children admitted to intensive care by sex were eligible for inclusion. The search strings were based on terms related to the population (those admitted into a paediatric intensive care unit), the exposure (sex), and the outcome (mortality). We used the search databases MEDLINE, Embase, and Web of Science as these cover relevant clinical publications. We assessed the reliability of included studies using a modified version of the risk of bias in observational studies of exposures (ROBINS-E) tool. We considered estimating a pooled effect if there were at least three studies with similar populations, periods of follow-up while in PICU, and adjustment variables.
Results: We identified 124 studies of which 114 reported counts of deaths by males and females which gave a population of 278,274 children for analysis, involving 121,800 (44%) females and 156,474 males (56%). The number of deaths and mortality rate for females were 5,614 (4.61%), and for males 6,828 (4.36%). In the pooled analysis, the odds ratio of female to male mortality was 1.06 [1.01 to 1.11] for the fixed effect model, and 1.10 [1.00 to 1.21] for the random effects model.
Discussion: Overall, males have a higher admission rate to PCU, and potentially lower overall mortality in PICU than females.
Systematic Review Registration: www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=203009, identifier (CRD42020203009).
Child mortality is a global measure of a nation's health and a top priority for the UK health system (1). Differences in child mortality rates between the sexes are well documented in almost all developed countries, showing higher female survival rates than males (2). Overall childhood mortality is very low in the UK, and in other developed countries [United Nations Inter-agency Group for Child Mortality Estimation (2021)]. Office for National Statistics (ONS) figures show downward mortality trends in the UK for both males and females since the 1950's, and levelling off since 2010.
Paediatric Intensive Care Unit (PICU) deaths account for about 15% of all UK childhood fatalities (3) and 86% of UK hospital deaths (4) thus provide a sizeable population to study childhood deaths. This led to the design and implementation of a longitudinal study of all infants admitted to UK PICUs over 11 years, which showed a higher PICU mortality rate for female over male infants (5). This difference is in the opposite direction to that seen in the overall population and could be due to differences in severity of disease on admission, despite both sexes having the same mean and median Paediatric Index of Mortality (PIM2), a proxy for severity of disease at the time of admission and mortality risk score. There are a number of published studies showing similar conclusions but there is no published systematic review which has collated and evaluated all the available evidence.
The aim of this systematic review was to study the differences in mortality, in either direction, between males and females from age 0 to <18 years, where the death event happens in PICU. This review is also part of a wider project using linked PICU and Hospital Episode Statistics (HES) data which aims to study differences in sex mortality and long term outcomes in England (6).
Using published data, our primary aim is to estimate the difference in mortality rates between males and females who die in PICU. This is to identify if male or female sex is associated with differences in mortality rates in PICU.
Our secondary aim is to quantify the rates of admission to PICU for males and females.
Our specific objectives are to report on the evidence with regards to:
• The difference (absolute or relative, as available) in sex mortality in PICU for all children aged 0 to any age <18 years, overall and separately by age groups.
• The rates of admission to PICU for all children aged 0 to any age <18 years by sex.
• The evidence summarised overall and by any primary diagnostic groups (sub-populations of PICU).
• Population Children of any age range <18 years old, and admitted to a Paediatric Intensive Care Unit.
• Exposure Sex.
• Comparison Comparing male and female mortality rates and their rates of admission to PICU.
• Outcome Death within a Paediatric Intensive Care Unit.
Our protocol was reported previously (7) using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines (8) and registered with the International prospective register of systematic reviews (PROSPERO) database, reference number CRD42020203009.
We conducted a systematic search of PubMed, Embase, and Web of Science using a controlled vocabulary (MeSH) and keywords, without date or language limitations. Our last search update was on 20th of December 2020 and our peer reviewed search strategy was described in the protocol and is reported in Supplementary Appendix 1 (Search Terms and Search Results).
We identified any studies that addressed the association between sex and PICU mortality in children, where sex was the primary exposure. Additionally, we identified all studies where PICU mortality was reported by sex, or where sex was used as a variable for statistical adjustment in the estimation of mortality rates in PICU. We did report but did not pool any estimate reported if sex was a variable for adjustment. This was to ensure we avoided the “Table 2 fallacy”, where effect estimates for any of the adjustment variables included in a regression model alongside the main exposure variable cannot be interpreted (9).
The search strings were based on terms related to the population (children in intensive care), the exposure (sex), and the outcome (in-PICU mortality).
The primary outcome is mortality in PICU by sex. Secondary outcomes are rates of admission to PICU, and length of stay in PICU, by sex.
Eligibility and inclusions criteria are presented in Table 1.
We included any observational study, clinical trial, or re-analysis of a clinical trial.
After the eligibility screening, we further scrutinised studies for any of the exclusion criteria listed in Table 1, and some additional criteria listed below.
Studies meeting at least one of the exclusion criteria were excluded as detailed in the full Table 1 Specifically, we excluded:
• Studies that were only published in abstract form, or were review articles.
• Potentially, studies not available in English, depending on the a priori specification to exclude non-English language studies if they comprised less than 20% of the full text records.
One reviewer screened the titles and abstracts of records after deduplication, and a second reviewer independently checked all the studies from this stage that were labelled “yes” and “maybe” and a sample of the ones labelled as “no”. The “no” sample was assigned to be twice the number of the “yes” total. A third reviewer resolved any disagreements. If all three reviewers gave different answers (Yes/No/Maybe) then the study was included.
For the studies included at the title and abstract level, we applied full text screening in two stages. Stage 1 was a rapid screening carried out by one reviewer to verify if the mortality outcome was reported by each sex. Stage 2 was applied to the studies included from stage 1, where we applied the remaining inclusion and exclusion criteria and this was done by two reviewers independently. See Figure 1.
The inclusion/exclusion decisions made by the reviewers on the basis of titles and abstract were compared and agreement summarised using kappa statistics. We calculated the level of agreement between rates at this stage using Cohen's weighted kappa. We used weights that reflected a disagreement of “maybe/yes” or “maybe/no” carries less weight than “yes/no”.
We adapted the DistillerSR software (10) for data extraction to capture specific features for our study. The resulting tool was piloted and rectified before full extraction was performed by one reviewer. Two additional reviewers independently checked the extracted data. The full data extraction sheet and risk of bias tool are available in Supplementary Appendix 2 (Tools used in screening, extraction, and quality assessment).
Studies where sex was the main exposure of interest were eligible for quality assessment using the “risk of bias in observational studies of exposures” (ROBINS-E) tool (11, 12), which scores studies to be of high, unclear and low risk of bias. Two reviewers independently assessed and checked eligible studies for quality, while a third reviewer resolved any disagreements between the first two reviewers.
We carried out a narrative synthesis of the data, with two final summary tables. The first is for studies with sex as the main exposure of interest, and the second is for all studies, including those where sex was used as a variable for adjustment or a variable for summary statistics.
Where we had three or more studies with a similar sub-population e.g., admissions due to sepsis, we present their results graphically in a forest plot. As a summary report, we combined all studies with death numbers reported by sex, regardless of their variability and types of sub-populations.
We categorised the reported age groups to enable pooling of some studies that have a similar population and with the same age group, see Table 2.
All analyses were carried out in R version 4.1.1.
In our protocol we planned to summarise mortality after PICU discharge in addition to mortality in PICU. However, after summarising the variability in the studies, we concluded that additional information on out of PICU mortality would not confer additional knowledge due to the variability in the reporting of post-PICU mortality.
Our search strategy identified 15,392 studies, of which 124 were eligible for inclusion, see Figure 2A. Overall, the 124 included studies had a total population of 866,620 children, 379,733 (44%) females and 486,887 (56%) males. Of the 124 studies, 114 reported counts of deaths by males and females which give a population of 278,274 children for analysis, specifically involving 121,800 (44%) females and 156,474 males (56%). The number of deaths and mortality rate for females was 5,614 (4.61%), and for males 6,828 (4.36%); thus there is a slightly higher proportion of deaths in females.
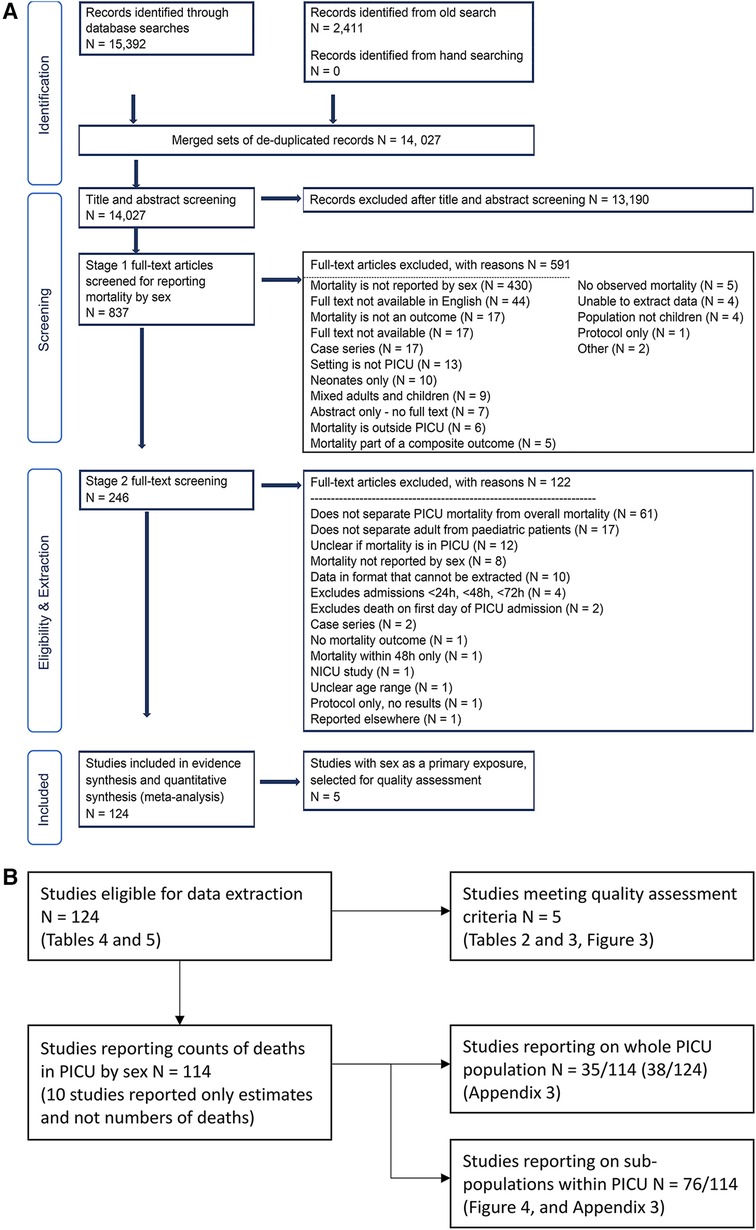
Figure 2. (A) PRISMA flowchart. Records identified from the old search are detailed in Appendix 1. (B) Supplement to PRISMA flowchart. Additions to the original PRISMA Flow Diagram, Copyright © 2020, Evidence Partners Inc., All Rights Reserved. Adapted from ”Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed1000097” For more information, visit: www.evidencepartners.com, www.prisma-statement.org.
One reviewer screened the titles and abstracts of 14,027 studies, and a second reviewer blindly double checked all the included studies (Yes = 863, Maybe = 406) from this stage and a sample of the excluded ones, totalling 2,562 double checks. The level of agreement and weighted Kappa was 68.7% and 0.62 respectively. This was driven mostly by the answers being yes/no/maybe, where a “maybe” answer was given if the abstract mentioned sex as a variable, but did not make clear if the mortality outcome was reported for each sex. This was also reflected in our exclusion reasons in Figure 2A, where we excluded 430 records out of 837 due lack of mortality numbers by sex. When we excluded the “maybe” records, the level of agreement and kappa were 88.5% and 0.69.
We were unable to retrieve the full text of 17 articles, and did not scrutinise the full text of the non-English articles. The non-English records were 44 out of 837 (5.3%) therefore excluded as they comprised <20% of the full text records eligible for screening. We retrieved the full text for the remaining 776 studies and applied the exclusion criteria in two stages. In stage 1, one reviewer rapidly assessed if the mortality outcome was reported by sex. In stage 2, a reviewer applied the exclusion criteria to the remaining 246 studies, and a second reviewer checked this process. The remaining 124 studies were eligible for data extraction. See Figures 2A,B for full details.
We report two types of summaries: first for all the studies meeting our extraction criteria (N = 124), and then for the subset of these studies where sex was the main exposure of interest and for which mortality was reported separately by sex (N = 5), see Table 3. To simplify the reporting, we split the summary of the 124 studies into two parts depending on the mortality outcomes for males and females, see Supplementary Appendix 3 (Summary tables of 124 studies meeting the inclusion criteria).
We report the measures of association between sex and mortality in two ways. If the crude numbers of deaths were reported by sex, we calculated the measure of association in terms of odds ratios. Otherwise, we present the reported measure of association and list any adjustment variables if used.
We report all the measures of association along with their confidence intervals (CIs), the type of sub-population, the age group, and the set of adjustment variables if used in each study. Only 18 of the 124 studies reported a measure of association of sex on mortality. All other studies reported numbers of deaths by sex as a summary statistic, see Supplementary Appendix 3 (Summary tables of 124 studies meeting the inclusion criteria). To summarise the results presented in these two tables, 68 studies reported higher female mortality, 6 studies reported equal mortality, and 50 studies reported higher male mortality.
Overall we found eight studies addressing sex as the primary exposure. Of these eight, three were excluded because PICU mortality was not reported separately from other mortality outcomes (18–20).
Table 3 summarises the five studies that met our criteria for quality assessment. There is considerable variability between these studies in terms of the age range, sub-population of PICU and baseline characteristics such as co-morbidities. Four of these studies did not include any score for severity of disease on admission; one reported the Paediatric Index of Mortality (PIM) score. Although all five studies specified sex as the primary exposure, in two of them PICU mortality was not the primary outcome. All studies reported a lower percentage of female admissions compared to males.
When we used the crude numbers to calculate the association between sex and mortality, three of the studies showed higher female mortality relative to males. In one of the two papers where male mortality was higher, the adjusted association reported by the authors showed the opposite, see Ghuman et al. (15).
Table 4 shows the quality assessment of the five studies using a modified version of the ROBINS-E tool. None of the studies achieved a high score for quality.
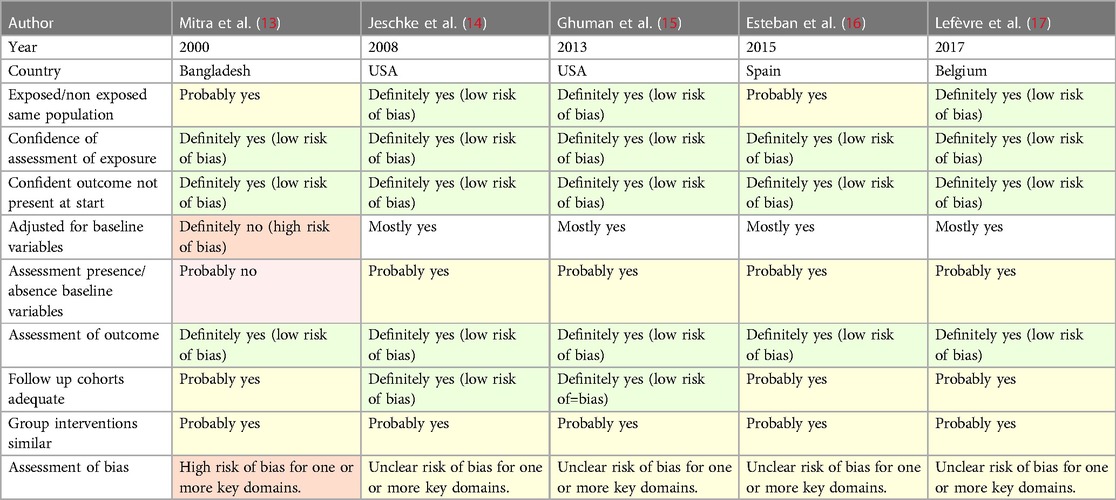
Table 4. Quality assessment of the five studies where sex was the main exposure, using the ROBINS-E tool.
In addition to the five studies where sex was the primary exposure, we summarised the results for a further 119 studies where the numbers of deaths for each sex were reported as a summary statistic, or sex was used as a variable for adjustment when studying mortality in PICU and estimated associations were reported for it. Supplementary Appendix 3 (Summary tables of 124 studies meeting the inclusion criteria).
Proportions of PICU admission by sex are reported in Supplementary Appendix 3 (Summary tables of 124 studies meeting the inclusion criteria). Out of 124 studies, 14 (11%) reported higher proportion of female admissions. However, the study by Ghuman et al. (15) reported on two age ranges showing a slightly higher admission rate for females compared to males in the 16–21 years age category relative to younger ages. As the former group is a mixture of adults and paediatric patients, it fell outside the criteria of inclusion for this review.
For the length of stay outcome, 118 studies did not report this outcome by sex. For the five studies meeting the quality assessment, we have reported a summary of this outcome in Table 3.
We found wide variability between the studies with regards to the sub-populations of PICU and their age range. It was therefore difficult to combine the results. Figures 3,4 summarise the numbers and proportions of population types we found in the studies which are summarised in Table 3 and Supplementary Appendix 3 respectively.
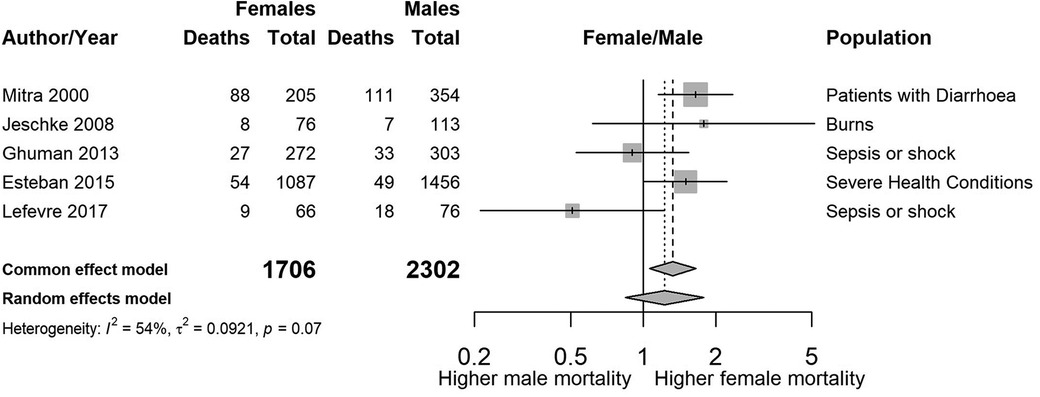
Figure 3. Forest plot showing the estimated unadjusted odds ratios of female to male mortality by study, sorted by year of publication.
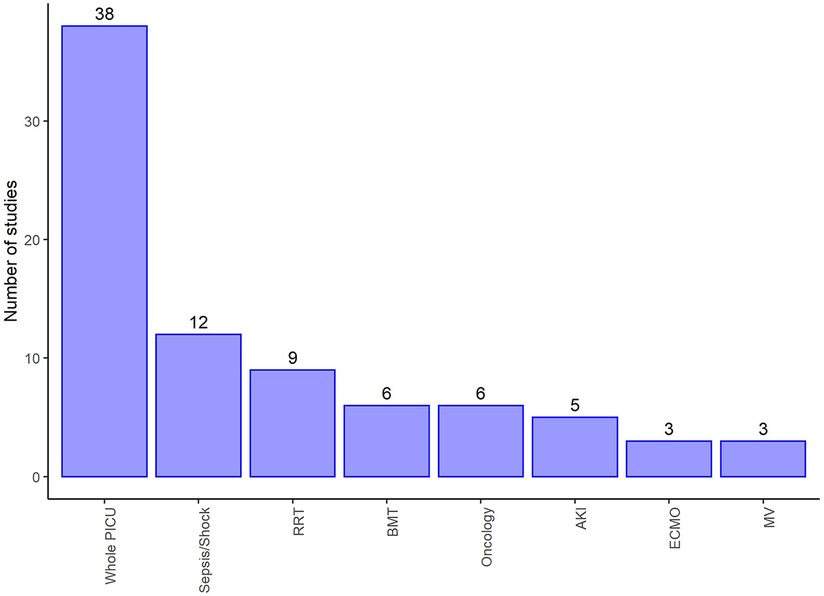
Figure 4. Number of studies by type of PICU admission of the reported studies summarised in Supplementary Appendix 3. Displays populations reported by at least three of the studies selected for extraction and make up 82/124 (66%) of these studies, and 72/124 (58%) reported counts of death by sex. RRT, Renal replacement therapy; BMT, Bone marrow transplant; AKI, Acute kidney injury; ECMO, Extra corporeal membrane oxygenation; MV, Mechanical ventilation.
As far as we could assess, we found very little evidence for publication bias in the reporting of studies. Figure 5 shows a funnel plot of the 27 studies of whole PICU population categorised into age group 1, showing negligible asymmetry. We focus on this subgroup of results because they should be more homogeneous in effect estimates.
Figure 3 shows a summary plot of the crude odds ratios for the five studies where sex was the primary exposure. We have not combined the estimates due to the large variability [I2 = 53.6% (0.0%–82.9%)] in sub-populations and age ranges between the studies.
From the remaining 119 studies that do not meet the quality assessment criteria, we report a summary plot of the estimated odds ratios of female to male mortality for the 27 studies which included whole PICU populations in age group 1 (see Figure 6). The unadjusted pooled OR of female to male mortality is 1.06 for the common (i.e., fixed) effect model, and 1.10 for the random effects model, with no strong evidence of heterogeneity (I2 = 29%).

Figure 6. Estimated odds ratios of female to male mortality for 27 studies that include the whole PICU population belonging to age group 1, sorted by the magnitude of the odds ratio.
Additional plots of sub-populations reported in three studies or more can be found in Supplementary Appendix 4 (Additional plots for some of the reported sub-populations).
When we combined the 114 studies reporting death counts in a pooled estimate, regardless of their heterogeneity, we had data on 278,274 individuals and 12,442 deaths. The unadjusted pooled OR of female to male mortality was 1.11 [95% CI 1.07–1.15] for the common (i.e., fixed) effect model, and 1.14 [95% CI 1.04–1.26] for the random effects model. The I2 statistic reflecting heterogeneity between studies was 58.9% [95% range 49.9%–66.6%] with a p-value of <0.001, indicating a high degree of heterogeneity. Hence these overall estimates are reported only as an indication of the possible direction of the association.
Our systematic review shows that whilst more male children are admitted to PICU, females tend to be more likely to die in PICU than males. Depending on the study, female mortality rates ranged from lower (OR 0.14) to higher (OR 5.06) than males, with a predominance (55%) of studies reporting higher female mortality. A number of studies (5%) reported similar mortality rates between sexes, in contrast to population mortality rates, where male mortality is higher.
Our review captured a wide range of studies in terms of design, size and variety of PICU sub-populations. This resulted in the full text scrutiny of over 837 studies and the inclusion of 124. However, we were only able to identify eight studies that reported sex as the primary exposure and only five eligible for data extraction. Nevertheless we were able to summarise the findings with a large number of participants, N = 866,620. For the majority of studies (n = 119), the publication year was after 2000 reflecting the clinical and reporting progress made in paediatric intensive care data capture over the last two decades.
A strength of this review is that there appears to be little publication bias since investigating the association between sex and mortality was not the primary aim of the majority of studies.
One of the limitations of our review is that it was not possible to combine the study estimates due to the large variability in the PICU sub-populations analysed, and the age ranges of the children included in these analyses. In studies where the association between sex and mortality was reported, and adjustments for confounders included, the variables used to statistically adjust the association between sex and mortality widely varied between studies. Studies reporting adjusted estimates for mortality did not justify the selection of variables used for their statistical adjustments and no two studies with adjusted mortality outcomes were comparable.
Furthermore, follow-up periods for reporting death in PICU were variable, with some studies reporting 7-day and 30-day outcomes in addition to the overall mortality.
We were only able to find five studies, none of good quality, where sex was addressed as the primary exposure. In some of these studies adjustment variables were used, but without rigorous justification for the set of variables used.
These findings show a paucity of evidence in relation to the effect of sex on mortality. Understanding the magnitude and direction of these differences can assist in improved identification of higher risk children and potentially improvements in follow up of high-risk children. A robust and sufficiently large study of PICU mortality in children is needed, where confounder identification and selection is carried out methodically to enable a mechanistic study of the relationship between sex and mortality in PICU.
The evidence we have collected show that, among children admitted to PICU, females appear to have a higher risk of PICU mortality than males, in contrast to a male excess of admissions to PICU. Investigating the reasons for these disparities may help improve insights into the needs of specific populations of critically ill children.
The number of children contributing to this review was large but the quality of the reporting studies were average or poor. Pooling of estimates was not possible in general due to their variability in design.
The data analyzed in this study is subject to the following licenses/restrictions: The data is gathered from published works. Requests to access these datasets should be directed to Ofran Almossawi,by5hbG1vc3Nhd2lAdWNsLmFjLnVr.
OA conceived the idea for the literature review and drafted the protocol. BD and RF reviewed and refined the protocol aims and objectives. BD, KH, RF, AF, and LP reviewed, contributed to, and approved the manuscript for the protocol. OA conducted the literature search, AF and LP reviewed the search strategy and approved it. OA, AF, LP, and SS screened all the titles and abstracts and resolved conflicts from the title and abstracts review. For the included full text publications, OA and SO extracted the data and completed the risk of bias tool then checked these steps. OA conducted the analysis and drafted the manuscript. All authors reviewed and approved the final draft. All authors contributed to the article and approved the submitted version.
OA is funded by an NIHR Fellowship grant, ICA-CDRF-2018-04-ST2-049. This project is sponsored by the joint Research and Development (R&D) office at UCL Great Ormond Street Institute of Child Health.
We would like to thank members of the Project Advisory Group for their contributions to the larger project and this manuscript in particular. We are grateful to our funder, the National Institute for Health Research, for their fellowship grant to Ofran Almossawi. This research was supported in part by the NIHR Great Ormond Street Hospital Biomedical Research Centre.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1225684/full#supplementary-material
1. Royal College of Paediatrics and Child Health, CHR-UK Programme of Work at the MRC Centre of Epidemiology for Child Health, & University College London Institute of Child Health. Child Health Reviews UK—Overview of child deaths in the four UK countries. (2013). https://www.rcpch.ac.uk/sites/default/files/CHR-UK_-_Retrospective_Epidemiological_Review_of_All-cause_Mortality_in_CYP.pdf
2. United Nations, Department of Economic and Social Affairs, Population Division. Sex Differentials in Childhood Mortality. (2011). http://www.un.org/esa/population/publications/SexDifChildMort/SexDifferentialsChildhoodMortality.pdf
3. Paediatric Intensive Care Audit Network. PICANet 2019 Annual Report Summary. (2019). https://www.picanet.org.uk/wp-content/uploads/sites/25/2019/12/PICANet-2019-Annual-Report-Summary_v1.0.pdf
4. Ramnarayan P, Craig F, Petros A, Pierce C. Characteristics of deaths occurring in hospitalised children: changing trends. J Med Ethics. (2007) 33:255–60. doi: 10.1136/jme.2005.015768
5. Almossawi O, O’Brien S, Parslow R, Nadel S, Palla L. A study of sex difference in infant mortality in UK pediatric intensive care admissions over an 11-year period. Sci Rep. (2021) 11:21838. doi: 10.1038/s41598-021-01173-x
6. Almossawi O, Harron K, Feltbower R, Nadel S, De Stavola B, Wong I. NHS health research authority confidentiality advisory group registers. Investigation of gender mortality differences in children admitted to UK Paediatric Intensive Care units. HRA CAG reference number 19/CAG/0164. (2023). Available from: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/confidentiality-advisory-group-registers/#:~:text=The%20register%20contains%20summary%20information%20about%20the%20activity%2C,Research%20Authority%20%28HRA%20approvals%20from%201%20April%202013%29.
7. Almossawi O, Friend A, Palla L, Feltbower R, Stavola BD. Is there a sex difference in mortality rates for deaths occurring in paediatric intensive care units? Systematic literature review protocol. BMJ Open. (2021) 11:e046794. doi: 10.1136/bmjopen-2020-046794
8. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J. (2015) 349:g7647. doi: 10.1136/bmj.g7647
9. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. (2013) 177:292–8. doi: 10.1093/aje/kws412
11. Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev. (2018) 7:242. doi: 10.1186/s13643-018-0915-2
12. Cochrane Methods. Tool to assess risk of bias in cohort studies. Available at: https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf.
13. Mitra AK, Rahman MM, Fuchs GJ. Risk factors and gender differentials for death among children hospitalized with diarrhoea in Bangladesh. J Health Popul Nutr. (2000) 18:151–6. PMID: 11262768.11262768
14. Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Przkora R, Kulp GA, et al. Gender differences in pediatric burn patients: does it make a difference? Ann Surg. (2008) 248:126–36. doi: 10.1097/SLA.0b013e318176c4b3
15. Ghuman AK, Newth CJL, Khemani RG. Impact of gender on sepsis mortality and severity of illness for prepubertal and postpubertal children. J Pediatr. (2013) 163:835–40.e1. doi: 10.1016/j.jpeds.2013.04.018
16. Esteban E, Bujaldon E, Esparza M, Jordan I, Esteban ME. Sex differences in children with severe health conditions: causes of admission and mortality in a pediatric intensive care unit. Am J Hum Biol. (2015) 27:613–9. doi: 10.1002/ajhb.22709
17. Lefèvre N, Noyon B, Biarent D, Corazza F, Duchateau J, Casimir G. Sex differences in inflammatory response and acid-base balance in prepubertal children with severe sepsis. Shock. (2017) 47:422–8. doi: 10.1097/SHK.0000000000000773
18. Morrison WE, Arbelaez JJ, Fackler JC, De Maio A, Paidas CN. Gender and age effects on outcome after pediatric traumatic brain injury*. Pediatr Crit Care Med. (2004) 5:145–51. doi: 10.1097/01.PCC.0000112373.71645.2A
19. Phelan HA, Shafi S, Parks J, Maxson RT, Ahmad N, Murphy JT, et al. Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J Trauma Acute Care Surg. (2007) 63:1127–31. doi: 10.1097/TA.0b013e318154c1b8
Keywords: child, critical care, paediatric intensive care, intensive care, mortality, sex differences
Citation: Almossawi O, Friend A, Palla L, Feltbower RG, Sardo-Infiri S, O’Brien S, Harron K, Nadel S, Saunders P and De Stavola B (2023) Is there a sex difference in mortality rates in paediatric intensive care units?: a systematic review. Front. Pediatr. 11:1225684. doi: 10.3389/fped.2023.1225684
Received: 19 May 2023; Accepted: 30 August 2023;
Published: 9 October 2023.
Edited by:
Michael Hermon, Medical University of Vienna, AustriaReviewed by:
Mary E. Hartman, Washington University in St. Louis, United States© 2023 Almossawi, Friend, Palla, Feltbower, Sardo-Infirri, O'Brien, Harron, Nadel, Saunders and De Stavola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ofran Almossawi by5hbG1vc3Nhd2lAdWNsLmFjLnVr
Abbreviations HES, Hospital Episode Statistics. 2; ONS, Office of National Statistics. 2; PICU, Paediatric Intensive Care Unit. 1–6, 8, 10, 22–25; PIM, Paediatric Index of Mortality. 10; PRISMA-P, Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols. 3; R&D, Research and Development.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.