
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 17 July 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1217650
 Camilla Fontana1,†
Camilla Fontana1,† Paola Schiavolin1,†
Paola Schiavolin1,† Giulia Ardemani2*
Giulia Ardemani2* Danila Angela Amerotti2
Danila Angela Amerotti2 Nicola Pesenti1,3
Nicola Pesenti1,3 Chiara Bonfanti1
Chiara Bonfanti1 Tiziana Boggini1
Tiziana Boggini1 Silvana Gangi1
Silvana Gangi1 Matteo Porro4
Matteo Porro4 Chiara Squarza1
Chiara Squarza1 Maria Lorella Giannì1,2
Maria Lorella Giannì1,2 Nicola Persico2,5
Nicola Persico2,5 Fabio Mosca1,2
Fabio Mosca1,2 Monica Fumagalli1,2
Monica Fumagalli1,2
Objective: To examine the effect of twin birth on long-term neurodevelopmental outcomes in a cohort of Italian preterm infants with very low birth weight.
Study design: We performed a retrospective cohort study on children born in a tertiary care centre. We included children born between 1 January 2007 and 31 December 2013 with a gestational age (GA) of ≤32 weeks and birth weight of <1,500 g. The infants born from twin pregnancies complicated by twin-to-twin transfusion syndrome and from higher-order multiple pregnancies were excluded. The children were evaluated both at 2 years corrected age and 5 years chronological age with Griffiths mental development scales revised (GMDS-R). The linear mixed effects models were used to study the effect of being a twin vs. being a singleton on GMDS-R scores, adjusting for GA, being born small for gestational age, sex, length of NICU stay, socio-economic status, and comorbidity score (CS) calculated as the sum of the weights associated with each of the major morbidities of the infants.
Results: A total of 301 children were included in the study, of which 189 (62.8%) were singletons and 112 (37.2%) were twins; 23 out of 112 twins were monochorionic (MC). No statistically significant differences were observed between twins and singletons in terms of mean general quotient and subscales at both 2 and 5 years. No effect of chorionicity was found when comparing scores of MC and dichorionic twins vs. singletons; however, after adjusting for the CS, the MC twins showed lower scores in the hearing and language and performance subscales at 5 years.
Conclusion: Overall, in our cohort of children born very preterm, twin infants were not at higher risk of neurodevelopmental impairment compared with singletons at pre-school age.
The rate of twin births has increased in the recent four decades across many high-income countries; this is mainly due to the augmented maternal age at childbearing and the increase of medically assisted reproduction (1, 2). According to national estimates, twin and multiple deliveries accounted for 1.5% of all deliveries and 9.6% of births from medically assisted pregnancies in 2021 (3). Compared with singletons, twins have an augmented risk of being born preterm (<37 weeks of gestational age (GA) (in Italy, it has been reported that more than 50% of twins or multiples are born preterm) (4) and have up to a 12-fold risk of very preterm (VPT) birth (<32 weeks of GA) (5). Among all preterm infants, 15%–20% are twins or multiples (6).
Preterm birth is associated with long-term neurodevelopmental impairments, with the youngest infants bearing the highest risk, even in the absence of overt brain damage in conventional neuroimaging studies (7, 8). Despite the improvement in the perinatal management of these fragile patients, the children born very preterm and very low birth weight (VLBW) are at risk of motor and cognitive delays (prevalence estimated to be 16.9% and 20.6%, respectively) and approximately 4.3% of these children develop cerebral palsy (CP) (9). The pathogenesis of developmental disorders is multifactorial and includes several dysmaturational events observed in the brains of preterm infants (10). The major risks for negative deviations of neurodevelopmental trajectories have been attributed to demographic factors, such as male sex, ethnicity, and lower level of parental education (11), as well as to the severity of postnatal morbidities, such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), sepsis and necrotising enterocolitis (NEC), intraventricular haemorrhage (IVH), and surgical diseases (12–14).
Within this framework, twin gestation has been traditionally associated with an increased risk of perinatal mortality and morbidity (15, 16). Moreover, it has been suggested that twins are at greater risk of adverse long-term neurodevelopmental outcomes than singletons, but the evidence is inconsistent (17). Moreover, longitudinal studies examining the neurodevelopmental outcomes among preterm twins and higher-order multiples are limited, and few of those studies had long follow-up durations. It remains unclear whether twin pregnancy itself is associated with an increased likelihood of adverse neurodevelopmental outcomes or if this effect is related to twin-induced prematurity. Notably, the disadvantages experienced by twins are mainly attributable to the risk associated with shorter gestational age and poor foetal growth (18). Recent studies (19, 20) have compared the neurodevelopmental outcomes of preterm multiples and preterm singletons and found no differences at a general level while a slightly lower mean score was observed for the locomotor and personal-social subscales (19) and for the verbal intelligence quotient (20).
Chorionicity may play an important role in the neurodevelopmental outcome of twins. Monochorionic (MC) twins share a single placenta with vascular anastomosis connecting the two foetal umbilical circulations and creating haemodynamic instability (21). MC gestation carries a higher risk for complications such as twin-to-twin transfusion syndrome (TTTS), which affects approximately 10%–15% of all MC twin pregnancies (22), selective intrauterine growth restriction (IUGR), and intrauterine death (IUD) of a co-twin (23–25). Interestingly, even in the absence of TTTS, MC twins, especially those born prematurely, seem to be at higher risk of morbidity than preterm dichorionic (DC) twins (26). Several studies have investigated the association between chorionicity and neurodevelopmental outcome, but the findings are controversial (16, 20).
The aim of the present study was to compare the neurodevelopmental outcomes at 2 and 5 years of age of singletons vs. uncomplicated twins from a cohort of premature infants with GA ≤32 weeks and birth weight (BW) <1,500 g. Furthermore, we explored the associations between different chorionicity features and neurodevelopmental outcomes.
We performed a retrospective cohort study. The children were considered eligible if they were born between 1 January 2007 and 31 December 2013 at the Tertiary Neonatal Intensive Care Unit of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan, Italy, with GA ≤32 weeks and BW <1,500 g and were admitted to the NICU within 6 h of life. The exclusion criteria were major congenital anomalies, metabolic and genetic syndromes, death before NICU discharge, transfer to another NICU within the first 24 h of life, infants born as singletons after intrauterine death of one or more foetuses, twin pregnancy complicated by TTTS, and higher-order multiple pregnancies. Only the children who underwent neurodevelopmental assessment at both 24 ± 6 months of corrected age and 5 ± 1 years of chronological age were included in the analysis. This study is a part of a larger cohort study evaluating the effects of very premature birth and related complications on long-term neurodevelopmental outcomes; the results regarding the effects of red blood cell transfusion have already been published (27). The cohort included in the present analysis partially overlaps with a previously published cohort (including all infants born between 2007 and 2011 at the same Institution independently from GA at birth) reporting only the data on early neonatal outcomes (28).
The neurodevelopment of the infants was assessed at the Paediatric Physical Medicine and Rehabilitation Unit of the same Foundation according to the local follow-up programme for preterm infants. Approval from the Ethical Committee of Foundation IRCCS Ca’ Granda Area 2 was obtained. Due to the retrospective nature of the study and the anonymisation of all the data collected, which are presented as aggregate data, the requirement for informed consent was waived.
The twins were classified as MC or DC twins according to chorionicity assessed by the first-trimester ultrasound scan (29, 30). The perinatal data, postnatal morbidities, treatments, and sociodemographic data from birth to discharge were collected from the electronic medical chart review. Small for gestational age (SGA) was defined as birth weight <10th percentile for GA (31), and the clinical risk index for babies (CRIB) II was calculated based on GA, BW, sex, temperature, and base excess at birth (32). Sepsis was defined by positive blood culture (33) with clinical signs of infection, BPD as requiring oxygen at 36 weeks of postmenstrual age (34). NEC was classified according to the modified Bell's staging criteria (35) and ROP according to the International Classification of ROP (36). Cerebral lesions as detected by cranial ultrasound were classified as follows: IVH grades according to Papile's classification (37), cystic periventricular leukomalacia (cPVL), cerebral focal lesion (defined as any other focal grey and white matter abnormalities as detected by cranial ultrasound), posthaemorrhagic ventricular dilatation (PHVD), cerebellar haemorrhage detectable by cranial ultrasound (>4 mm in size), and major cerebral malformations. Socio-economic status (SES) was calculated with the Hollingshead four-factor index (38).
The follow-up data were retrieved from the clinical files of the infants. Neurodevelopmental assessments at 24 ± 6 months of corrected age and 5 ± 1 years of chronological age were assessed using the Griffiths mental development scales revised (GMDS-R) (39) and extended revised (GMDS-ER) (40) for ages 0–2 and 2–8 years, respectively, and were performed by trained developmental specialists who were blinded to the clinical histories of the infants but were aware of the child being a twin and the type of chorionicity. The Griffiths scales consist of 5 subscales (6 for the extended version): locomotor, personal-social, hearing and language, eye and hand coordination, performance, and practical reasoning (only for the extended version). The locomotor subscale evaluates gross motor skills. The personal-social subscale assesses the level of independency in daily living activities and social development. The hearing and language subscale investigates both receptive and expressive language. The eye and hand coordination subscale measures fine motor skills, visual monitoring skills, and manual dexterity. The performance subscale investigates the ability to reason through performance tests, mainly focusing on visuospatial skills. The practical reasoning subscale covers a range of reasoning skills, including learning numerical concepts and time and space orientation. A composite general quotient [GQ, mean 100, standard deviation (SD) 12] and separate standardised quotients for each subscale (mean = 100, SD = 16) can be calculated. Accordingly, the children were classified as having the following: typical development if their GQ was 88 or higher and their subquotients were 84 or higher; a developmental delay if their GQ was lower than 88 and their subquotients were lower than 84. Because the normative data of the GMDS-R are not available in our country, we referred to the 1996 United Kingdom norms (41).
The statistical analyses were performed using R Software (R Foundation for Statistical Computing, Vienna, Austria) (42). The descriptive statistics of twins and singletons are presented. The continuous variables following normal or non-normal distribution were reported as the mean (SD) or median (IQR) and compared among groups with the Student's t-test or Mann–Whitney U test. The categorical variables are presented as the absolute frequency (percentage) and were compared with χ2 test or Fisher's exact test (when some of the cells had counts fewer than 5). To control for between-family variability, the linear mixed effects models were used to study the effect of being a twin vs. being a singleton on GMDS-R at 2 and 5 years of age, and the family was considered a random effect. The models were adjusted for GA, SGA, sex, length of NICU stay (LoS), SES, and comorbidity score (CS).
The CS (43) was calculated as the sum of the weights associated with each of the major morbidities of the infants: severe BPD, sepsis, ROP grade 3–4, NEC (both medical and surgical), severe brain injury (IVH grade 3 or 4, cPVL, PHVD, cerebellar haemorrhage or cerebral focal lesions), and need for major surgery. The weights were determined using a linear regression model for each outcome of interest (dependent variables) and comorbidities as independent variables. The effect of being MC or DC twins vs. being singletons on GMDS-R at 2 and 5 years of age was also studied.
All tests were two-tailed, and a p-value <0.05 was considered significant.
During the study period, a total of 772 infants were born with GA ≤32 weeks and birth weight <1,500 g. A total of 312 infants were excluded due to predefined criteria (Figure 1). A total of 460 preterm infants were enrolled in the larger cohort study; 159 out of 460 were lost to follow-up (91 at 24 months and 68 at 60 months). A total of 301 infants were eligible for this part of the study, including 189 (62.8%) singletons and 112 (37.2%) twins; 23 out of 112 twins (20.5%) were MC. The flowchart of the study is reported in Figure 1. The clinical characteristics of the included infants of this part of the study are presented in Tables 1–3.
The mean GMDS-R GQ was similar in singletons and twins at both 24 ± 6 months of corrected age (singletons = 89.8, SD = 15.0 and twins = 88.4, SD = 13.4; p = 0.421) and 5 ± 1 years (singletons = 88.4, SD = 11.2 and twins = 88.4, SD = 10.9; p = 0.998). No significant differences were observed between singletons and twins in the GMDS-R subquotients (Table 4). In the model adjusted for GA, SGA, sex, LoS, SES, and comorbidity score, no differences emerged between the two groups at both short- and long-term neurodevelopmental assessments (Table 4).
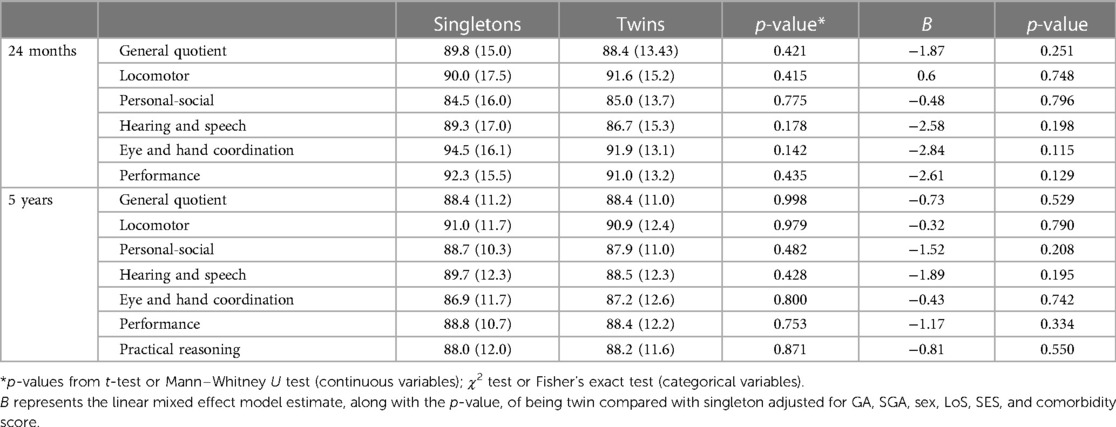
Table 4. Griffiths scores at 24 ± 6 months and at 5 ± 1 years expressed as mean (SD) for singletons vs. twins.
The mean GMDS-R GQ was similar in singletons and MC twins at both 24 ± 6 months of corrected age (singletons = 89.8, SD = 15 and MC twins = 88.9, SD = 16.4; p = 0.778) and 5 ± 1 years (singletons = 88.4, SD = 11.2 and MC twins = 86.7, SD = 10.9; p = 0.507). No significant differences were observed between MC twins and singletons in the GMDS-R subquotients when not corrected for the main clinical characteristics (Table 5). However, after adjusting for GA, SGA, sex, LoS, SES, and comorbidity score, a significant difference between the two groups emerged at the assessment after 5 ± 1 years in the hearing and language and in the performance subscales (Table 5), with MC twins showing lower scores. No differences between DC twins vs. singletons were observed at 24 months and 5 years, in GQ and subscales, even after adjustment (Table 6).

Table 5. Griffiths scores at 24 ± 6 months and at 5 ± 1 years expressed as mean (SD) for singletons vs. MC.
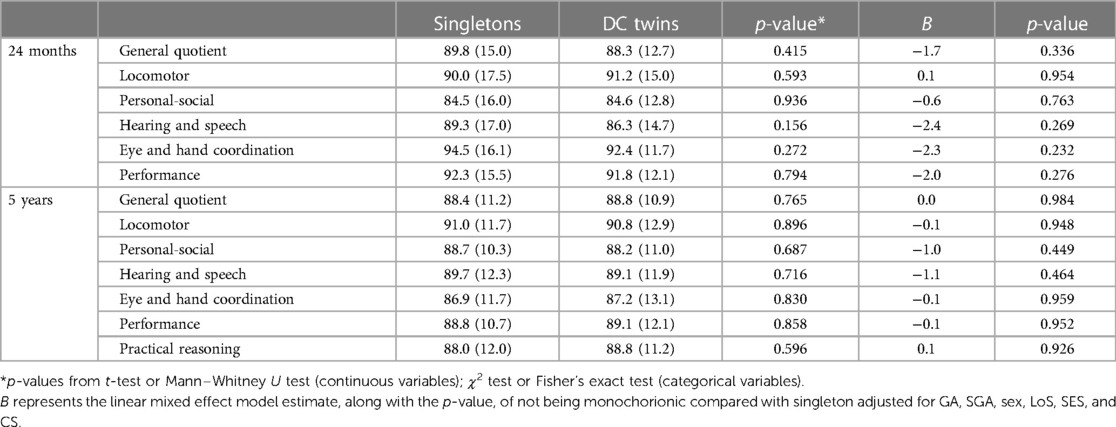
Table 6. Griffiths scores at 24 ± 6 months and at 5 ± 1 years expressed as mean (SD) for singletons vs. DC.
The impact of twin birth on the long-term neurodevelopment of prematurely born infants is still a matter of debate. Our findings show that preterm twins have similar neurodevelopment at 2 and 5 years of age compared with singleton preterm-born children, supporting the hypothesis that being born twin is not an additional risk factor for neurodevelopmental impairment and that the neurodevelopmental outcomes are mainly related to prematurity itself and consequential morbidities.
The available evidence on this topic is controversial, and previous studies have reported contrasting results. Consistent with our findings, Gnanendran et al. (44) observed that the neurodevelopmental outcomes at 2–3 years of multiples (twins, triplets, quadruplets) compared with singleton extremely preterm infants <29 weeks GA were similar. Ylijoki et al. (20) evaluated cognitive development, neuropsychological performance, and neurodevelopmental impairments at 5 years in a population of VLBW and VPT infants born singleton and twins and concluded that twins had similar neuropsychological outcomes to singletons, despite having a slightly lower verbal intelligence quotient. In contrast, Bodeau-Livinec et al. (45) found that VLBW twins had higher mortality and slightly lower cognitive scores than singletons after adjustment for sex, GA, IUGR, and SES but no difference with respect to severe deficiencies at 5 years of age. Nevertheless, this study included twin pregnancies complicated by TTTS, potentially affecting the results.
In their review, Lorenz (46) highlighted that several older population-based studies reported a higher prevalence of delay in the achievement of cognitive neurodevelopmental milestones among twins compared with singletons, but not all the studies were adjusted for GA and BW, which are two of the most important risk factors for long-term neurodevelopmental impairments (46); furthermore, they did not exclude TTTS. Therefore, we decided to consider GA and BW as part of the inclusion criteria to compare two homogeneous groups of infants. The neurodevelopment of preterm infants is affected by a dense interplay of causal factors. In our population, singletons had lower GA (mean = 28.6, SD = 2.3 weeks) than twins (mean = 29.2, SD = 2.3 weeks), were more likely to be born SGA (36.5% of singletons vs. 20.8% of twins) and male (51.3% vs. 39.0%), had a higher CRIB score (mean = 8.4, SD = 3.5 vs. 6.9 SD = 3.7), and had a longer LoS (median 68 vs. 56 days). Despite these covariates, we did not observe any differences in the neurodevelopmental outcome at 2 and 5 years of age between singletons and twins, even after adjusting for the main confounding factors.
Interestingly, our data concerning perinatal characteristics of singletons and twins are somewhat in contrast with the literature. Kalikkot Thekkeveedu et al. (47) found the highest percentage of premature birth among twins compared with singletons, with twins having a lower mean GA at birth. Moreover, in their study, the number of SGA neonates was significantly higher among twins than among singletons, whereas the median LoS was significantly shorter for singleton within each GA category. Our hospital is one of the national referral centres for high-risk pregnancies, and singletons were more likely to be born prematurely due to an iatrogenically induced preterm delivery because of maternal or foetal diseases (maternal hypertension, pre/eclampsia, foetal growth restriction) rather than spontaneous preterm labour per se, resulting in sicker preterm infants at birth. These observations may at least partially explain the differences compared with the other studies; unfortunately, due to the retrospective nature of our study, the data regarding maternal or obstetric complications were not available to support this hypothesis.
Finally, in the review of Babatunde et al. (17) on neurodevelopment differences between twins and singletons, including the studies published between 2011 and 2017, five of the eight articles showed no significant difference in the neurodevelopmental outcomes between twins and singletons while two showed that singletons had better academic outcomes than twins. However, they did not conduct a meta-analysis due to the low number of the studies included and the heterogenicity of the study populations and instruments to measure the neurodevelopmental outcomes.
We decided to further investigate the impact of twin birth by considering the role of chorionicity as a risk factor for adverse neurodevelopmental outcomes. We a priori decided to exclude MC pregnancies complicated by TTTS, which represents a well-known neurological risk factor, with both donor and recipient survivors at risk for antenatally acquired brain damage. A higher risk of neurological morbidities (26) in MC pregnancies has also been reported in cases of single IUD and selective IUGR (48). However, even in the absence of these complications, the typical placental architecture in MC pregnancies may lead to a transitory cardiovascular imbalance responsible for cerebral hypoperfusion and subsequent brain damage (26, 49). Nonetheless, evidence is still scarce, as few studies have focused on twin pregnancies without complication. In our study, despite the overall similarity with singletons, after adjusting for the major comorbidities, uncomplicated MC twins at 5 years revealed a less favourable neurodevelopmental outcome in the hearing and speech and performance GMDS-ER subscales, compared with singletons; conversely, no differences emerged in the DC population compared with singletons.
Several studies previously compared MC twins with DC twins but not with singletons. Hack et al. (50) did not observe any differences in developmental outcome between uncomplicated MC infants and DC infants at 22 months of corrected age, except for a higher incidence of mild delay in hearing and language in uncomplicated MC infants. However, in this study, the mean GA of the population was higher (approximately 35 weeks) than that of our population. Similarly, Ichinomiya et al. (16) described a significant verbal disability and social impairment at 3 years in VLBW MC twins vs. VLBW DC twins, assuming that the cerebral areas associated with verbal and language functions may be vulnerable to suboptimal placental flows that are typical of the MC pregnancies. However, this difference was not observed when only MC twins without TTTS were considered. In contrast, Adegbite et al. (51) found that neurologic morbidity in MC infants at 2 years was 7-fold higher than that in DC infants; however, in this study, the TTTS, IUD, and discordant birth weight were the main determinants of neurodevelopmental impairments. Finally, in a recent study, Tosello et al. (52) found no differences in the neurodevelopmental outcome between MC, including pregnancies complicated by TTTS, and DC twins less than 34 weeks of GA (incidence of survival without neurosensory impairment >96%).
The major limitations of our work are the small sample of the MC cohort and the retrospective design of the study. The latter limited the possibility of retrieving obstetric variables that could affect the neurodevelopmental outcomes in twin pregnancies, such as information about assisted reproductive technology pregnancy, evidence and severity of placental blood flow alteration, and weight discordance fluctuation between the foetuses. In particular, we were not able to investigate the effect of selective IUGR, which is a known condition potentially affecting the neurodevelopment outcome. In addition, although we adjusted for severe comorbidities and socio-economic status, it was not possible to deepen the analysis of the family condition, as we could not gather information on the presence of other siblings and, more generally, on the home environment, which is known to play a key role in child neurodevelopment (53). Lastly, the developmental specialists performing the follow-up assessment were not blind to whether the child was a twin or a singleton and to the chorionicity of twins.
One of the main strengths of the study was the relatively large cohort of VPT and VLBW infants assessed longitudinally up to 5 years of age. Indeed, the previous studies mainly focused on short-term developmental outcomes (19, 54) that, as observed here, might not be predictive of the long-term neurodevelopmental profile in this group of infants. In addition, all infants were enrolled at the same institute, thereby limiting the confounding factors related to the potential differences in medical assistance among centres.
In conclusion, in our cohort of children born very preterm, twin birth was not associated with an increased risk of neurodevelopmental impairment compared with singletons, both at 24 months and 5 years of age. This confirms that twin birth itself is not an added risk factor for an unfavourable developmental outcome, although uncomplicated MC twins seem to have a somewhat less favourable neurodevelopmental outcome at 5 years of age supporting the need of a close neurodevelopmental follow-up. Although the present study may be useful when counselling the parents of twin foetuses at risk for preterm delivery, it is important to note that the number of infants in our MC group was too small to draw a definite conclusion on this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee Milano Area 2-Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was partially funded by the Italian Ministry of Health, Current research IRCCS.
The authors thank the preterms’ follow-up research group and paediatric rehabilitation unit for their contribution. In particular, a special thanks to Laura Gardon and Andrea Frigerio, neurodevelopmental therapists; Odoardo Picciolini, Laura Messina, and Marta Macchi, MD. Preliminary results were presented as an abstract at the Pediatric Academic Societies Meeting in 2020 and were presented as a poster at the XXVIII National Congress of the Italian Society of Neonatology in Florence (Italy) in October 2022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor GL declared a past collaboration with the author FM at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BPD, bronchopulmonary dysplasia; CP, cerebral palsy; CS, comorbidity score; cPVL, cystic periventricular leukomalacia; CRIB II, clinical risk index for babies II; DC, dichorionic; GA, gestational age; GMDS-ER, Griffiths mental development scales extended revised; GMDS-R, Griffiths mental development scales revised; GQ, general quotient; IQR, interquartile range; IVH, intraventricular haemorrhage; LoS, length of NICU stay; MC, monochorionic; NEC, necrotising enterocolitis; PHVD, posthaemorrhagic ventricular dilatation; ROP, retinopathy of prematurity; SES, socio-economic status; SD, standard deviation; SGA, small for gestational age; TTTS, twin-to-twin transfusion syndrome; VLBW, very low birth weight; VPT, very preterm.
1. Pison G, Monden C, Smits J. Twinning rates in developed countries: trends and explanations. Popul Dev Rev. (2015) 41:629–49. doi: 10.1111/j.1728-4457.2015.00088.x
2. Kulkarni AD, Jamieson DJ, Jones HW, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. (2013) 369:2218–25. doi: 10.1056/NEJMoa1301467
3. Boldrini R, Di Cesare M, Basili F, Campo G, Moroni R, Romanelli M, et al. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita—Anno 2021. Available at: www.salute.gov.it/statistiche (Accessed June 9, 2023).
4. Penna L, Salemi M, Arnofi A. Convegno: I gemelli in età pediatrica: epigenetica, epidemiologia e clinica. Istituto Superiore di Sanità. Roma, 4 e 5 ottobre 2013. Atti. Rapporti ISTISAN 14/22. Roma, Istituto Superiore di Sanità (2014).
5. Heino A, Gissler M, Hindori-Mohangoo AD, Blondel B, Klungsøyr K, Verdenik I, et al. Variations in multiple birth rates and impact on perinatal outcomes in Europe. PLoS One. (2016) 11:e0149252. doi: 10.1371/journal.pone.0149252
6. Goldenberg RL, Culhane JF, Iams JD, Romero R. Preterm birth 1 epidemiology and causes of preterm birth. Lancet. (2008) 371:10. doi: 10.1016/S0140-6736(08)60074-4
7. Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. (2009) 124:717–28. doi: 10.1542/peds.2008-2816
8. Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. Br Med J. (2012) 345:e7961. doi: 10.1136/bmj.e7961
9. Pascal A, Naulaers G, Ortibus E, Oostra A, De Coen K, Michel S, et al. Neurodevelopmental outcomes of very preterm and very-low-birthweight infants in a population-based clinical cohort with a definite perinatal treatment policy. Eur J Paediatr Neurol. (2020) 28:133–41. doi: 10.1016/j.ejpn.2020.06.007
10. Volpe JJ. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr Neurol. (2019) 95:42–66. doi: 10.1016/j.pediatrneurol.2019.02.016
11. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. (2015) 169:1162. doi: 10.1001/jamapediatrics.2015.2175
12. Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. (2008) 153:170–5.e1. doi: 10.1016/j.jpeds.2008.02.033
13. Glass TJA, Chau V, Gardiner J, Foong J, Vinall J, Zwicker JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F532–7. doi: 10.1136/archdischild-2016-312533
14. Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. (2012) 160:409–14. doi: 10.1016/j.jpeds.2011.09.009
15. Porta R, Capdevila E, Botet F, Verd S, Ginovart G, Moliner E, et al. SEN1500 network. Morbidity and mortality of very low birth weight multiples compared with singletons. J Matern Fetal Neonatal Med. (2019) 32:389–97. doi: 10.1080/14767058.2017.1379073
16. Ichinomiya K, Maruyama K, Koizumi A, Inoue F, Fukuda K, Kaburagi K, et al. Comparison of neurodevelopmental outcomes between monochorionic and dichorionic twins with birth weight ≤1500 g in Japan: a register-based cohort study. J Perinatol. (2018) 38:1407–13. doi: 10.1038/s41372-018-0190-z
17. Babatunde OA, Adebamowo SN, Ajayi IO, Adebamowo CA. Neurodevelopmental outcomes of twins compared with singleton children: a systematic review. Twin Res Hum Genet. (2018) 21:136–45. doi: 10.1017/thg.2018.3
18. Zeltzer J, Shand AW, Kelly P, Hopper JL, Scurrah KJ, Nassar N. Early birth is a key factor in educational disadvantage of twins: a data linkage study. Acta Paediatr. (2020) 109:534–40. doi: 10.1111/apa.14966
19. Squarza C, Gardon L, Giannì ML, Frigerio A, Gangi S, Porro M, et al. Neurodevelopmental outcome and adaptive behavior in preterm multiples and singletons at 1 and 2 years of corrected age. Front Psychol. (2020) 11:1653. doi: 10.3389/fpsyg.2020.01653
20. Ylijoki M, Haataja L, Lind A, Ekholm E, Lehtonen L. Neurodevelopmental outcome of preterm twins at 5 years of age. Pediatr Res. (2020) 87:1072–80. doi: 10.1038/s41390-019-0688-x
21. Bamberg C, Hecher K. Update on twin-to-twin transfusion syndrome. Best Pract Res Clin Obstet Gynaecol. (2019) 58:55–65. doi: 10.1016/j.bpobgyn.2018.12.011
22. Gheorghe CP, Boring N, Mann L, Donepudi R, Lopez SM, Chauhan SP, et al. Neonatal outcomes and maternal characteristics in monochorionic diamniotic twin pregnancies: uncomplicated versus twin-to-twin transfusion syndrome survivors after fetoscopic laser surgery. Fetal Diagn Ther. (2020) 47:165–70. doi: 10.1159/000500858
23. Hack K, Derks J, Elias S, Franx A, Roos E, Voerman S, et al. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: clinical implications of a large Dutch cohort study. BJOG. (2007) 115:58–67. doi: 10.1111/j.1471-0528.2007.01556.x
24. Oldenburg A, Rode L, Bødker B, Ersbak V, Holmskov A, Jørgensen FS, et al. Influence of chorionicity on perinatal outcome in a large cohort of Danish twin pregnancies. Ultrasound Obstet Gynecol. (2012) 39:69–74. doi: 10.1002/uog.10057
25. Townsend R, Khalil A. Fetal growth restriction in twins. Best Pract Res Clin Obstet Gynaecol. (2018) 49:79–88. doi: 10.1016/j.bpobgyn.2018.02.004
26. Lewi L, Schoubroeck DV, Gratacós E, Witters I, Timmerman D, Deprest J. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol. (2003) 15:177–94. doi: 10.1097/00001703-200304000-00013
27. Fontana C, Raffaeli G, Pesenti N, Boggini T, Cortesi V, Manzoni F, et al. Red blood cell transfusions in preterm newborns and neurodevelopmental outcomes at 2 and 5 years of age. Blood Transfus. (2022) 20:40–9. doi: 10.2450/2020.0207-20
28. Fumagalli M, Schiavolin P, Bassi L, Groppo M, Uccella S, De Carli A, et al. The impact of twin birth on early neonatal outcomes. Am J Perinatol. (2015) 33:63–70. doi: 10.1055/s-0035-1556881
29. Carroll SGM, Soothill PW, Abdel-Fattah SA, Porter H, Montague I, Kyle PM. Prediction of chorionicity in twin pregnancies at 10-14 weeks of gestation. BJOG. (2002) 109:182–6. doi: 10.1111/j.1471-0528.2002.01172.x
30. Stenhouse E, Hardwick C, Maharaj S, Webb J, Kelly T, Mackenzie FM. Chorionicity determination in twin pregnancies: how accurate are we? Ultrasound Obstet Gynecol. (2002) 19:350–2. doi: 10.1046/j.1469-0705.2002.00679.x
31. Zeve D, Regelmann MO, Holzman IR, Rapaport R. Small at birth, but how small? The definition of SGA revisited. Horm Res Paediatr. (2016) 86:357–60. doi: 10.1159/000449275
32. Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. (2003) 361:1789–91. doi: 10.1016/S0140-6736(03)13397-1
33. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. (2017) 390:1770–80. doi: 10.1016/S0140-6736(17)31002-4
34. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:7. doi: 10.1164/ajrccm.163.1.hh11-00
35. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
36. Gole GA, Ells AL, Katz X, Holmstrom G, Fielder AR, Capone A, et al. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123:991. doi: 10.1001/archopht.123.7.991
37. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–43. doi: 10.1016/s0022-3476(78)80282-0
39. Battaglia F, Savoin M. GMDS-R: Griffiths mental development scales, revised: 0–2 years. Italian edition. Firenze: Giunti O.S., Organizzazioni speciali (2007). p. 134.
40. Cianchetti C, Fancello GS. GMDS-ER: Griffiths mental development scales, extended revised: 2 -8 anni: manuale di somministrazione / D. Luiz et al.; edizione italiana a cura di Carlo Cianchetti e Giuseppina Sannio Fancello, Oxford, United Kingdom: Giunti O.S. Firenze (2007). p. 106.
41. Griffiths R, Huntley M. The Griffiths mental development scales revised manual: from birth to 2 years. High Wycombe: ARICD (1996).
42. R Core Team (2021). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2021). Available at: https://www.R-project.org/ (Accessed January 31, 2023).
43. Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient–based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. (2016) 79:22–8. doi: 10.1016/j.jclinepi.2016.03.031
44. Gnanendran L, Bajuk B, Oei J, Lui K, Abdel-Latif ME. Neurodevelopmental outcomes of preterm singletons, twins and higher-order gestations: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F106–14. doi: 10.1136/archdischild-2013-305677
45. Bodeau-Livinec F, Zeitlin J, Blondel B, Arnaud C, Fresson J, Burguet A, et al. Do very preterm twins and singletons differ in their neurodevelopment at 5 years of age? Arch Dis Child Fetal Neonatal Ed. (2013) 98:F480–7. doi: 10.1136/archdischild-2013-303737
46. Lorenz JM. Neurodevelopmental outcomes of twins. Semin Perinatol. (2012) 36:201–12. doi: 10.1053/j.semperi.2012.02.005
47. Kalikkot Thekkeveedu R, Dankhara N, Desai J, Klar AL, Patel J. Outcomes of multiple gestation births compared to singleton: analysis of multicenter KID database. Matern Health Neonatol Perinatol. (2021) 7:15. doi: 10.1186/s40748-021-00135-5
48. Spruijt MS, Lopriore E, Steggerda SJ, Slaghekke F, Van Klink JMM. Twin-twin transfusion syndrome in the era of fetoscopic laser surgery: antenatal management, neonatal outcome and beyond. Expert Rev Hematol. (2020) 13:259–67. doi: 10.1080/17474086.2020.1720643
49. Khalil A. Call to action: long-term neurodevelopment in monochorionic twins. Ultrasound Obstet Gynecol. (2021) 58:5–10. doi: 10.1002/uog.23591
50. Hack KEA, Koopman-Esseboom C, Derks JB, Elias SG, de Kleine MJK, Baerts W, et al. Long-term neurodevelopmental outcome of monochorionic and matched dichorionic twins. PLoS One. (2009) 4:e6815. doi: 10.1371/journal.pone.0006815
51. Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. (2004) 190:156–63. doi: 10.1016/j.ajog.2003.07.004
52. Tosello B, Garbi A, Blanc J, Lorthe E, Foix-L’Hélias L, D’Ercole C, et al. The impact of chorionicity on pregnancy outcome and neurodevelopment at 2 years old among twins born preterm: the EPIPAGE-2 cohort study. BJOG. (2021) 128:281–91. doi: 10.1111/1471-0528.16170
53. Bush NR, Wakschlag LS, LeWinn KZ, Hertz-Picciotto I, Nozadi SS, Pieper S, et al. Family environment, neurodevelopmental risk, and the environmental influences on child health outcomes (ECHO) initiative: looking back and moving forward. Front Psychiatry. (2020) 11:547. doi: 10.3389/fpsyt.2020.00547
Keywords: twins, monochorionic, dichorionic, prematurity, neurodevelopmental outcome
Citation: Fontana C, Schiavolin P, Ardemani G, Amerotti DA, Pesenti N, Bonfanti C, Boggini T, Gangi S, Porro M, Squarza C, Giannì ML, Persico N, Mosca F and Fumagalli M (2023) To be born twin: effects on long-term neurodevelopment of very preterm infants—a cohort study. Front. Pediatr. 11:1217650. doi: 10.3389/fped.2023.1217650
Received: 5 May 2023; Accepted: 26 June 2023;
Published: 17 July 2023.
Edited by:
Gianluca Lista, Ospedale dei Bambini Vittore Buzzi, ItalyReviewed by:
Katsusuke Ozawa, National Center for Child Health and Development (NCCHD), Japan© 2023 Fontana, Schiavolin, Ardemani, Amerotti, Pesenti, Bonfanti, Boggini, Gangi, Porro, Squarza, Giannì, Persico, Mosca and Fumagalli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Ardemani Z2l1bGlhLmFyZGVtYW5pQHVuaW1pLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.