- Department of Pediatric Surgery, West China Hospital of Sichuan University, Chengdu, China
Introduction: Kaposiform lymphangiomatosis (KLA) is a rare and complex lymphatic anomaly with a poor prognosis. There is no standard treatment, and drug therapies are the most common therapeutic method. However, some patients' symptoms become gradually aggravated despite medical treatment. Splenectomy may be an alternative option when pharmacological therapies are ineffective.
Materials and Methods: We reviewed and evaluated the cases of 3 patients with KLA who ultimately underwent splenectomy. Results: The lesions were diffusely distributed and involved the lungs and spleens of the 3 patients. Laboratory examinations revealed that all three patients had thrombocytopenia and reduced fibrinogen levels. All patients underwent symptomatic splenectomy after the medication failed. Surprisingly, their symptoms greatly improved. Histopathological investigation of the splenic lesions of the three patients confirmed the diagnosis of KLA. Immunohistochemical staining showed positivity for CD31, CD34, podoplanin, Prox-1 and angiopoietin 2 (Ang-2).
Discussion: This study aimed to review the features of KLA patients treated by splenectomy and explore the underlying link between splenectomy and prognosis. The reason for the improvement after splenectomy may be related to increased Ang-2 levels and platelet activation in patients with KLA. Future research should seek to develop more targeted drugs based on molecular findings, which may give new hope for the treatment of KLA.
1. Introduction
Kaposiform lymphangiomatosis (KLA), which is a novel subtype of a generalized lymphatic anomaly (GLA), is a rare and devastating lymphatic anomaly (1–3). It often occurs in children, and the first reported case series found that the mean interval time from diagnosis to death was 2.75 years and the 5-year survival was 51% (1). KLA exhibits a complex, diverse, and long-term course. The mediastinum, lung, and pleura are the prevalent sites of intrathoracic involvement, while the spleen and bones are the frequently affected extrathoracic sites (1, 4, 5). Owing to its various and vague symptoms, the diagnosis and confirmation are difficult and can easily be delayed, leading to poor prognosis and significant mortality.
There is no standard treatment, and the appropriate treatment regimen remains controversial. Previously reported pharmacotherapies, which are used alone or in combination, include vincristine, sirolimus, interferon-alpha, corticosteroids, and trametinib (5). However, patients with KLA have variable responses to pharmacotherapies. Almost half of KLA cases may involve the spleen (1). Splenectomy may be considered in severely affected patients with life-threatening KLA and splenic infiltration and has been reported to ameliorate coagulation and hematological parameters (4, 6–8).
Here, we report three KLA patients with pulmonary symptoms; the patients underwent splenectomy after ineffective medical treatment. We reviewed the clinical and pathological features of these three cases, with the goals of better understanding the natural history of KLA and exploring the mechanism of the positive effect of splenectomy on prognosis.
2. Materials and methods
This study was performed after obtaining permission from the Institutional Review Board of West China Hospital of Sichuan University. The parents of all patients gave written informed consent.
The study reviewed 20 patients diagnosed with KLA between April 2018 and December 2022. The diagnosis was based on clinical data, imaging studies, and biopsy (1, 6, 9). The following are the inclusion criteria: the diagnosis was confirmed by pathological examination, and a splenectomy was performed. The patients were excluded if detailed clinical data were absent or insufficient. A total of four patients underwent splenectomy. Due to the loss of follow-up, one patient was not included in this study; so finally, we included three patients. For pathological comparison, the spleens of the patients with traumatic splenic rupture were included. The children with traumatic splenic rupture received a partial splenectomy. We analyzed the spleen tissues of the KLA patients and the spleen tissues of the children with traumatic splenic rupture. Tissue sections were stained with hematoxylin and eosin. Immunohistochemistry followed our previous process (10). Primary antibodies against CD31 (1:800, CST#3528, Cell Signaling Technology), CD34 (1:50, CST#3569, Cell Signaling Technology), podoplanin (1:400, 11629-1-AP, Proteintech), Ki67 (1:800, CST#9449, Cell Signaling Technology), α-SMA (1:400, CST #19245, Cell Signaling Technology), and angiopoietin 2 (Ang-2) (1:50, ET1705-6, HUABIO) were added. These tissue sections were incubated overnight at 4°C. The slides were washed and incubated with secondary antibodies for 30 min at room temperature. The images were obtained through a microscope camera (Leica Microsystems, Wetzlar, Germany).
3. Results
3.1. Baseline characteristics
A total of three KLA patients, consisting of two males and one female who all underwent splenectomy, were included in the study. The age of onset ranged from 9.0 to 43.0 months, with an average of 21.3 months. All patients had respiratory symptoms of varying degrees, including cough, shortness of breath, dyspnea, and even pneumonia. Two patients had pleural effusion, and two patients had pericardial effusion. One patient had gastrointestinal bleeding at the time of presentation. The characteristics of the patients, consisting of basic information, treatment methods, and prognosis, are summarized in Table 1.
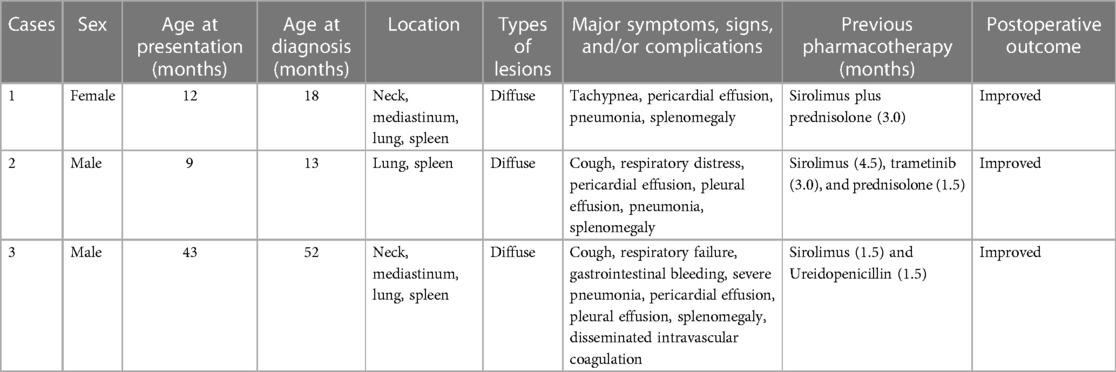
Table 1. A summary of the general information, treatment intervention, and prognosis of patients with KLA.
All patients were first treated regularly with medication. All patients achieved therapeutic levels of sirolimus during the attempted treatment period. The starting dose of oral sirolimus was 0.8 mg/m2 administered twice daily. Subsequently, oral sirolimus was titrated to achieve trough levels of 10–15 ng/ml. Prednisolone was administered at 2 mg/kg orally once daily. Trametinib was administered at 0.1 mg/kg orally once daily. However, the medication treatment was ineffective. Persistent breathing difficulties in the three patients were recorded. All patients had sustained clinical deteriorations in their platelet counts, even after receiving pharmacological treatment. The conditions of the patients were severe, and splenectomy was recommended to save their lives. All patients received a 13-valent pneumococcal conjugate vaccine, the quadrivalent (ACWY) meningococcal and Haemophilus influenzae type b vaccination, before surgery. Medications were discontinued after splenectomy, and all patients underwent regular follow-up after surgery.
Here, we report Patient 1 in detail. The child was a 12-month-old female who was admitted due to tachypnea. Then, she was diagnosed with KLA after admission and evaluation of her medical history and imaging examination. Because the child had dyspnea and a low platelet count, she was given sirolimus (0.8 mg/m2, po, bid) combined with prednisolone (2 mg/kg, po, qd). During the medication treatment process, the platelet count of the patient briefly increased but quickly decreased. In addition, she had orthopnea accompanied by a large amount of pericardial effusion. To alleviate the symptoms, pericardiocentesis was performed to drain a total of 355 ml of bloody fluid. There was a slight improvement in her respiratory symptoms after the surgery. However, many lesions in the chest cavity did not improve during the medication period. The platelet count of the patient continued to decrease, and her breathing was difficult. Due to the progression of her condition and ineffective drug treatment, the patient ultimately underwent splenectomy. The postoperative recovery of the child was remarkable, with an increased platelet count and stable vital signs. Two weeks after the surgery, the platelet count of the patient reached its peak at 1,035 × 109/L, and the D-dimer level also returned to normal at 2.57 mg/L. Afterward, the follow-up examinations were conducted at regular intervals. The platelet counts of the patient were slightly higher than normal, and the D-dimer levels were normal. The MRI images at each follow-up also indicated that the postoperative chest lesions in the patient were alleviated, but there were occasional inflammatory manifestations.
The condition of the other two children was similar, but the type and duration of medication varied depending on the situation. Patient 2 was given sirolimus, prednisolone, and trametinib. However, the patient failed to achieve the expected effect, and the disease progressed rapidly. Patient 3 had severe respiratory symptoms and abdominal distention. He received sirolimus monotherapy and several symptomatic treatments to ease his symptoms (e.g., multiple gastrointestinal decompressions). The treatment remained difficult because these two patients showed no responses to medical therapies. Patient 2 had dyspnea and a large amount of pleural effusion during the disease course. Patient 3 was unable to maintain oxygen saturation and had significant dyspnea during the medication process. After a tracheal intubation, an invasive ventilation was performed. At the same time, the coagulation function of the patient could not be corrected, and it was speculated to be related to splenic lesions. Pleural effusion in Patient 2 and Patient 3 required drainage to relieve the symptoms. The thoracic liquid discharged was milky white or hemorrhagic. Patient 3 also had drainage of pericardial effusion. To alleviate these symptoms, there was an urgent need to apply an alternative plan. There were numerous nodular lesions in the spleen with splenomegaly. Combined with previous medication and splenic lesions, a multidisciplinary discussion regarding the disease and treatment plans of the three patients led the team to recommend splenectomy. The postoperative recovery of all patients was good, and follow-up is ongoing.
3.2. Imaging features
All patients underwent detailed imaging examinations, both for preoperative and postoperative evaluation. Computed tomography (CT) and magnetic resonance imaging (MRI) are the main methods of auxiliary modes. KLA was characterized by infiltrating soft tissue thickening, low-attenuation mass, or different degrees of pleural effusion on CT (Figure 1A), and MRI showed interstitial thickening and mediastinal infiltration (Figure 1B). When the lesion involved the spleen, the volume of the spleen usually increased, and there were many abnormal nodule signal foci (Figure 1C). The lesions of the three patients were diffuse with varying degrees of involvement of the lung and spleen (Figures 1D–F).
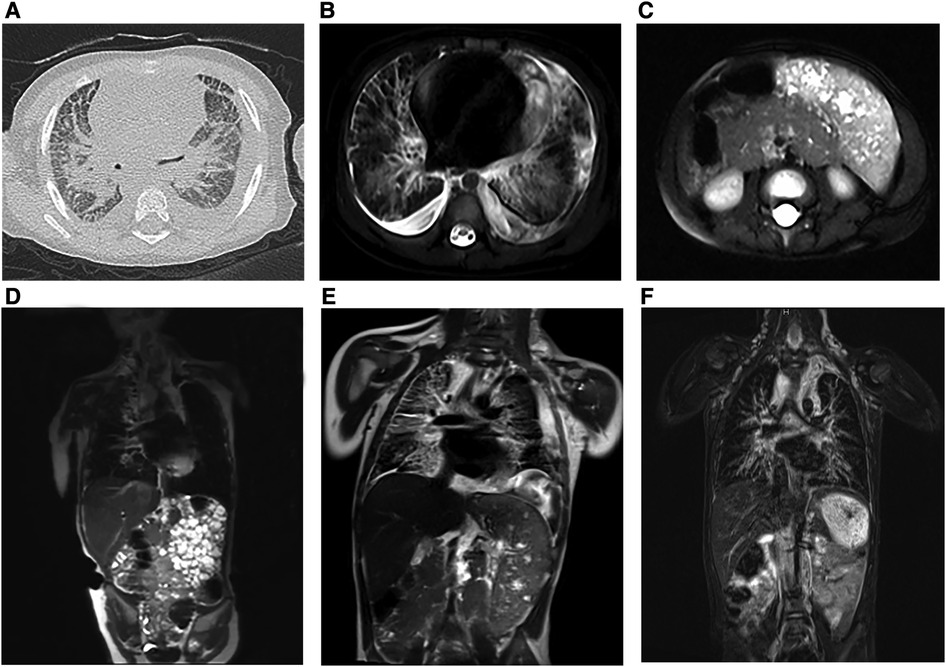
Figure 1. Preoperative radiologic findings in KLA (Patients 1–3). (A) (Case 2) The CT scan image showed multiple patches and stripes and suspicious thickening of interlobular septa in both lungs with bilateral pleural effusion. (B) (Case 2) Horizontal T2-weighted MRI of the chest revealed scattered striped abnormalities and pericardial effusion. (C) (Case 2) Horizontal T2-weighted MRI showed enlargement of the spleen and multiple nodular lesions in the spleen parenchyma. (D) (Case 1) Coronal T2-weighted MRI images showed bilateral hilar enlargement, no enlargement of the hilar and mediastinal lymph nodes, and no displacement of the mediastinum. The heart was enlarged with a small amount of pericardial effusion. (E) (Case 2) Coronal T2-weighted MRI showed that the soft tissue shadows in the cervical root, mediastinum, and right hilar area increased; the boundary was unclear; the bronchial wall in the upper and lower lobes of the right lung was thickened; and the interlobular septa of the right lung was thickened. (F) (Case 3) Significant interstitial changes in both lungs were accompanied by a large amount of pleural effusion on the left side and a small amount of pleural effusion on the right side. The spleen was enlarged, the lower margin reached the pelvic cavity, and numerous nodules could be seen in it (coronal T2-weighted MRI).
3.3. Laboratory examinations
In this study, we regularly monitored the routine hematological parameters and coagulation function of all patients. Three patients had fibrinogen reduction, and the lowest concentration was 0.56 g/L. All patients had thrombocytopenia, and the lowest platelet count was 13 × 109/L. The detailed monitoring data can be found in Table 2.
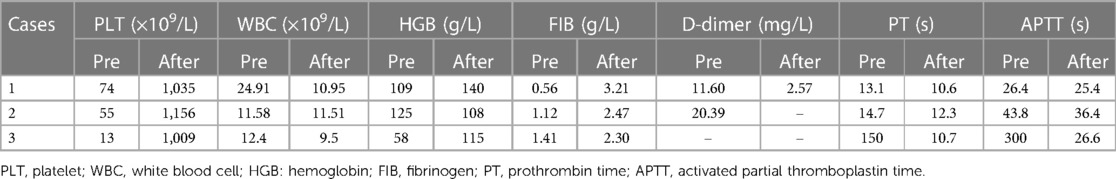
Table 2. Summary of hematological parameters of patients with KLA at the time of the lowest recorded platelet count and at 2 weeks after splenectomy.
3.4. Pathological findings
The spleens in the KLA patients were enlarged with macroscopic cystic spaces. We performed splenectomy on the patients. In the three cases, the splenic lesions were examined pathologically. The pathological findings are shown in Figures 2A–H. The lesions were immunopositive for CD31, CD34, podoplanin, and Prox-1. Minor immunopositivity for SMA and Ki67 was observed. Immunohistochemistry was performed on the spleen of the KLA patients, and for comparison, we also added immunohistochemical staining of Ang-2 in the spleen of the children with traumatic splenic rupture. A strong positive expression of Ang-2 was detected in the spleen of the KLA patients. Ang-2 was negative in the spleen partially removed due to traumatic rupture (Figures 2I,J).
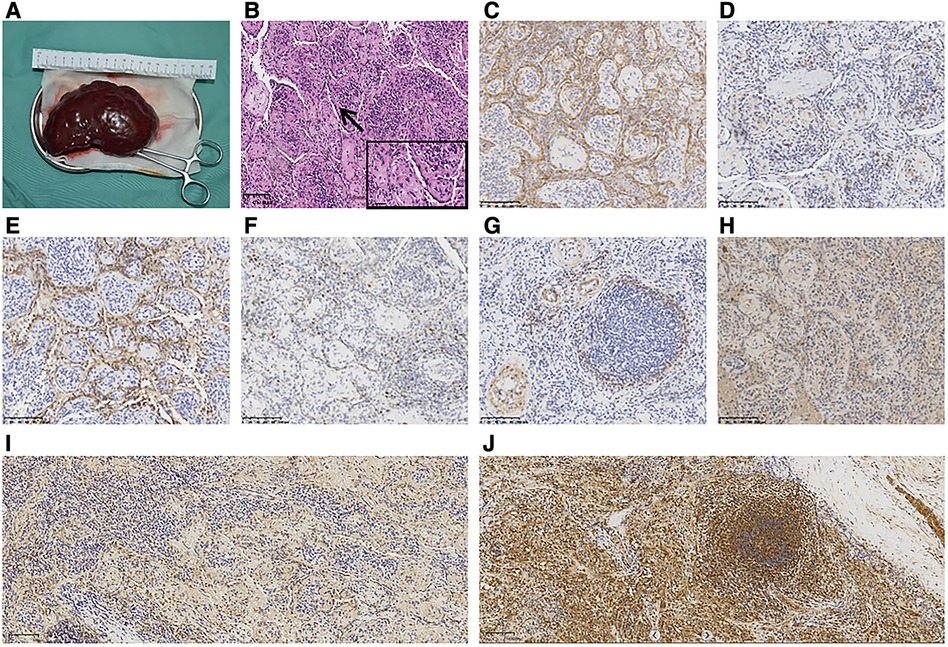
Figure 2. Pathological results of KLA with lung and spleen involvement (Patient 1). (A) Macroscopic view of the spleen excised from the patient. (B) Diffuse proliferation of abnormal, dilated lymphatics and small fascicles of hemosiderin-laden spindled lymphatic endothelial cells (HE). (C–E) Spindle-like cells were strongly immunopositive for CD31 (C), CD34 (D), and podoplanin (E). (F) Slight positive staining of Ki67. (G) α-SMA was focally positive and mainly concentrated in the blood vessel wall around the tumor focus (G). (H) Prox-1 was strongly positive. (I) Negative staining of spleen tissue in children with splenic rupture for Ang-2. (J) Positive staining of the spleen of KLA for Ang-2.
3.5. Prognosis
Fortunately, the conditions of the three patients improved, and they were followed up at the outpatient department weekly for 2 weeks, then every other week for 2 weeks, then monthly for 2 months, and then every 3 months thereafter or as clinically indicated. Examinations mainly included chest and abdomen MRI, routine blood tests, blood biochemical examinations, and coagulation function tests. The follow-up continued until December 2022, and the three patients were separately followed up for 56, 16, and 35 months. The chest MRI images of the three patients are shown in Figure 3. The postoperative vital signs of all patients were stable. To better track the changes in the hematology parameters of the patients before and after treatment, we collected their data throughout the entire process, from admission to discharge and to follow-up. For Patient 1, the indicators monitored were the platelet count and D-dimer level. There was no routine monitoring of D-dimer in the early stages for Patients 2 and 3, whereas fibrinogen was used for monitoring alternatively. In a subsequent follow-up, the hematological parameters of the patients remained basically normal (Figure 4).
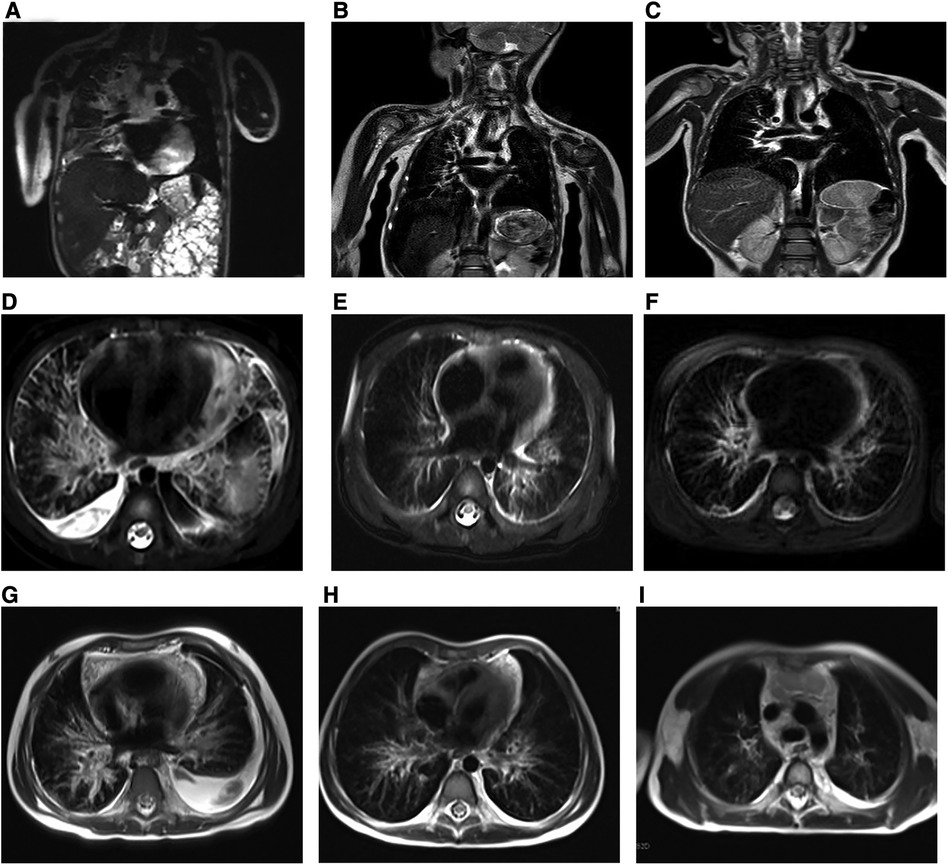
Figure 3. Preoperative and postoperative radiologic findings in KLA (Patients 1–3). (A–C) (Case 1) (A) Preoperative image. Coronal T2-weighted MRI of the chest revealed that 6 (B) and 12 months (C) after surgery, the inflammation of the patient improved, and the pericardial effusion decreased significantly. (D–F) (Case 2) Preoperative image (D) and postoperative images at 6 (E) and 12 months (F). The inflammation improved, and the fluid accumulation decreased compared with the previous period. All images are horizontal T2-weighted MRI images. (G–I) (Case 3) The presented images are all horizontal T2-weighted MRI images. The child had a large amount of pleural effusion and significant pulmonary inflammation before surgery (G). The symptoms of the child improved gradually, and the pleural effusion had mostly disappeared by 6 months (H) after the operation. The inflammation improved 12 months after surgery (I).
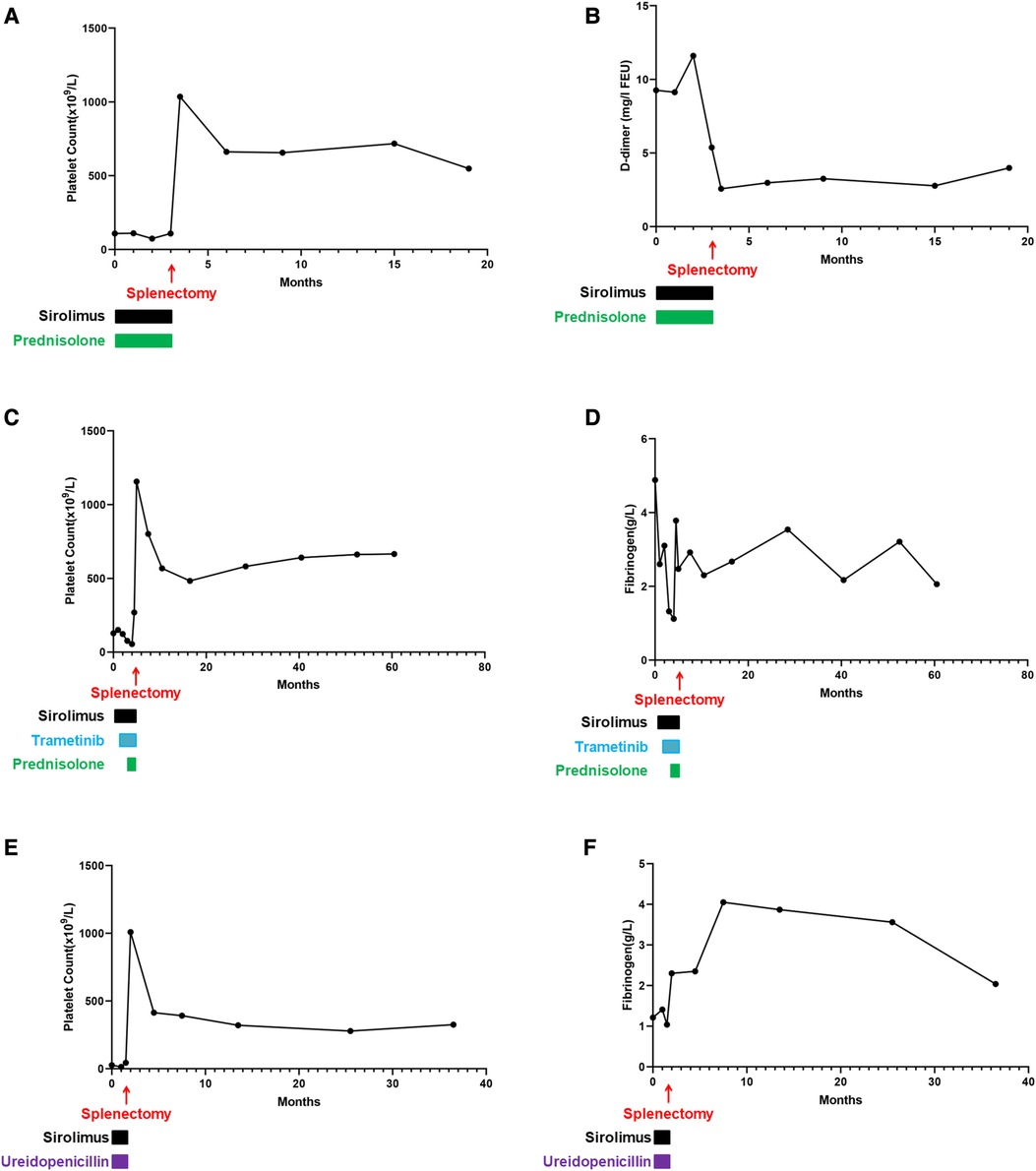
Figure 4. Hematology parameters of all patients throughout the disease. (A) Platelet count of Case 1. After surgical treatment, the index increased and fluctuated consistently in a high range thereafter. (B) D-dimer of Case 1. The changes in D-dimer were consistent with the trend of the platelet count changes. (C) Platelet count of Case 2. During medication, the platelet count did not increase. After splenectomy, the platelet count increased significantly and was maintained at a high level throughout the follow-up. (D) Fibrinogen of Case 2. During drug treatment, this value fluctuated outside the normal range, and after splenectomy, it fluctuated within the normal range. (E) Platelet count of Case 3. During the medication period, there was no increase in the platelet count, but after splenectomy, the platelet count remained elevated and remained constant. (F) Fibrinogen of Case 3. During postoperative follow-up, this indicator fluctuated within the normal range.
4. Discussion
As an extremely rare, invasive, and diffuse lymphangitic disease, KLA exhibits malformations in the histologic appearance, and it also exhibits more invasive features, such as vascular tumors (1, 3, 11, 12). KLA usually involves the thoracic cavity, bone, retroperitoneum, and viscera, with various clinical manifestations (4, 9). The unique imaging characteristics are intrathoracic lesions with deteriorating respiratory symptoms and hemorrhagic effusions (6, 12). In our cases, all the presenting features were respiratory symptoms, including tachypnea, cough, and dyspnea.
Due to the diversity of symptoms, the diagnosis of KLA has been challenging, and there is no standardized diagnostic method. Indeed, the diagnosis of KLA is often delayed because KLA usually mimics GLA or kaposiform hemangioendothelioma (KHE) (1, 6, 13). Thus, imaging examinations are very important (4, 14–16). Imaging studies combined with medical history can help us make a clinical diagnosis. A pathological examination of the lesion is the gold standard for the diagnosis of KLA (1, 5). Under the microscope, clustered or flaky spindle lymphatic endothelial cells show abnormal expansion and proliferation, accompanied by scattered red blood cells. Hemosiderin deposits can be seen inside the cells. The immunoreactive markers podoplanin, LYVE-1, and Prox-1 are positive (17, 18).
There are no unified treatment approaches for KLA. Drug treatment is relatively common. Current research confirms that sirolimus may have a positive effect on a subset of KLA patients (12, 19–21). If patients have confirmed mutations, treatment with certain targeted drugs, such as trametinib, can be administered (12, 16, 22–24). Unfortunately, drug treatment is not always effective for KLA. The deaths of KLA patients are usually caused by cardiopulmonary failure or coagulation disorders (6, 25). Symptomatic treatments such as thoracic catheter drainage, pericardial puncture, and platelet transfusion are administered for pleural effusion, pericardial effusion, and low platelet counts. When the means of treatment are limited, splenectomy is an optional palliative treatment that also has certain effects (1, 4, 7). In recent years, we have treated a total of 20 patients. Among them, 11 patients had splenic lesions. All our patients were initially treated with medication, but four patients ultimately underwent splenectomy. Finally, we reported three patients with complete follow-up data.
The drug treatments for our three children were ineffective, and the conditions of the patients progressed rapidly. Ultimately, splenectomy was performed to save the lives of the patients. Surprisingly, during follow-up to date, we have seen an improvement in the symptoms in the three children who underwent splenectomy. Interestingly, when KLA cases were first reported, there were also three patients who underwent splenectomy. In that study, one patient experienced symptom relief, and two patients had only short-term improvement (1). Regrettably, the initial situation and prognosis of the three children who underwent splenectomy were not very detailed. The preoperative and postoperative data of our three patients supplement the prognosis of splenectomy. In addition, when considering this treatment approach, we should consider the risk of infection after splenectomy. In the previous studies, to prevent postoperative infections in these patients, vaccinations were recommended (26–30). Considering the possibility of infection in the children, it is necessary for our three children to receive vaccination before splenectomy. Because splenectomy is not the initial treatment regimen, vaccination was not originally planned. In the present study, all the three children received splenectomy after vaccination. After receiving a splenectomy, the symptoms of our patients improved remarkably and were relieved continuously. The lung inflammation of the three patients significantly reduced, the pleural or pericardial effusion decreased, the respiratory symptoms were significantly relieved, and the platelet counts increased. All three children are still being followed up. We have tried to find some links between splenectomy and continuous relief of symptoms and provide some ideas for the improvement and treatment of symptoms in KLA patients.
KLA is characterized by abnormal lymphatic vessels, mainly involving the thoracic cavity. The lymphatic system is the basis of interstitial circulation and immunity. Abnormal structure and function of the lymphatic system can lead to edema, hydrops, and infection. In the previous studies, Ang-2 was found to be elevated in the serum of KLA patients and could also be used as a serum marker to participate in the diagnosis and prognosis (31, 32). Our preliminary research in the field of vascular diseases also suggests the role of Ang-2 in the occurrence and development of diseases (33, 34). Our study revealed that Ang-2 is also strongly positive in splenic lesions. According to the previous studies and our results, we speculate that the main factors of symptom relief after splenectomy in KLA children depend, at least partially, on platelet activation and Ang-2 levels.
The platelet counts in KLA patients often decrease, and this decrease is often accompanied by fibrinogen reduction, which is similar to that observed in KHE with the Kasabach–Merritt phenomenon (KMP) (1, 5, 6, 13). The mechanisms of thrombocytopenia in patients with KLA and KHE are unknown. KMP is defined as a platelet count below 100 × 109/L, accompanied by a consumptive coagulation disorder and hypofibrinogenemia (34). In the previous studies, there have been many hypotheses regarding the occurrence of KMP, mostly related to lesion sizes, platelet activation, and high expression of podoplanin (35, 36). Our previous research revealed that the larger the lesion in KHE patients, the higher the risk of developing KMP (37). The spleen lesions in all three patients were diffuse. In view of this finding, we speculated that the presence of numerous cystic lesions in the spleen of KLA patients accelerates the abnormal activation and destruction of platelets. In KLA patients with spleen involvement, multiple lesions in the spleen are accompanied by splenomegaly. In such patients, the rate of platelet destruction increases. However, the degree of thrombocytopenia in KLA cannot be explained solely by splenomegaly. A study showed a significant increase in thrombomodulin in splenic lesions of KLA, which is a sign of endothelial cell destruction (17). Under normal circumstances, platelets do not interact with the intima of vascular endothelial cells (38). However, in abnormal situations such as vascular injury, inflammation, and tumors, platelets may abnormally activate and release many factors that affect endothelial cell function. Platelets in patients with KLA adhere to the abnormal lymphatic endothelium, and platelets are abnormally activated (1, 34, 39–41). At the same time, cystic lesions in the spleen also capture platelets, and platelets that accumulate in the lesions are also abnormally activated. Many abnormally activated platelets are captured by the diseased spleen, and the serum platelet levels decrease. Abnormally activated platelets result in inflammation and leakage and even affect the abnormal production of blood vessels and lymph vessels (34, 42, 43).
Angiopoietin 1 (Ang-1) is largely stored in platelets and maintains endothelial stability during normal physiological functions (44, 45). Ang-2 antagonizes the effect of Ang-1 under normal conditions. It is stored in the Weibel–Palade bodies of endothelial cells and rapidly mobilized and released during endothelial activation (46). Among lymphatic endothelial cells, it plays a more intriguing role in lymphangiogenesis (47). A previous study of Ang-2-deficient mice suggested that Ang-2 plays an important role in the development of lymph and angiogenic remodeling in vivo (48). A large dose of Ang-2 can promote lymphatic endothelial cell proliferation and cell survival (49). Based on histological findings, we speculate that the reason for the elevated level of Ang-2 is that similar to KHE, the normality and integrity of the vasculature in KLA patients are disrupted (50–52). The diseased spleen destroys many platelets and releases many factors, causing Ang-2 to accumulate at the lesion sites and be released into the serum. An elevated Ang-2 may promote the leakage of abnormal lymphatic vessels, resulting in pleural and peritoneal effusion. Nonetheless, further studies are needed to verify our hypothesis.
After splenectomy, splenic lesions were removed, followed by a decrease in platelet destruction and abnormal activation. Subsequently, there was a significant decrease in Ang-2 release. The previous studies have shown that a further decrease in Ang-2 during treatment is associated with a normalization of the platelet count and reduction of D-dimer (53). This achieved the goal of relieving conditions such as elevated platelet counts and decreased pleural effusion. Although the three patients had a good prognosis and we obtained some useful information, there are still some limitations in our research. These limitations include the unknown specific mechanism of splenectomy in the treatment of KLA, the unclear role of Ang-2 and platelets in vascular diseases, and our short-term follow-up. We will continue to explore the mechanisms and follow up with patients for more information.
In conclusion, due to the absence of comprehensive clinical guidelines and the complex nature of patient conditions, the treatment of KLA necessitates interdisciplinary collaboration. The interval between the onset and diagnosis of the disease is too long to lead to a good prognosis. In addition, KLA can impose a significant social burden on the patients and their families. Given the complexity of the disease and the long-term nature of treatment, good doctor‒patient communication and appropriate patient education are extremely essential. This can help them better comply with treatment plans and achieve greater benefits. Despite some progress, the treatment is still challenging. Therefore, there is an urgent need to determine the pathogenesis of KLA to explore and find new and more-effective drugs to help the patients. Despite the current difficulties in treatment, with the progress of genetic testing technology and the deepening of research related to lymphatic development, there will be more means to treat KLA in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were approved by the Institutional Review Board of West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individuals and minors’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization: YL, JZ, and YJ; data collection: YL, JZ, TQ, and XG; writing—original draft preparation: YL and JZ; writing—review and editing: YL, JZ, TQ, XG, and YJ; supervision: YJ. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 82273556), the Key Project in the Science & Technology Program of Sichuan Province (grant numbers 2022YFS0233, 2022YFS0225 and 2019YFS0322), the Project of “0 to 1” of Sichuan University (grant number 2022SCUH0033), Med-X Center for Informatics funding project (YGJC004), the 1·3·5 Project for Disciplines of Excellence-Clinical Research Incubation Project of West China Hospital of Sichuan University (grant numbers 2023HXFH004, 2020HXFH048 and 2019HXFH056), and the 1·3·5 Project for Disciplines of Excellence-Clinical Research Interdisciplinary Innovation Project of West China Hospital of Sichuan University (ZYJC21060).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Croteau SE, Kozakewich HP, Perez-Atayde AR, Fishman SJ, Alomari AI, Chaudry G, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. (2014) 164(2):383–8. doi: 10.1016/j.jpeds.2013.10.013
2. ISSVA Classification. Available at: https://www.issva.org/classification (Accessed 10 July 2023).
3. Sadick M, Muller-Wille R, Wildgruber M, Wohlgemuth WA. Vascular anomalies (part I): classification and diagnostics of vascular anomalies. Rofo. (2018) 190(9):825–35. doi: 10.1055/a-0620-8925
4. Goyal P, Alomari AI, Kozakewich HP, Trenor CC, Perez-Atayde AR, Fishman SJ, et al. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol. (2016) 46(9):1282–90. doi: 10.1007/s00247-016-3611-1
5. McDaniel CG, Adams DM, Steele KE, Hammill AM, Merrow AC, Crane JE, et al. Kaposiform lymphangiomatosis: diagnosis, pathogenesis, and treatment. Pediatr Blood Cancer. (2023) 70(4):e30219. doi: 10.1002/pbc.30219
6. Ji Y, Chen S, Peng S, Xia C, Li L. Kaposiform lymphangiomatosis and kaposiform hemangioendothelioma: similarities and differences. Orphanet J Rare Dis. (2019) 14(1):165. doi: 10.1186/s13023-019-1147-9
7. Ricci KW, Iacobas I. How we approach the diagnosis and management of complex lymphatic anomalies. Pediatr Blood Cancer. (2022) 69(Suppl 3):e28985. doi: 10.1002/pbc.28985
8. Glaser K, Dickie P, Dickie BH. Proliferative cells from kaposiform lymphangiomatosis lesions resemble mesenchyme stem cell-like pericytes defective in vessel formation. J Pediatr Hematol Oncol. (2018) 40(8):e495–504. doi: 10.1097/MPH.0000000000001284
9. Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham–Stout disease. Pediatr Blood Cancer. (2016) 63(5):832–8. doi: 10.1002/pbc.25914
10. Yang K, Zhang X, Chen L, Chen S, Ji Y. Microarray expression profile of mRNAs and long noncoding RNAs and the potential role of PFK-1 in infantile hemangioma. Cell Div. (2021) 16(1):1. doi: 10.1186/s13008-020-00069-y
11. Zhou J, Yang K, Chen S, Ji Y. Sirolimus in the treatment of kaposiform lymphangiomatosis. Orphanet J Rare Dis. (2021) 16(1):260. doi: 10.1186/s13023-021-01893-3
12. Adams DM, Ricci KW. Vascular anomalies: diagnosis of complicated anomalies and new medical treatment options. Hematol Oncol Clin North Am. (2019) 33(3):455–70. doi: 10.1016/j.hoc.2019.01.011
13. Fernandes VM, Fargo JH, Saini S, Guerrera MF, Marcus L, Luchtman-Jones L, et al. Kaposiform lymphangiomatosis: unifying features of a heterogeneous disorder. Pediatr Blood Cancer. (2015) 62(5):901–4. doi: 10.1002/pbc.25278
14. Nakamura F, Kato H, Ozeki M, Matsuo M. CT and MRI findings of focal splenic lesions and ascites in generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham–Stout disease. J Clin Imaging Sci. (2021) 11:44. doi: 10.25259/JCIS_101_2021
15. Cho YJ, Kwon H, Kwon YJ, Kim SC, Kim DY, Namgoong JM. Effects of sirolimus in the treatment of unresectable infantile hemangioma and vascular malformations in children: a single-center experience. J Vasc Surg Venous Lymphat Disord. (2021) 9(6):1488–94. doi: 10.1016/j.jvsv.2021.03.014
16. Allen-Rhoades W, Al-Ibraheemi A, Kohorst M, Tollefson M, Hull N, Polites S, et al. Cellular variant of kaposiform lymphangiomatosis: a report of three cases, expanding the morphologic and molecular genetic spectrum of this rare entity. Hum Pathol. (2022) 122:72–81. doi: 10.1016/j.humpath.2022.02.010
17. Suarez-Vilela D, Izquierdo FM, Honrado E, Diez-Tascon C. Splenic lesions and other findings in kaposiform lymphangiomatosis. Am J Surg Pathol. (2023) 47(5):631–3. doi: 10.1097/PAS.0000000000002026
18. Perez-Atayde AR, Debelenko L, Al-Ibraheemi A, et al. Kaposiform lymphangiomatosis: pathologic aspects in 43 patients. Am J Surg Pathol. (2022) 46(7):963–76. doi: 10.1097/PAS.0000000000001898
19. Adams DM, Trenor CC, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. (2016) 137(2):e20153257. doi: 10.1542/peds.2015-3257
20. Wiegand S, Wichmann G, Dietz A. Treatment of lymphatic malformations with the mTOR inhibitor sirolimus: a systematic review. Lymphat Res Biol. (2018) 16(4):330–9. doi: 10.1089/lrb.2017.0062
21. Ji Y, Chen S, Yang K, Zhou J, Zhang X, Jiang X, et al. A prospective multicenter study of sirolimus for complicated vascular anomalies. J Vasc Surg. (2021) 74(5):1673–81.e3. doi: 10.1016/j.jvs.2021.04.071
22. Barclay SF, Inman KW, Luks VL, McIntyre JB, Al-Ibraheemi A, Church AJ, et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet Med. (2019) 21(7):1517–24. doi: 10.1038/s41436-018-0390-0
23. Chowers G, Abebe-Campino G, Golan H, Vivante A, Greenberger S, Soudack M, et al. Treatment of severe kaposiform lymphangiomatosis positive for NRAS mutation by MEK inhibition. Pediatr Res. (2022). doi: 10.1038/s41390-022-01986-0. [Epub ahead of print].35246606
24. Foster JB, Li D, March ME, Sheppard SE, Adams DM, Hakonarson H, et al. Kaposiform lymphangiomatosis effectively treated with MEK inhibition. EMBO Mol Med. (2020) 12(10):e12324. doi: 10.15252/emmm.202012324
25. Bundy JJ, Ootaki Y, McLean TW, Hays BS, Miller M, Downing T. Thoracic duct embolization in kaposiform lymphangiomatosis. J Vasc Surg Venous Lymphat Disord. (2020) 8(5):864–8. doi: 10.1016/j.jvsv.2020.03.013
26. Lenti MV, Luu S, Carsetti R, Osier F, Ogwang R, Nnodu OE, et al. Asplenia and spleen hypofunction. Nat Rev Dis Primers. (2022) 8(1):71. doi: 10.1038/s41572-022-00399-x
27. Hernandez MC, Khasawneh M, Contreras-Peraza N, Lohse C, Stephens D, Kim BD, et al. Vaccination and splenectomy in Olmsted county. Surgery. (2019) 166(4):556–63. doi: 10.1016/j.surg.2019.04.046
28. Freeman JJ, Yorkgitis BK, Haines K, Koganti D, Patel N, Maine R, et al. Vaccination after spleen embolization: a practice management guideline from the Eastern Association for the Surgery of Trauma. Injury. (2022) 53(11):3569–74. doi: 10.1016/j.injury.2022.08.006
29. Lee GM. Preventing infections in children and adults with asplenia. Hematology Am Soc Hematol Educ Program. (2020) 2020(1):328–35. doi: 10.1182/hematology.2020000117
30. Kanhutu K, Jones P, Cheng AC, Grannell L, Best E, Spelman D. Spleen Australia guidelines for the prevention of sepsis in patients with asplenia and hyposplenism in Australia and New Zealand. Intern Med J. (2017) 47(8):848–55. doi: 10.1111/imj.13348
31. Le Cras TD, Mobberley-Schuman PS, Broering M, Fei L, Trenor CC, Adams DM. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. (2017) 20(1):163–73. doi: 10.1007/s10456-016-9537-2
32. Ozeki M, Nozawa A, Kawamoto N, Fujino A, Hirakawa S, Fukao T. Potential biomarkers of kaposiform lymphangiomatosis. Pediatr Blood Cancer. (2019) 66(9):e27878. doi: 10.1002/pbc.27878
33. Ji Y, Chen S, Li K, Li L, Xu C, Xiang B. Signaling pathways in the development of infantile hemangioma. J Hematol Oncol. (2014) 7:13. doi: 10.1186/1756-8722-7-13
34. Ji Y, Chen S, Yang K, Xia C, Li L. Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis. (2020) 15(1):39. doi: 10.1186/s13023-020-1320-1
35. O’Rafferty C, O’Regan GM, Irvine AD, Smith OP. Recent advances in the pathobiology and management of Kasabach–Merritt phenomenon. Br J Haematol. (2015) 171(1):38–51. doi: 10.1111/bjh.13557
36. Debelenko LV, Perez-Atayde AR, Mulliken JB, Liang MG, Archibald TH, Kozakewich HP. D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. (2005) 18(11):1454–60. doi: 10.1038/modpathol.3800444
37. Zhou J, Lan Y, Qiu T, Gong X, Zhang Z, He C, et al. Impact of age and tumor size on the development of the Kasabach–Merritt phenomenon in patients with kaposiform hemangioendothelioma: a retrospective cohort study. Precis Clin Med. (2023) 6(2):pbad008. doi: 10.1093/pcmedi/pbad008
38. Etulain J, Schattner M. Glycobiology of platelet-endothelial cell interactions. Glycobiology. (2014) 24(12):1252–9. doi: 10.1093/glycob/cwu056
39. Shulkin BL, Argenta LC, Cho KJ, Castle VP. Kasabach–Merritt syndrome: treatment with epsilon-aminocaproic acid and assessment by indium 111 platelet scintigraphy. J Pediatr. (1990) 117(5):746–9. doi: 10.1016/S0022-3476(05)83334-7
40. Zhou J, Li Y, Qiu T, Gong X, Yang K, Zhang X, et al. Long-term outcomes of sirolimus treatment for kaposiform hemangioendothelioma: continuing successes and ongoing challenges. Int J Cancer. (2023).
41. Ji Y, Chen S, Zhou J, Yang K, Zhang X, Xiang B, et al. Sirolimus plus prednisolone vs sirolimus monotherapy for kaposiform hemangioendothelioma: a randomized clinical trial. Blood. (2022) 139(11):1619–30. doi: 10.1182/blood.2021014027
42. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. (2017) 36(2):249–62. doi: 10.1007/s10555-017-9673-1
43. Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood. (2000) 96(13):4216–21. doi: 10.1182/blood.V96.13.4216
44. Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. (2013) 319(9):1271–80. doi: 10.1016/j.yexcr.2013.03.011
45. Oluboyo AO, Chukwu SI, Oluboyo BO, Odewusi OO. Evaluation of angiopoietins 1 and 2 in malaria-infested children. J Environ Public Health. (2020) 2020:2169763. doi: 10.1155/2020/2169763
46. Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel–Palade bodies. Blood. (2004) 103(11):4150–6. doi: 10.1182/blood-2003-10-3685
47. Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. (2019) 8(5):471. doi: 10.3390/cells8050471
48. Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. (2002) 3(3):411–23. doi: 10.1016/S1534-5807(02)00217-4
49. Nguyen VP, Chen SH, Trinh J, Kim H, Coomber BL, Dumont DJ. Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC Cell Biol. (2007) 8:10. doi: 10.1186/1471-2121-8-10
50. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand S, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. (1997) 277(5322):55–60. doi: 10.1126/science.277.5322.55
51. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. (2013) 328(1):18–26. doi: 10.1016/j.canlet.2012.08.018
52. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol. (2009) 10(3):165–77. doi: 10.1038/nrm2639
Keywords: kaposiform lymphangiomatosis, splenectomy, angiopoietin 2, platelet, angiopoietin 1
Citation: Lan Y, Zhou J, Qiu T, Gong X and Ji Y (2023) Refractory kaposiform lymphangiomatosis relieved by splenectomy. Front. Pediatr. 11:1203336. doi: 10.3389/fped.2023.1203336
Received: 10 April 2023; Accepted: 2 August 2023;
Published: 17 August 2023.
Edited by:
Chihaya Imai, Niigata University, JapanReviewed by:
Michio Ozeki, Gifu University, JapanFriedrich Georg Kapp, University Medical Center Freiburg, Germany
© 2023 Lan, Zhou, Qiu, Gong and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Ji SmlqaXl1YW55dWFuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yuru Lan
Yuru Lan Jiangyuan Zhou
Jiangyuan Zhou Tong Qiu
Tong Qiu Xue Gong
Xue Gong Yi Ji
Yi Ji