- 1Department of Clinical Pharmacy, King Fahad Medical City Hospital, Riyadh, Saudi Arabia
- 2Department of Neonatal Intensive Care Unit, Maternity and Children Hospital, Dammam, Saudi Arabia
- 3Department of Pharmacy, Maternity and Children Hospital, Ministry of Health, Jouf, Saudi Arabia
- 4Department of Medicine, Ministry of Health, Abha, Saudi Arabia
- 5Department of Medicine, Ministry of Health, Khamis Mushait, Saudi Arabia
- 6Department of Medicine, King Khaled University, Abha, Saudi Arabia
- 7College of Medicine, King Khalid University, Al Fara, Saudi Arabia
- 8Department of Pharmacy, Dr. Sulaiman Al-Habib Hospital, Khobar, Saudi Arabia
- 9Department of Nursing Office, Hail General Hospital, Hail, Saudi Arabia
- 10Department of Pharmacy, Al-Dawaa Medical Services Company Limited, Unaizah, Saudi Arabia
- 11Department of Pharmacy, Qassim University Medical City Hospital, Buraidah, Saudi Arabia
Background: Neonatal early onset sepsis (NEOS) is a serious and potentially life-threatening condition affecting newborns within the first few days of life. While the diagnosis of NEOS was based on clinical signs and symptoms in the past, recent years have seen growing interest in identifying specific diagnostic factors and optimizing therapy outcomes. This study aims to investigate the diagnostic and risk factors and therapy outcomes of neonatal EOS in ICU patients in Saudi Arabia, with the goal of improving the management of neonatal EOS in the country.
Methods: This method outlines the protocol development, search strategy, study selection, and data collection process for a systematic review on neonatal early onset sepsis in Saudi Arabian ICU patients, following the PRISMA 2020 guidelines. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is a well-established guideline that provides a framework for conducting systematic reviews and meta-analyses in a transparent and standardized manner. It aims to improve the quality and reporting of such research by ensuring clear and comprehensive reporting of study methods, results, and interpretations. The search strategy included electronic databases (PubMed, Embase, Google Scholar, Science Direct, and the Cochrane Library) and manual search of relevant studies, and data were extracted using a standardized form.
Results: The systematic review included 21 studies on neonatal sepsis in Saudi Arabia, with varying study designs, sample sizes, and prevalence rates of sepsis. Group B streptococcus and E. coli were the most commonly isolated pathogens. Various diagnostic factors and risk factors were reported, including hematological parameters, biomarkers, and blood cultures. The quality of the included studies was assessed using the Newcastle-Ottawa Scale and Joanna Briggs Institute critical checklist.
Conclusions: The review identified a number of risk and diagnostic factors and therapy outcomes for neonatal sepsis. However, most of the studies were having small scale cohort groups. Further research with controlled study designs is needed to develop effective prevention and management strategies for neonatal sepsis in Saudi Arabia.
1. Introduction
Neonatal early onset sepsis (NEOS) is a serious and potentially life-threatening condition that affects newborns within the first few days of life (1, 2). It is characterized by systemic infection and inflammation, which can lead to septic shock, multiple organ failure, and death if not promptly and effectively treated (3, 4). The pathophysiology predominantly involves ascending colonisation of the uterine cavity and maternal vaginal tract by the typical bacterial flora of the genitourinary and gastrointestinal tracts, which leads to eventual colonisation and infection of the foetus or newborn (5). NEOS is a major health concern worldwide, with an estimated incidence of 0.5–1.5 cases per 1,000 live births in developed countries, and up to 5 cases per 1,000 live births in low- and middle-income countries (LMICs) (6, 7). In 3%–4% of newborns who have NEOS, there may be serious systemic disease and even deaths are possible outcomes (8, 9).
In the past, the diagnosis of NEOS was based on clinical signs and symptoms, such as fever, lethargy, poor feeding, and respiratory distress, and was often treated empirically with broad-spectrum antibiotics (1, 10, 11). However, this approach led to overuse of antibiotics, increased healthcare costs, and the emergence of antibiotic-resistant bacteria (12). In recent years, there has been growing interest in identifying specific diagnostic factors that can accurately predict the presence or absence of NEOS, and in optimizing therapy outcomes through targeted antibiotic therapy and supportive measures (13, 14). The risk factors for NEOS include maternal factors such as maternal fever, chorioamnionitis, and prolonged rupture of membranes, as well as neonatal factors such as low birth weight, prematurity, and invasive procedures (2, 4, 15–17). In addition, laboratory parameters such as elevated C-reactive protein (CRP) levels, low platelet count, and abnormal white blood cell (WBC) count have been shown to be useful in predicting the presence of NEOS (13, 14).
Despite the availability of diagnostic tools and risk stratification algorithms, the management of neonatal EOS remains challenging, particularly in neonatal intensive care unit (NICU) patients (18). Guidelines for the prevention of NEOS are provided by the Centers for Disease Control and Prevention (CDC), the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists, and provide algorithms for the diagnosis and management of high risk NEOS patients (19, 20). These recommendations are based on epidemiological data collected prior to the widespread use of intrapartum antibiotic prophylaxis in obstetrics, when NEOS incidence was 5–10-fold greater than presently found (21–23). These recommendations lead to a significant number (15%–20%) of term and late preterm babies being examined for sepsis and getting 5%–8% of empirical antibiotics (24). The optimal duration of antibiotic therapy, the choice of antibiotics, and the use of adjunctive therapies such as intravenous immunoglobulin (IVIG) and granulocyte-colony stimulating factor (G-CSF) remain topics of debate (25, 26).
Saudi Arabia is a high-income country with a well-established healthcare system, including specialized NICUs. However, the incidence and outcomes of neonatal EOS in Saudi Arabia, along with other Gulf countries, remain insufficiently characterized (27, 28). Nevertheless, NEOS is a major health concern in neonatal intensive care units (NICUs) in Saudi Arabia. Identifying diagnostic factors and optimizing therapy outcomes are crucial for the management of EOS in these patients. This study aims to investigate the diagnostic factors and therapy outcomes of neonatal EOS in ICU patients in Saudi Arabia. The study explores the clinical presentation of EOS, laboratory parameters, and risk factors associated with the development of EOS. The findings of this study will contribute to improving the management of neonatal EOS in Saudi Arabia and provide insight into optimizing therapy outcomes for this vulnerable patient population.
2. Materials and methods
2.1. Protocol development
The methodology for this study on followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines to ensure transparent reporting and reduce bias in the study. PRISMA is a well-established guideline that provides a framework for conducting systematic reviews and meta-analyses in a transparent and standardized manner. It aims to improve the quality and reporting of such research by ensuring clear and comprehensive reporting of study methods, results, and interpretations. The study protocol was developed before the commencement of the study, outlining the research question, search strategy, inclusion and exclusion criteria, data collection and analysis methods, and ethical considerations.
2.2. Search strategy
A comprehensive search of electronic databases such as PubMed, Embase, Google Scholar, Science Direct, and the Cochrane Library was conducted to identify studies relevant to the research question. The search included keywords such as “neonatal sepsis,” “early onset sepsis,” “ICU patients,” “diagnostic factors,” “risk factors,” and “therapy outcomes.” The search strategy also include MeSH terms and keywords related to neonatal EOS, ICU patients, and Saudi Arabia. A manual search of the reference lists of relevant studies was also performed. The search strategy was designed by two authors who are expert in this field and reviewed by another research team among co-authors. The details are given in Supplementary Table S1 as supplementary file.
2.3. Study selection
Two independent reviewers screened the titles and abstracts of the identified studies to determine their eligibility for inclusion. The full texts of potentially relevant studies were assessed for eligibility based on predefined inclusion and exclusion criteria. Studies that meet the following criteria were included in the study: (1) conducted in Saudi Arabia, (2) neonates diagnosed with early onset sepsis, (3) admitted to ICU, (4) diagnostic and risk factors and therapy outcomes reported, and (5) observational or interventional studies. Studies that did not meet the inclusion criteria were excluded. Any discrepancies between the reviewers were resolved through discussion and consensus.
2.4. Data collection
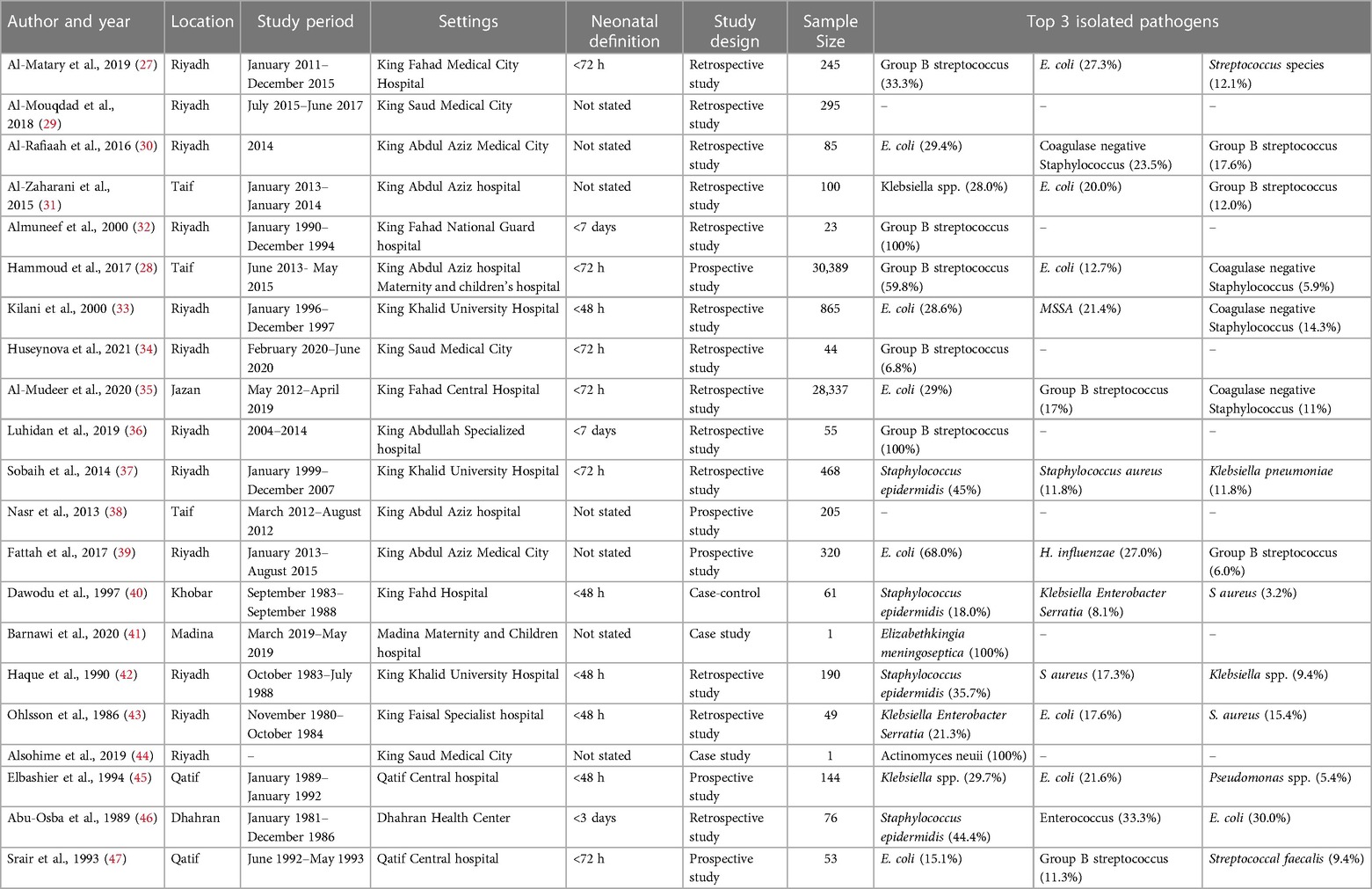
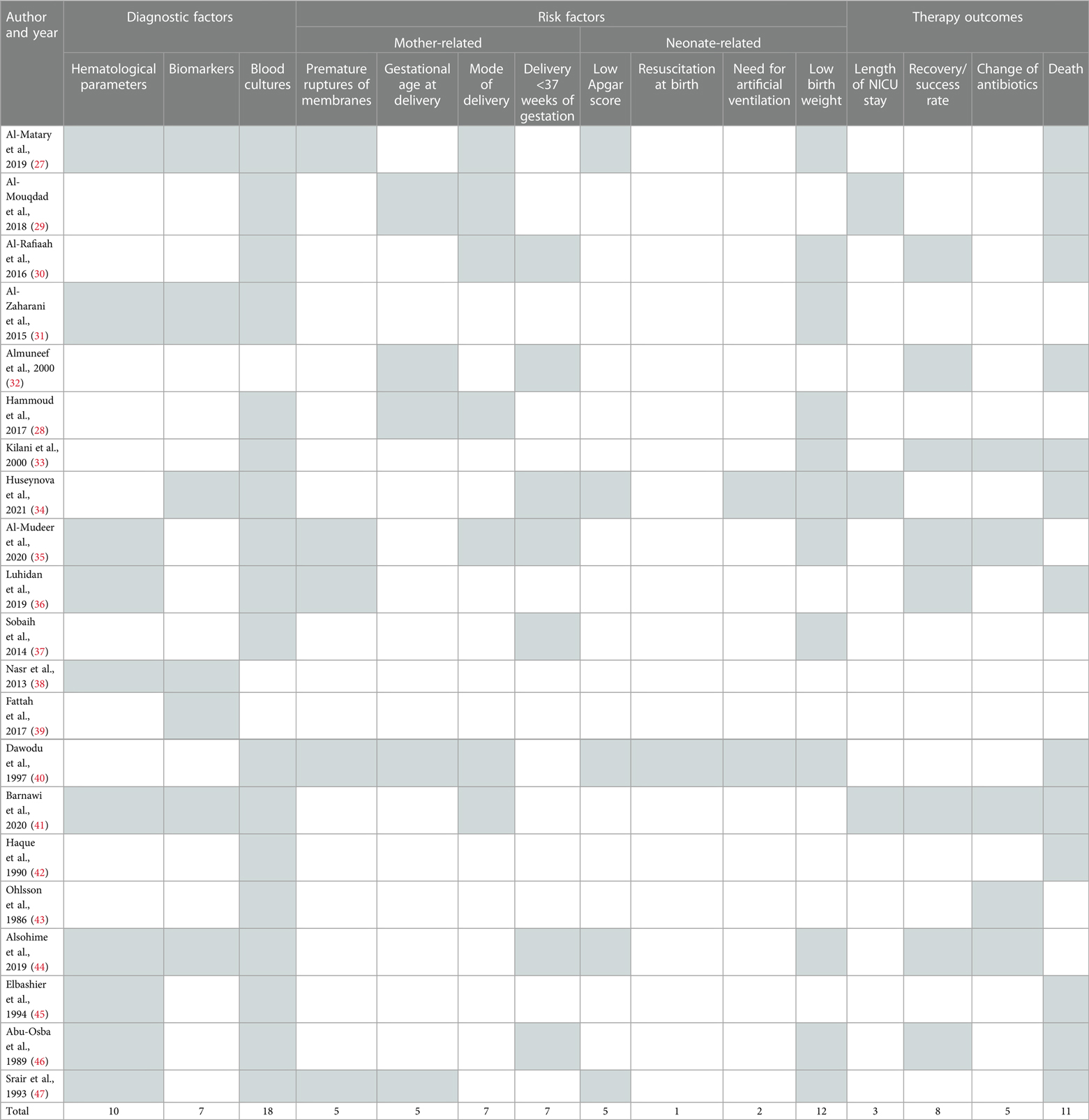
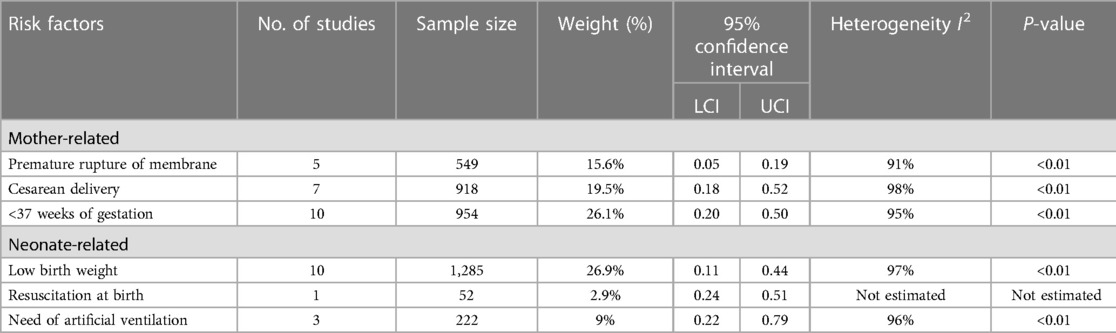
Data were extracted from the eligible studies using a standardized data extraction form. The data in Table 1 included information on author and year, location, study period and settings, NEOS definition, study design, sample size and microbiological data on common causative pathogens. In Table 2, information was included related to diagnostic factors (hematological parameters, biomarkers, blood cultures), mother-related risk factors (premature ruptures of membranes, gestational age at delivery, mode of delivery and delivery <37 weeks of gestation), neonate-related risk factor (low apgar score, resuscitation at birth, need for artificial ventilation, low birth weight) and therapy outcome parameters (length of nicu stay, recovery/success rate, change of antibiotics and death). This review was performed between January and April 2023.
2.5. Meta-analysis
The statistical analysis was performed using RStudio software (RStudio Team, Boston, MA; version R, 2023.03.0) using Meta Package and Metaprop command. The proportion test was performed to estimate the proportion of risk factors. I-squared (I2) index statistic was employed to evaluate the assertiveness heterogeneity in choice of effects, considering the randomized effect for the analysis. A threshold of 0.05 was considered significant.
3. Results
3.1. Included studies
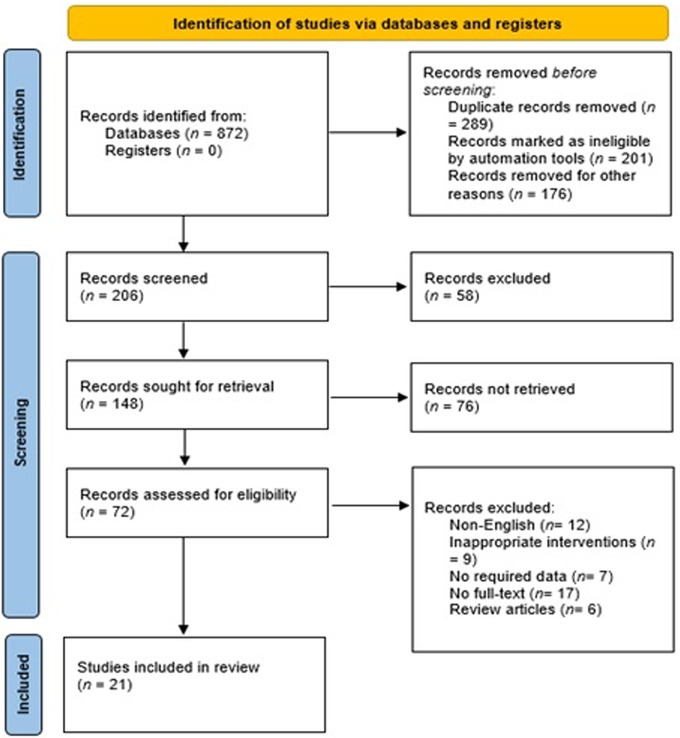
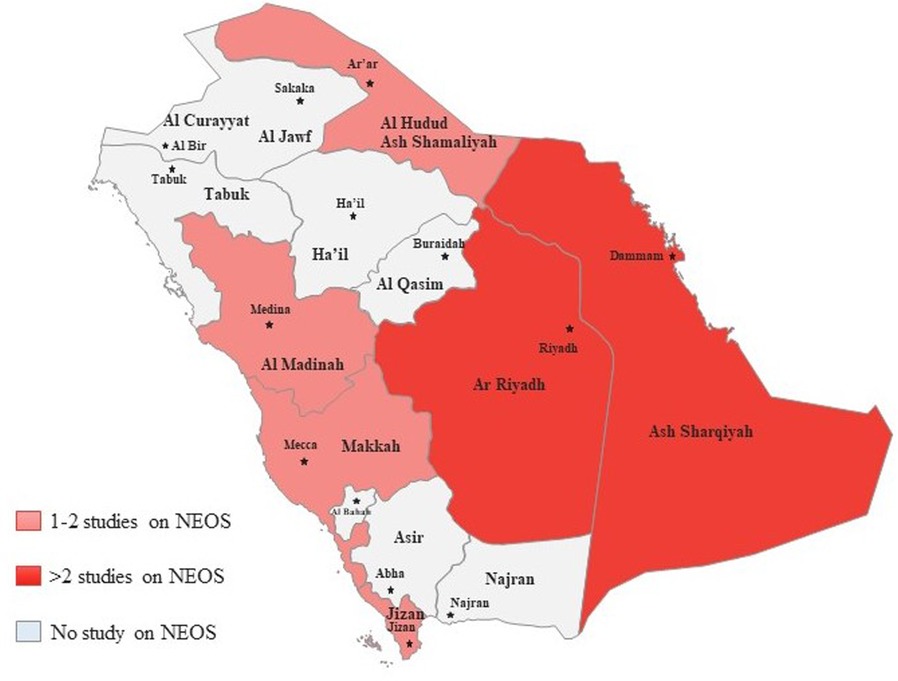
The PRISMA 2020 flow diagram is shown in Figure 1 which is a graphical representation of the systematic review process. It outlines the steps involved in identifying, screening, and selecting studies for inclusion in a review. In this particular study, the review included searches of databases only. The diagram starts with the identification of studies via databases and registers, where 872 records were identified from databases and none from registers. After identifying the records, the next step was to remove duplicate records, which accounted for 289 records. In addition, 201 records were marked as ineligible by automation tools, and 176 records were excluded based on the initial screening of titles and abstracts. These studies did not meet the predefined inclusion criteria for our systematic review. The reasons for exclusion at this stage included irrelevant topics, unrelated interventions, and studies conducted in settings other than Saudi Arabia. Of the remaining 206 screened records, 58 were excluded further during the full-text assessment phase. These exclusions were due to the identification of commentaries, guidelines, and book chapters, which were not eligible for inclusion in our systematic review. The remaining 148 records were sought for retrieval, but 76 records were not retrieved. The 72 retrieved records were assessed for eligibility, where 12 non-English studies, 9 studies with inappropriate interventions, 7 studies with no required data, 17 studies with no full-text, and 6 review articles were excluded. Finally, 21 studies were included in the review. These studies met the eligibility criteria and were included in the systematic review. Figure 2 showed that most of the studies were conducted from Riyadh and eastern provinces of KSA.
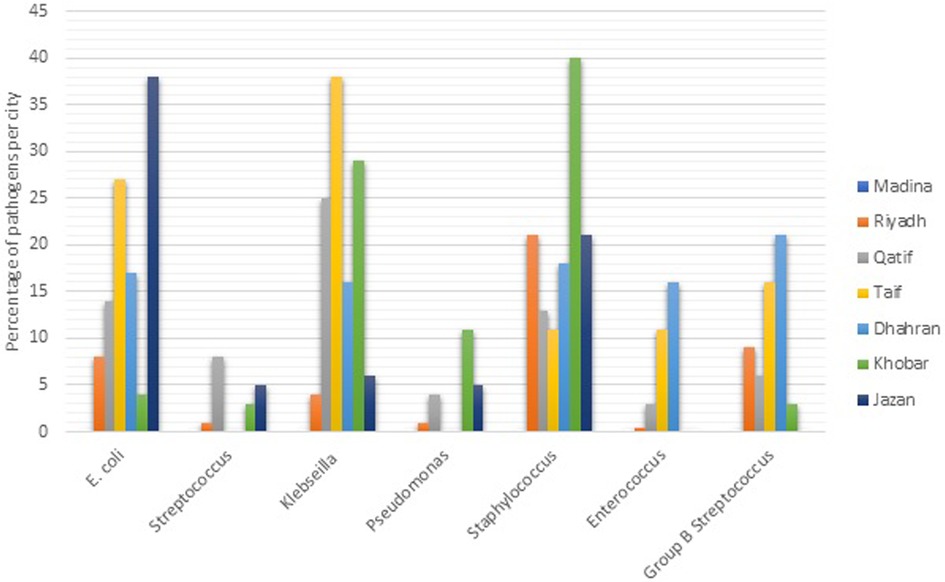
The studies used different study designs, including retrospective, prospective, case-control, and case studies, and varied in their neonatal definitions of NEOS, study period, and sample sizes. The Table 1 included the results of 21 different studies on neonatal sepsis conducted in different locations and settings in Saudi Arabia. The studies varied in terms of study design, sample size, and prevalence of neonatal sepsis (NOS). The sample sizes of the studies varied widely, ranging from 1 in case study to 30,389 in prospective study. The larger sample sizes were reported by Al-Mudeer et al. (2020) and Hammoud et al. (2017), both of which had over 28,000 participants. The studies were conducted in different settings, including medical centers, hospitals, and maternity and children's hospitals. The top three isolated pathogens varied across the studies. Group B streptococcus (GBS) and E. coli were the most commonly isolated pathogens, with GBS being the most commonly isolated pathogen in 5 of the 21 studies. Other pathogens that were commonly isolated include Coagulase negative Staphylococcus (CNS), Klebsiella spp., and Staphylococcus epidermidis. The distribution of pathogens in different cities of KSA is presented in Figure 3.
The present study also aimed to investigate the diagnostic and risk factors and therapy outcomes of NEOS in ICU patients in Saudi Arabia as shown in Table 2. The analysis of the results obtained from the 21 selected studies revealed that various factors were used for the diagnosis of EOS, including hematological parameters, biomarkers and blood cultures, The use of hematological parameters, such as white blood cell count, absolute neutrophil count, and immature to total neutrophil ratio, was reported by some studies as a diagnostic factor for EOS. In contrast, biomarkers such as C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) were reported by several studies as useful tools for the diagnosis of EOS. Blood cultures are considered the gold standard for the diagnosis of NEOS and several studies reported that blood cultures were used as a diagnostic factor for NEOS. Regarding the mother and neonate related risk factors, gestational age at delivery was reported as a significant risk factor for NEOS by some studies. Preterm delivery is known to increase the risk of NEOS due to the immature immune system of preterm infants. Moreover, premature rupture of membranes was reported by some studies as a significant risk factor for EOS. Low Apgar score at birth, need for artificial ventilation, and low birth weight were also reported by some studies as significant risk factors for NEOS. The length of NICU stay was reported as a neonate-related risk factor by some studies. Mode of delivery including vaginal delivery and caesarean section was reported by some studies as a significant risk factor for NEOS. In terms of therapy outcomes, the recovery or success rate of therapy was reported by several studies. The change of antibiotics during treatment was reported by some studies. The overall death rate was reported by most of the studies. Nevertheless, the present study revealed that there is heterogeneity among the diagnostic factors and risk factors reported by the selected studies. This heterogeneity may be due to differences in the study population.
3.2. Meta-analysis
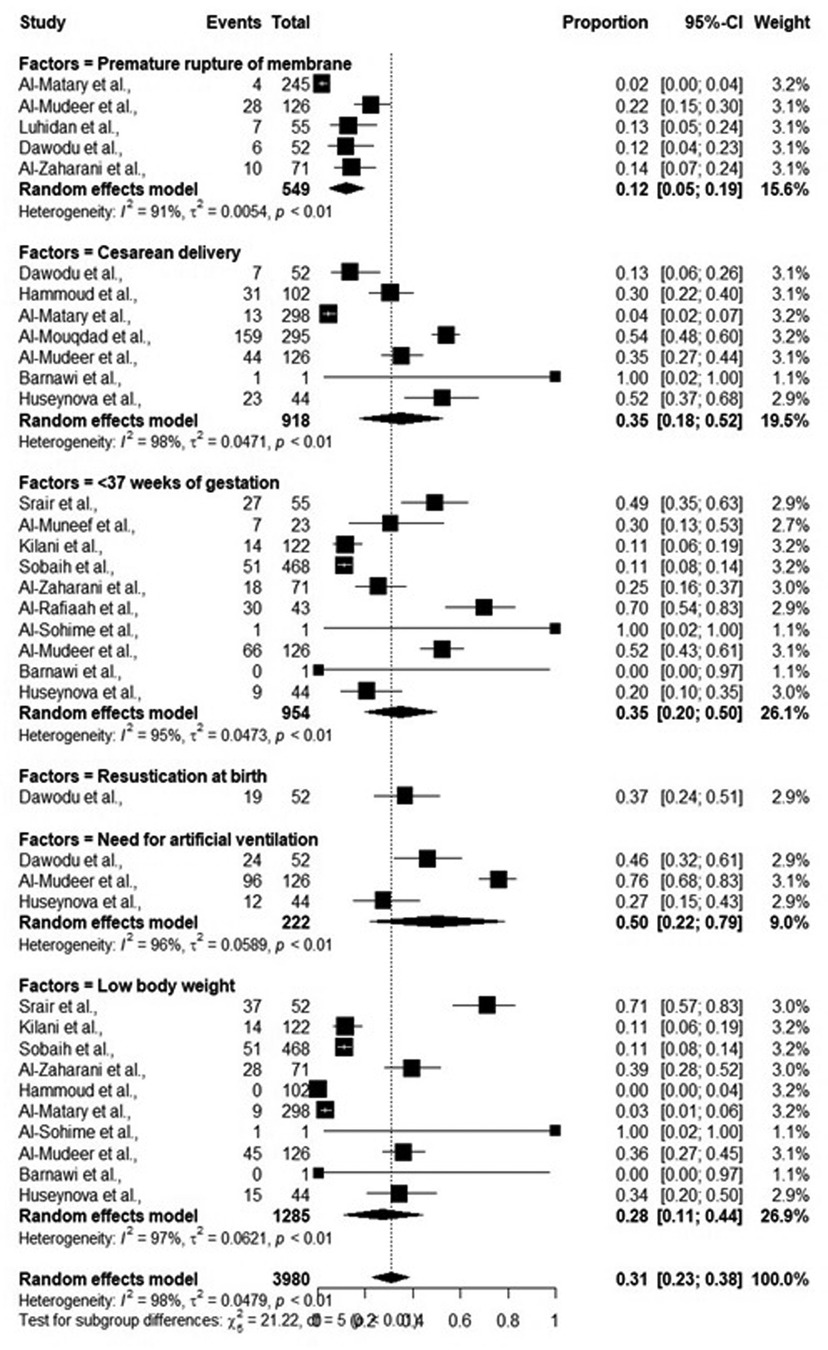
While performing meta-analysis, significant heterogeneity between included studies, as shown by I2 (>90%) was observed, thus all the data were analyzed following random effect models. Overall, the risk factors including mother-related and neonate-related increased the chances of early onset sepsis (EOS). Most of the studies reported that pre-term neonates (95% CI: 0.20–0.50) and low birth weight neonates (95% CI: 0.11–0.44) were at elevated risk of EOS. The detailed results are shown in Table 3 and Figure 4.
3.3. Quality assessment
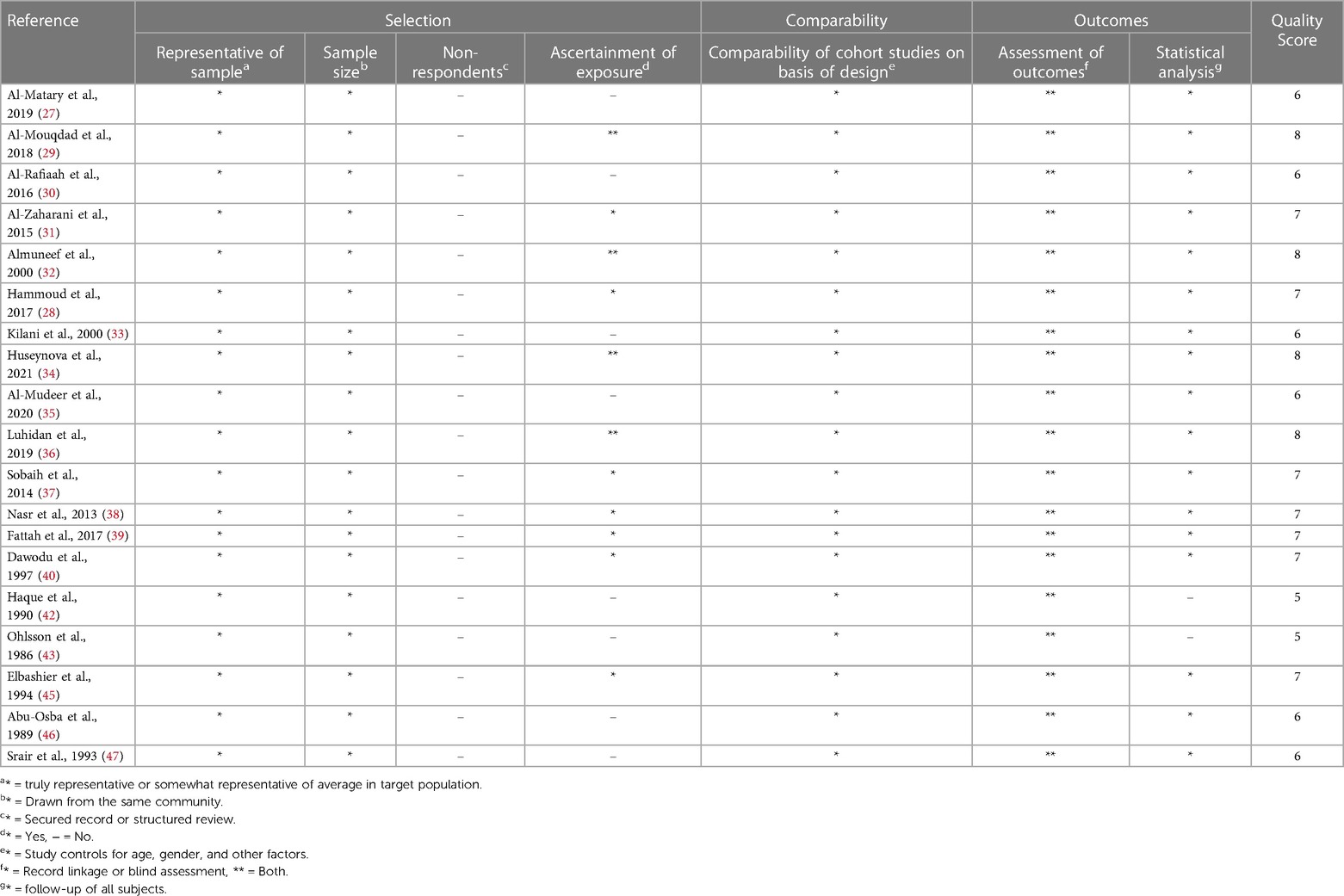
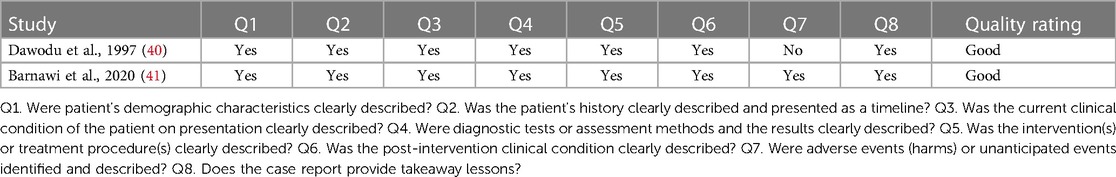
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies and Joanna briggs Institute (JBI) critical checklist for case study. For cohort studies, the NOS was used to assess the methodological quality of each study. This scale evaluates the risk of bias in three domains: selection of study groups, comparability of groups, and the ascertainment of exposure/outcome. Each study is awarded a maximum of nine stars based on its quality in these three domains, with higher scores indicating lower risk of bias. This checklist includes criteria such as clear identification of the problem, description of the context, adequate data analysis, and appropriate conclusions. Each study was evaluated against these criteria to determine its methodological quality any discrepancies between the reviewers were resolved through discussion and consensus. The details are mentioned in Tables 4, 5.
4. Discussion
This systematic review is one of the broadest studies to show the pattern, risk and diagnostic factors, and clinical outcome of newborn with NEOS from Saudi Arabia. Findings of 21 studies were included which are consistent with observations from other research indicating the proportion of NEOS is significantly higher (48, 49). The risk factors identified are similar to other previous studies, focusing on the significance of premature rupture of membrane, premature birth, and low birth weight. According to a study, NEOS is more common in newborns with extremely low birth weights, decreased respiratory function at delivery, and mother related risk factors (50). These results may be explained by preterm babies' weak innate immune system, which increase their risk of developing NEOS (5). NEOS develops in the uterus as a result of maternal blood infection or, more frequently because of infection of the placenta and the amniotic fluid (51). Patients may present with fetal distress, pneumonia, newborn asphyxia or sepsis owing to aspiration of contaminated amniotic fluid or secretions after delivery (52). A comprehensive study looked at whether newborn clinical presentation might be used to rule out EOS in babies born to moms who had chorioamnionitis. According to the investigation, EOS can happen in infants who had initially positive clinical status (53). A range of services are available in different healthcare settings included in this review, to manage high-risk newborns who are prone to infection. Nevertheless, in our review, we discovered a variety of variables, including a low Apgar score, in a sizable number of neonates as a risk factor for NEOS. This finding is consistent with other studies that revealed a low Apgar score to be strongly related with newborn sepsis (48, 49, 54). Low Apgar ratings in newborns make them more vulnerable to infection because they are less able to handle stress from outside sources (55). Furthermore, the findings revealed that NEOS patients had low birth weights, which is consistent with a finding from other research (56). The leading maternal risk factors for NEOS were found to be multiparty and caesarean birth. This was also the main maternal risk factor for NEOS according to a research by Al Dasoky et al. in a tertiary care hospital in Jordan (57). A valuable measure of the health of the newborn and the severity of the illness is the total WBC count. Our review revealed that the patients' WBC counts were disturbed which is a sign of a severe illness in the newborn (58). The fact that NEOS occurs while the mother and child are still confined to the hospital and that there is a significant risk of hospital acquired infection (59).
In developed and underdeveloped countries, different pathogens are responsible for causing newborn sepsis including both Gram-negative and positive bacteria (60). Nonetheless, the present initiatives for maternal intrapartum antibiotic prophylaxis against GBS may be responsible for the more prevalence of Gram-negative microbes (61). A variety of pathogens are responsible for neonatal EOS, depending on multiple factors, such as hospital practices, geographical region, and changes in microbial resistance pattern. Overall, Group B streptococcus and E. coli are the leading pathogens for EOS. However, the patterns of pathogens may vary from state to state. According to our findings, GBS was the most prevalent etiologic agent in NEOS, followed by E. coli. Our study reported that Staphylococcus species are responsible for EOS in Khobar, E. coli in Jazan and Klebsiella spp. in Taif. Stoll et al., reported that E. coli associated sepsis was increased from 3.2 to 6.8 per 1,000 live births. Likewise, the most frequently identified agent in NEOS in European countries is GBS, followed by Gram-negative bacilli and staphylococci (62). Moreover, GBS, staphylococci, gram-negative bacteria, and candida were identified in NEOS patients in other regions as well (6, 7, 9, 16, 49). According to earlier research that is in line with our results, infant sepsis mortality is also very high and had a mortality rate for NEIOS 10%–20% (63). Neonates are more susceptible to infections in ICU settings because of their immature immune system, and exposure to invasive medical procedures.
To the best our knowledge, this is first systematic review carried out from the KSA and is notable because it represents a comprehensive complete database evaluated to identify the pattern, diagnostic and risk factors, and therapy outcomes of NEOS in different healthcare institutes in KSA. It is crucial to undertake more controlled research studies since neonatal safety is so important. Incidence or prevalence of NEOS in the KSA population must be quantified. Moreover, larger trials are necessary to assess the safety and effectiveness of antibiotic therapy. However, no adverse drug reactions were seen, indicating that even if a negative effect existed, it would likely be rare and would need to be considered in relation to the unfavourable consequences of sepsis assessments and antibiotic exposure. Prior research studies from other parts of world have demonstrated the need of clinical monitoring as a component of any NEOS approach by showing that GBS-specific NEOS persists in children delivered to moms who screen erroneously negative for GBS without additional intrapartum risk factors for EOS (64–66). Nonetheless, this systematic review provides a valuable baseline data to start in KSA. Future studies could explore further questions.
5. Conclusions
This systematic review highlighted the various substantial risk factors associated with development of neonatal early onset sepsis in ICU patients. The prevalence of EOS is high in neonates admitted to ICU with multiple maternal and neonatal risk factors. Therefore, by identifying such factors, the healthcare experts can implement targeted preventive strategies and monitor these risk factors, thus reducing the prevalence of EOS and improving the therapeutic outcomes. Moreover, high-quality studies and better diagnostics are required especially in hospitals with high neonatal mortality to evaluate the prevalence of EOS, identify the associated risk factors and clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
RA was employed by Al-Dawaa Medical Services Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1206389/full#supplementary-material
References
1. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. (2014) 27(1):21–47. doi: 10.1128/CMR.00031-13
2. Chacko B, Sohi I. Early onset neonatal sepsis. Indian J Pediatr. (2005) 72(1):23–6. doi: 10.1007/BF02760574
3. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. (2002) 347(4):240–7. doi: 10.1056/NEJMoa012657
4. Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. (2000) 105(1):21–6. doi: 10.1542/peds.105.1.21
5. Glaser MA, Hughes LM, Jnah A, Newberry D, Harris-Haman PA. Neonatal sepsis: a review of pathophysiology and current management strategies. Adv Neonatal Care. (2021) 21(1):49–60. doi: 10.1097/ANC.0000000000000769
6. Braye K, et al. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006-2016. PloS One. (2019) 14(4):e0214298. doi: 10.1371/journal.pone.0214298
7. Joshi NS, Huynh K, Lu T, Lee HC, Frymoyer A. Epidemiology and trends in neonatal early onset sepsis in California, 2010–2017. J Perinatol. (2022) 42(7):940–6. doi: 10.1038/s41372-022-01393-7
8. Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. (2011) 30(11):937. doi: 10.1097/INF.0b013e318223bad2
9. Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. (2011) 127(5):817–26. doi: 10.1542/peds.2010-2217
10. Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis using a hematologic scoring system. J Pediatr. (1988) 112(5):761–7. doi: 10.1016/S0022-3476(88)80699-1
11. Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. (2001) 107(1):97–104. doi: 10.1542/peds.107.1.97
12. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. (2019) 12:3903–10. doi: 10.2147/IDR.S234610
13. Stocker M, van Herk W, El Helou S, Dutta S, Schuerman FA, van den Tooren-de Groot RK, et al. C-reactive protein, procalcitonin, and white blood count to rule out neonatal early-onset sepsis within 36h: a secondary analysis of the neonatal procalcitonin intervention study. Clin Infect Dis. (2021) 73(2):e383–90. doi: 10.1093/cid/ciaa876
14. Snoek L, van Kassel MN, Krommenhoek JF, Achten NB, Plötz FB, van Sorge NM, et al. Neonatal early-onset infections: comparing the sensitivity of the neonatal early-onset sepsis calculator to the Dutch and the updated NICE guidelines in an observational cohort of culture-positive cases. EClinicalMedicine. (2022) 44. doi: 10.1016/j.eclinm.2021.101270
15. Oddie S, Embleton ND. Risk factors for early onset neonatal group B streptococcal sepsis: case-control study. Br Med J. (2002) 325(7359):308. doi: 10.1136/bmj.325.7359.308
16. Klinger G, Levy I, Sirota L, Boyko V, Reichman B, Lerner-Geva L, et al. Epidemiology and risk factors for early onset sepsis among very-low-birthweight infants. Am J Obstet Gynecol. (2009) 201(1):38.e1–6. doi: 10.1016/j.ajog.2009.03.006
17. Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the national institute of child health and human development neonatal research network, 2002–2003. Pediatr Infect Dis J. (2005) 24(7):635–9. doi: 10.1097/01.inf.0000168749.82105.64
18. Fileccia L, Wood T, Guthrie A, Ronoh C, Sleeth C, Kamath-Rayne BD, et al. Comparison of early-onset sepsis risk-stratification algorithms in neonates in a Kenyan nursery. Hosp Pediatr. (2022) 12(10):876–84. doi: 10.1542/hpeds.2021-006228
19. American College of Obstetricians and Gynecologists. ACOG committee opinion No. 485. Prevention of earlyonset group B streptococcal disease in newborns. Obstet Gynecol. (2011) 117(4):1019–27. doi: 10.1097/AOG.0b013e318219229b
20. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep. (2010) 59(RR-10):1–36.21088663
21. Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics. (2010) 125(5):e1031–8. doi: 10.1542/peds.2009-1573
22. Moore MR, Schrag SJ, Schuchat A. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect Dis. (2003) 3(4):201–13. doi: 10.1016/S1473-3099(03)00577-2
23. Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. (2005) 116(3):595–602. doi: 10.1542/peds.2005-0552
24. Kuzniewicz MW, Walsh EM, Li S, Fischer A, Escobar GJ. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt Comm J Qual Patient Saf. (2016) 42(5):232–9. doi: 10.1016/S1553-7250(16)42030-1
25. Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. (2012) 129(5):1006–15. doi: 10.1542/peds.2012-0541
26. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. (2017) 43(3):304–77. doi: 10.1007/s00134-017-4683-6
27. Al-Matary A, Heena H, AlSarheed AS, Ouda W, AlShahrani DA, Wani TA, et al. Characteristics of neonatal sepsis at a tertiary care hospital in Saudi Arabia. J Infect Public Health. (2019) 12(5):666–72. doi: 10.1016/j.jiph.2019.03.007
28. Hammoud MS, Al-Taiar A, Al-Abdi SY, Bozaid H, Khan A, AlMuhairi LM, et al. Culture-proven early-onset neonatal sepsis in Arab states in the gulf region: two-year prospective study. Int J Infect Dis. (2017) 55:11–5. doi: 10.1016/j.ijid.2016.12.006
29. Al-Mouqdad MM, Aljobair F, Alaklobi FA, Taha MY, Abdelrahim A, Asfour SS, et al. The consequences of prolonged duration of antibiotics in premature infants with suspected sepsis in a large tertiary referral hospital: a retrospective cohort study. Int J Pediatr Adolesc Med. (2018) 5(3):110–5. doi: 10.1016/j.ijpam.2018.08.003
30. Alrafiaah AS, Al Shaalan M, Alshammari FO, Almohaisani AA, Bawazir AS, Omair A, et al. Neonatal sepsis in a tertiary care hospital in Saudi Arabia. Int J Adv Res. (2016) 4:1713–20. doi: 10.21474/IJAR01/2265
31. Al-Zahrani AK, Ghonaim MM, Hussein YM, Eed EM, Khalifa AS, Dorgham LS, et al. Evaluation of recent methods versus conventional methods for diagnosis of early-onset neonatal sepsis. J Infect Dev Ctries. (2015) 9(04):388–93. doi: 10.3855/jidc.5950
32. Almuneef M, Alalola S, Ahmed S, Memish Z, Khan MY, Alshaalan M, et al. The changing spectrum of group B streptococcal (GBS) infection in infants of Saudi Arabia. J Chemother. (2000) 12(1):48–52. doi: 10.1179/joc.2000.12.1.48
33. Kilani RA, Basamad M. Pattern of proven bacterial sepsis in a neonatal intensive care unit in Riyadh-Saudi Arabia: a 2-year analysis. J Med Liban. (2000) 48(2):77–83. 11028154.11028154
34. Huseynova R, Mahmoud LB, Aljobair FH, Huseynov O, Jaganathan PP, Abdelrahim A, et al. Use of early-onset sepsis risk calculator for neonates ≥34 weeks in a large tertiary neonatal centre, Saudi Arabia. Cureus. (2021) 13(4). doi: 10.7759/cureus.14620
35. Almudeer AH, Alibrahim MA, Gosadi IM. Epidemiology and risk factors associated with early onset neonatal sepsis in the south of KSA. J Taibah Univ Medical Sci. (2020) 15(6):509–14. doi: 10.1016/j.jtumed.2020.08.009
36. Al Luhidan L, Madani A, Albanyan EA, Al Saif S, Nasef M, AlJohani S, et al. Neonatal group B streptococcal infection in a tertiary care hospital in Saudi Arabia: a 13-year experience. Pediatr Infect Dis J. (2019) 38(7). doi: 10.1097/INF.0000000000002269
37. Sobaih BH, Al-Mandeel H. Early and late onset neonatal sepsis in very low birth weight infants in a tertiary center in Saudi Arabia. J Neonatal Biol. (2014) 3(5):2–4. doi: 10.4172/2167-0897.1000159
38. Nasr A, Allam G, Al-Zahrani A, Alsulaimani A. Neonatal infections in Saudi Arabia: association with C-reactive protein, CRP -286 (C>T>A) gene polymorphism and IgG antibodies. BMC Immunol. (2013) 14(1):38. doi: 10.1186/1471-2172-14-38
39. Fattah MA, Al Fadhil AO, Asaif S, Manlulu R, Karar T, Ahmed A, et al. Utility of cytokine, adhesion molecule and acute phase proteins in early diagnosis of neonatal sepsis. J Nat Sci Biol Med. (2017) 8(1):32. doi: 10.4103/0976-9668.198362
40. Dawodu A, Al Umran K, Twum-Danso K. A case control study of neonatal sepsis: experience from Saudi Arabia. J Trop Pediatr. (1997) 43(2):84–8. doi: 10.1093/tropej/43.2.84
41. Barnawi AI, Kordy FN, Almuwallad OK, Kassarah KA. Early neonatal sepsis and meningitis caused by Elizabethkingia meningoseptica in Saudi Arabia. Saudi Med J. (2020) 41(7):753. doi: 10.15537/smj.2020.7.25720
42. Haque KN, Chagia AH, Shaheed MM. Half a decade of neonatal sepsis, Riyadh, Saudi Arabia. J Trop Pediatr. (1990) 36(1):20–3. doi: 10.1093/tropej/36.1.20
43. Ohlsson A, Bailey T, Takieddine F. Changing etiology and outcome of neonatal septicemia in Riyadh, Saudi Arabia. Acta Pædiatrica. (1986) 75(4):540–4. doi: 10.1111/j.1651-2227.1986.tb10246.x
44. Alsohime F, Assiri RA, Al-Shahrani F, Bakeet H, Elhazmi M, Somily AM, et al. Premature labor and neonatal sepsis caused by Actinomyces neuii. J Infect Public Health. (2019) 12(2):282–4. doi: 10.1016/j.jiph.2018.04.001
45. Elbashier AM, Abusrair H, Owa JA. Aetiology of neonatal septicaemia in Qatif, Saudi Arabia. Early Child Dev Care. (1994) 98(1):31–8. doi: 10.1080/0300443940980104
46. Abu-Osba YK, Mallouh AA, Hann RW, Tolley S. Pattern of and risk factors for neonatal sepsis in Saudi Arab newborn. J Obstet Gynaecol. (1989) 10(sup1):S23–4. doi: 10.3109/01443618909151292
47. Srair HA, Owa JA, El-Bashier AM, Aman H. Genital flora, prolonged rupture of the membranes and the risk of early onset neonatal septicemia in Qatif Central Hospital, Kingdom of Saudi Arabia. Early Child Dev Care. (1993) 93(1):79–85. doi: 10.1080/0030443930930108
48. Sundaram V, Kumar P, Dutta S, Mukhopadhyay K, Ray P, Gautam V, et al. Blood culture confirmed bacterial sepsis in neonates in a north Indian tertiary care center: changes over the last decade. Jpn J Infect Dis. (2009) 62(1):46–50. doi: 10.7883/yoken.JJID.2009.46
49. Jabiri A, Wella HL, Semiono A, Saria A, Protas J. Prevalence and factors associated with neonatal sepsis among neonates in Temeke and Mwananyamala Hospitals in Dar es Salaam, Tanzania. Tanzan J Health Res. (2016) 18(4). doi: 10.4314/thrb.v18i4.4
50. Naher H, Khamael A. Neonatal sepsis; the bacterial causes and the risk factors. Int Res J Medical Sci. (2013) 1(6):19–22.
51. Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. (1993) 36(4):795–808. doi: 10.1097/00003081-199312000-00004
52. De Cunto A, Paviotti G, Demarini S. Neonatal aspiration: not just meconium. J Neonatal Perinatal Med. (2013) 6(4):355–7. doi: 10.3233/NPM-1372613
53. Wortham JM, Hansen NI, Schrag SJ, Hale E, Van Meurs K, Sánchez PJ, et al. Chorioamnionitis and culture-confirmed, early-onset neonatal infections. Pediatrics. (2016) 137(1). doi: 10.1542/peds.2015-2323
54. Adatara P, Afaya A, Salia SM, Afaya RA, Konlan KD, Agyabeng-Fandoh E, et al. Risk factors associated with neonatal sepsis: a case study at a specialist hospital in Ghana. Sci World J. (2019) 2019. doi: 10.1155/2019/9369051
55. Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med. (2020) 383(1):49–57. doi: 10.1056/NEJMoa1915075
56. Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med. (2005) 6(3):S45–9. doi: 10.1097/01.PCC.0000161946.73305.0A
57. Al Dasoky HA, Al Awaysheh FN, Kaplan NM, Al Rimawi HA, Agha R, Abu-Setteh M, et al. Risk factors for neonatal sepsis in tertiary hospital in Jordan. JRMS. (2009) 16(3):16–9.
58. Hornik CP, Benjamin DK, Becker KC, Benjamin DK Jr, Li J, Clark RH, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. (2012) 31(8):799–802. doi: 10.1097/INF.0b013e318256905c
59. Srivastava S, Shetty N. Healthcare-associated infections in neonatal units: lessons from contrasting worlds. J Hosp Infect. (2007) 65(4):292–306. doi: 10.1016/j.jhin.2007.01.014
60. Bas AY, Demirel N, Zenciroglu A, Göl N, Tanir G, et al. Nosocomial blood stream infections in a neonatal intensive care unit in Ankara, Turkey. Turk J Pediatr. (2010) 52(5):464.21434530
61. Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. (2000) 342(1):15–20. doi: 10.1056/NEJM200001063420103
62. Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatric Clinics. (2004) 51(4):939–59. doi: 10.1016/j.pcl.2004.03.009
63. Turhan EE, Gürsoy T, Ovalı F. Factors which affect mortality in neonatal sepsis. Turk Arch Pediatr. (2015) 50(3):170. doi: 10.5152/TurkPediatriArs.2015.2627
64. Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns ≥34 weeks’ gestation. Pediatrics. (2014) 133(1):30–6. doi: 10.1542/peds.2013-1689
65. Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. (2005) 115(5):1240–6. doi: 10.1542/peds.2004-2275
Keywords: risk factors, diagnostic factors, therapy outcome, neonatal early onset sepsis, ICU patients, Saudi Arabia
Citation: Alshammari MK, Alsanad AH, Alnusayri RJ, Alanazi AS, Shamakhi FQ, Alshahrani KM, Alshahrani AM, Yahya G, Alshahrani AA, Alshahrani TS, Sultan HS, Alshahrani FM, Alreshidi FA, Alnigaidan RA and Almazyad AA (2023) Risk and diagnostic factors and therapy outcome of neonatal early onset sepsis in ICU patients of Saudi Arabia: a systematic review and meta analysis. Front. Pediatr. 11:1206389. doi: 10.3389/fped.2023.1206389
Received: 15 April 2023; Accepted: 3 August 2023;
Published: 23 August 2023.
Edited by:
Shi Yuan, Children’s Hospital of Chongqing Medical University, ChinaReviewed by:
Nie Chuan, Guangdong Women and Children Hospital, ChinaMuhammad Fawad Rasool, Bahauddin Zakariya University, Pakistan
© 2023 Alshammari, Alsanad, Alnusayri, Alanazi, Shamakhi, Alshahrani, Alshahrani, Yahya, Alshahrani, Alshahrani, Sultan, Alshahrani, Alreshidi, Alnigaidan and Almazyad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed K. Alshammari aWlfa2FuYW4xMDFAb3V0bG9vay5jb20=
†ORCID Mohammed K. Alshammari orcid.org/0000-0001-6687-0461 Abdulmajeed S. Alanazi orcid.org/0009-0006-1890-9679 Fatmah Q. Shamakhi orcid.org/0009-0008-4686-1320 Khaled M. Alshahrani orcid.org/0009-0008-5192-7902 Abdullah M. Alshahrani orcid.org/0009-0008-1213-4870 Ghaliah Yahya orcid.org/0009-0009-4715-8909 Abdulaziz A. Alshahrani orcid.org/0009-0005-9410-1375 Turki S. Alshahrani orcid.org/0009-0002-4991-4105 Hamad S. Sultan orcid.org/0009-0000-3750-3144
 Mohammed K. Alshammari
Mohammed K. Alshammari Ahlam H. Alsanad2
Ahlam H. Alsanad2 Rawan J. Alnusayri
Rawan J. Alnusayri Turki S. Alshahrani
Turki S. Alshahrani