- 1Institute of Medicine and Nursing, Hubei University of Medicine, Shiyan, China
- 2Precision Medical Lab Center, People’s Hospital of Yangjiang Affiliated to Guangdong Medical University, Yangjiang, China
- 3Department of Neonatology, People’s Hospital of Yangjiang Affiliated to Guangdong Medical University, Yangjiang, China
- 4Precision Medical Center, Chaozhou Central Hospital Affiliated to Southern Medical University, Chaozhou, China
- 5School of Life Science and Food Engineering, Hanshan Normal University, Chaozhou, China
- 6Yangjiang Branch, Biochip Beijing National Engineering Research Center, Yangjiang, China
Objective: Glucose 6-phosphate dehydrogenase (G6PD) deficiency increases the risk of neonatal hyperbilirubinemia. The aim of this study is to evaluate the risk factors associated with hyperbilirubinemia in infants from the western part of Guangdong Province, and to assess the contribution of G6PD deficiency to neonatal jaundice.
Methods: The term infants with neonatal hyperbilirubinemia in People's Hospital of Yangjiang from June 2018 to July 2022 were recruited for the retrospective analysis. All the infants underwent quantitative detection of the G6PD enzyme. The etiology was determined through laboratory tests and clinical manifestations.
Results: Out of 1,119 term infants, 435 cases presented with jaundice. For the etiology analysis, infection was responsible for 16.09% (70/435), G6PD deficiency accounted for 9.66% (42/435), of which 3 were complicated with acute bilirubin encephalopathy), bleeding accounted for 8.05% (35/435), hemolytic diseases accounted for 3.45% (15/435), and breast milk jaundice accounted for 2.53% (11/435). One case (0.23%) was attributed to congenital hypothyroidism, multiple etiologies accounted for 22.3% (97/435), and 35.63% (155/435) were of unknown etiology. Of the jaundiced infants, 19.54% (85/435) had G6PD deficiency, while only 10.23% (70/684) of non-jaundiced infants had G6PD deficiency; this difference was found to be statistically significant (P < 0.001). Furthermore, the hemoglobin levels in the jaundiced infants with G6PD deficiency (146.85 ± 24.88 g/L) were lower than those without G6PD deficiency (156.30 ± 22.07 g/L) (P = 0.001). 65 jaundiced infants with G6PD deficiency underwent G6PD mutation testing, and six different genotypes were identified, including c.95A > G, c.392G > T, c.1024C > T, c.1311C > T, c.1376G > T, c.1388G > A, c.871G > A/c.1311C > T, c.392G > T/c.1388G > A, and c.1376G > T/c.1311C > T.65iciency
Conclusion: In newborns in Yangjiang, G6PD deficiency, infection, and neonatal hemolytic disease were identified as the main causes of hyperbilirubinemia and acute bilirubin encephalopathy. Specifically, Hemolytic factors in infants with G6PD deficiency may lead to reduced hemoglobin and increased bilirubin levels in jaundiced infants.
1. Introduction
Neonatal jaundice, characterized by yellow skin, sclera and conjunctiva due to hyperbilirubinemia, is a common condition in Chinese newborns. Bilirubin can cross the blood-brain barrier, leading to severe hyperbilirubinemia, acute bilirubin encephalopathy, nuclear jaundice, and even permanent brain damage (1). In China, acute bilirubin encephalopathy in neonates is a significant concern, making it crucial to identify and intervene in risk factors for jaundice promptly. This approach can help reduce the incidence and severity of hyperbilirubinemia and acute bilirubin encephalopathy (2).
Neonatal hyperbilirubinemia (NHB) can arise from various causes, including infections as the primary cause, followed by perinatal and hemolytic factors (3, 4). Hereditary factors can also contribute to neonatal hyperbilirubinemia (5). This study aimed to comprehensively analyze the etiology of jaundiced infants in a hospital in the western part of Guangdong province, exploring the various factors causing this disease. Additionally, this study aimed to evaluate the contribution of glucose-6-phosphate dehydrogenase (G6PD) deficiency to neonatal jaundice.
2. Material and methods
2.1. Subjects
Full-term infants with a gestational age of >37 weeks, birth weight ≥2,500 g, and who underwent quantitative detection of G6PD enzyme were included in this study. The age of admission ranged from 1 to 28 days. Jaundiced neonates were identified as those whose maximum total serum bilirubin (TSB) reached or exceeded the 95th percentile of the hour-specific TSB nomogram established by the Chinese Multicenter Study Coordination Group for Neonatal Hyperbilirubinemia in 2015 (6). Demographic and clinical data of the participants were collected from their medical records. The diagnostic standard for severe hyperbilirubinemia was TSB ≥ 342 μmol/L (6).
This study obtained approval from the Ethics Committee of the People's Hospital of Yangjiang in 2022 (No.20220063). The patients' data was analyzed anonymously, and blood samples were used after clinical diagnosis; therefore, the Ethics Committee of the People's Hospital of Yangjiang granted a waiver for written consent.
2.2. Methods
General information was gathered, including the infant's sex, gestational age, birth weight, age and weight at admission, delivery mode, feeding pattern, as well as treatment measures received. Hemolytic disease of the newborn (ABO and Rh incompatibility), glucose-6-phosphate dehydrogenase (G6PD) deficiency, infections (sepsis, pneumonia, and urinary tract infections), extravascular hemorrhage (intracranial hematoma, scalp hematoma, gastrointestinal, and other bleedings), congenital hypothyroidism (diagnosed through neonatal screening), and breast milk jaundice were among the major clinical etiologies examined. Breast milk jaundice was diagnosed by exclusion, in infants who were exclusively breastfed and older than 14 days, with no other identifiable pathogenic factors for jaundice; As a result, breast milk jaundice was not considered as a contributing factor in the combined analysis. If a patient was found to have two or more contributing factors for jaundice, they were documented in both the combined group and the respective single factor groups. Information about acute bilirubin encephalopathy (typical clinical symptoms of the nervous system and/or MRI imaging) and criteria for blood exchange [as recommended by the American Academy of Pediatrics in 2022 (7)] was also documented.
ABO incompatibility hemolysis was tested by three serological antibody tests using commercial three-cell panel (LIBO biotechnology, China, Co, Ltd.) by gel technique. Three tests included red blood cells direct antiglobulin test (direct Coombs test), free antibody test (free) and antibody release test. Serological diagnostic criteria for ABO incompatibility hemolysis were as follows: (1) confirmed cases were neonates with two positive results of the three tests or with a positive result on the antibody elution test; (2) suspected cases were only positive for either direct Coombs test or serum free antibody test. Antibody elution test was the final confirmed diagnosis for neonatal hemolysis disease (8).
The confirmation of G6PD enzyme deficiency was made by measuring the production rate of NADPH, using a detection kit (Beijing Antu Bioengineering Co., Ltd., China) following the manufacturer's protocols. Infants with a production rate of NADPH lower than 2,500 U/L were categorized as G6PD-deficient (9).
To determine the frequency of G6PD deficiency in Yangjiang, routine body check-ups were conducted on male adults at our hospital, and they were also tested for G6PD deficiency using the same detection kit mentioned above. The deficiency was defined as G6PD enzyme activity lower than 1,300 U/L.
2.2.1. Molecular diagnosis of G6PD deficiency
Blood specimens collected from G6PD-deficient patients were subjected to molecular analysis. For newborns with jaundice, whole blood was prospectively collected after clinical diagnosis and stored at −40°C in a biobank. DNA was extracted using a DNA extraction kit (HYBRIBIO Co, Ltd., China) according to the instructions and the quantity and purity were measured by NanoDrop One (Thermo Fisher Scientific Co., Ltd.). Amplification of G6PD gene was performed by PCR using VeritiTM Dx 96-Well Thermal Cycler (Thermo Fisher Scientific), followed by detection of the G6PD gene variant through reverse dot blot method (HYBRIBIO Co., Ltd., China) for 13 common G6PD mutation types, including c.95A > G (G6PD Gaohe), c.392G > T (G6PD Qing Yan), c.487G > A (G6PD Mahidol), c.493A > G (G6PD Taipei), c.592G > T (G6PD Coimbra), c.871G > A (G6PD Viangchan), c.1004C > T (G6PD Fushan), c.1024C > T (G6PD Chinese-5), c.1360C > T (G6PD Union), c.1376G > T (G6PD Canton), c.1387C > T (G6PD Keelung), c.1388G > A (G6PD Kaiping), c.1381G > A (G6PD Yunan) and c.1311C > T (polymorphism) (10).
2.3. Statistical analysis
The data was analyzed by SPSS 23.0 (IBM SPSS 23.0) and shown as mean ± standard deviation. Differences in continuous variables between the two groups were analyzed by the Mann–Whitney nonparametric test. The significance of differences in the categorical variables was determined by the Chi-square test or Fisher's exact test. P < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
From June 2018 to July 2022, a quantitative assay of G6PD enzyme was conducted on 1,828 in-hospital infants. Of these, 1,119 were term infants, with 435 presenting with jaundice and 684 presenting normal bilirubin levels. G6PD deficiency accounted for 9.66% (42/435) hyperbilirubinemia cases, while multiple etiologies, including infection (pneumonia, sepsis, and upper respiratory tract infection), hemorrhage (delivery injury, scalp hematoma, gastrointestinal bleeding), ABO incompatibility hemolysis, and breast-milk jaundice were responsible for 22.3% (97/435), 16.09% (70/435), 8.05% (35/435), 3.45% (15/435), and 2.53% (11/435) of cases, respectively. One case (0.23%, 1/435) was attributed to congenital hypothyroidism, and 2.07% (9/435) of cases were caused by other factors such as thalassemia, mothers with diabetes, and hereditary spherocytosis. The etiology was unknown in 35.63% (155/435) of cases (Figure 1).
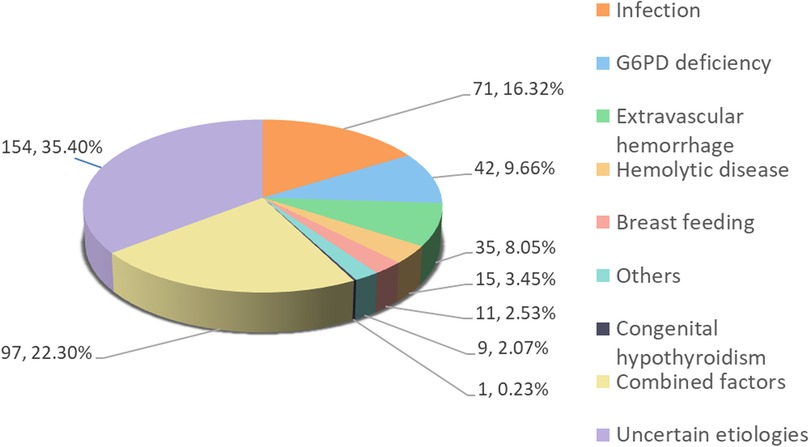
Figure 1. Clinical causes in 435 cases of jaundice. Combined factors, with 2 or more clinical causes.
Among the 435 cases of jaundice, severe hyperbilirubinemia (TSB ≥342 μmol/L) was observed in 50 cases, 8 cases of which presented with acute bilirubin encephalopathy (2 males and 6 females), and 7 cases required exchange transfusions. The primary causes of acute bilirubin encephalopathy were G6PD deficiency (3 cases) and hemorrhage (3 cases), followed by infection (2 cases of sepsis and 1 case of pneumonia), ABO incompatibility hemolysis (2 cases), and congenital hypothyroidism (1 case) (Table 1). One female infant with acute bilirubin encephalopathy was admitted at the age of 17 days, who had congenital hypothyroidism combined with G6PD deficiency and had not undergone neonatal screening at birth (Table 1).
3.2. G6PD deficiency prevalence
Of the 435 cases with jaundice, 19.54% (60 males, 25 females) were found to be G6PD-deficient, while only 10.23% (47 males, 23 females) of the 684 cases with normal bilirubin levels were G6PD-deficient. The prevalence of G6PD deficiency was significantly higher in infants with jaundice compared to their counterparts (P < 0.001) (Table 2). The prevalence of G6PD-deficient in severe hyperbilirubinemia (TSB ≥ 342 µmol/L) and mild-medium jaundice (TSB < 342 µmol/L) was 30% (15 out of 50, 11 males, 4 females) and 18.18% (70 out of 385, 49 males, 11 females), respectively. A statistically significant difference was observed between the two groups (P = 0.041) (Table 3). In comparison, G6PD deficiency was present in 8.75% (42/480) of male adults during routine body check-ups.
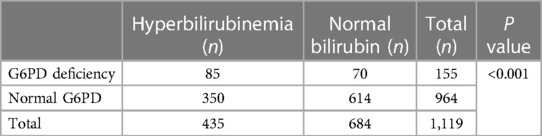
Table 2. The comparison of G6PD deficiency in the infants with jaundice and group with normal bilirubin levels.
3.3. Comparison of bilirubin and hemoglobin levels in infants with jaundice
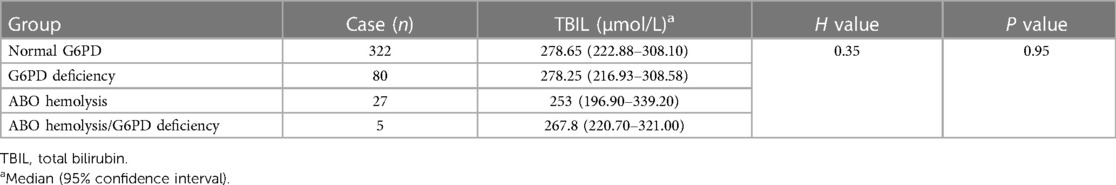
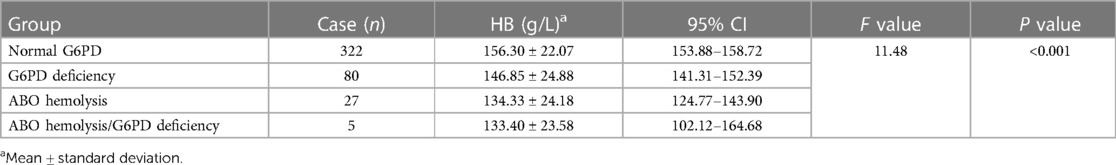
Of the 435 infants with jaundice, one case was excluded due to the lack of hemoglobin data. The remaining cases were grouped according to jaundice causes—normal, G6PD-deficient, ABO incompatibility hemolysis, and ABO incompatibility hemolysis combined with G6PD-deficiency. The difference in total bilirubin levels among these four groups was compared (Table 4), and the peak bilirubin values were not statistically significant (P = 0.95). Among infants with jaundice, the hemoglobin levels of 80 G6PD-deficient infants were 146.85 ± 24.88 g/L, which was significantly lower than that of infants with normal G6PD (156.30 ± 22.07 g/L) (P = 0.001) (Table 5). Meanwhile, the hemoglobin levels of 27 infants with ABO incompatibility hemolysis were at 134.33 ± 24.18 g/L, slightly lower than that of infants with G6PD deficiency (146.85 ± 24.88 g/L) (P = 0.014).
3.4. G6PD genotypes distribution
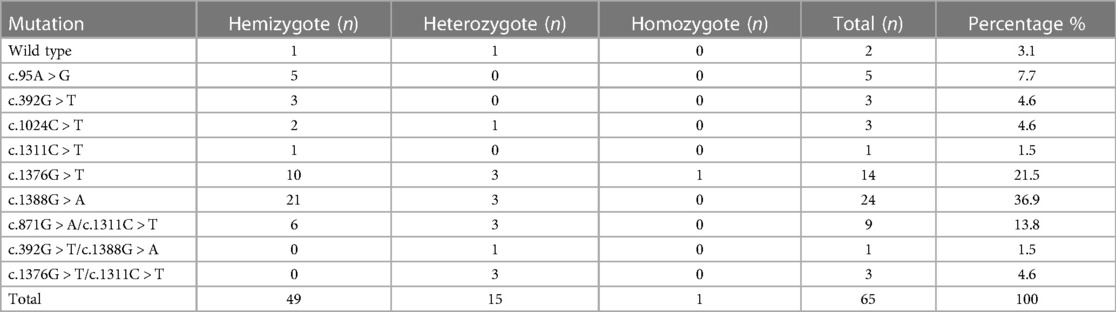
A total of 65 blood samples from jaundiced infants with G6PD deficiency were analyzed for G6PD genotypes using reverse dot hybridization (10). Five types of gene mutations and one polymorphism were detected in infants with jaundice, namely c.95A > G, c.392G > T, c.1024C > T, c.1376G > T, c.1388G > A, and c.1311C > T (polymorphism). Furthermore, three kinds of compound heterozygous mutations were identified, namely c.871G > A/c.1311C > T, c.392G > T/c.1388G > A, and c.1376G > T/c.1311C > T (Table 6). Specifically, c.871G > A was consistently linked to 1311C > T in all nine cases (Table 6). Moreover, one G6PD-deficient infant developed acute bilirubin encephalopathy with compound heterozygous mutations of c.392G > T and c.1388G > A.
4. Discussion
The pathogenic factors of neonatal hyperbilirubinemia are multifaceted, and different cases can have a single or mixed etiology. Common causes include extravascular hemorrhage, hemolytic diseases, infection, G6PD deficiency, breastfeeding, and maternal disease factors. Furthermore, the etiological composition of neonatal hyperbilirubinemia can vary in different regions. Among the 435 full-term infants with hyperbilirubinemia in our study, the main causes were combined factors (22.3%), infection (16.32%), G6PD deficiency (9.66%), hemorrhage (8.05%), hemolytic diseases (3.45%), breast milk jaundice (2.53%), other factors (2.07%), and congenital hypothyroidism (0.23%).
A study conducted in the eastern region of China reported that the top five pathogenic factors of neonatal unconjugated hyperbilirubinemia were combined factors (16%), infections (15%), breast milk jaundice (11%), hemorrhage (10%), hemolytic diseases (7%), and G6PD deficiency (1%) (3). In comparison, the cohort in our study had a higher prevalence of G6PD deficiency, likely due to the high prevalence of G6PD deficiency in the population of western Guangdong province. Nevertheless, infections, hemolytic diseases, and hemorrhage remained the main pathogenic factors of neonatal jaundice in our study.
In this study, the prevalence of G6PD deficiency was 19.54% in the jaundiced group, while it was 10.23% in the control group with normal bilirubin levels. In Chaozhou of eastern Guangdong province, among 882 neonates with hyperbilirubinemia, 74 cases (8.39%) were G6PD-deficient (11), and in Fujian province, the prevalence of G6PD deficiency among neonates with hyperbilirubinemia was 7% (12). As such, the prevalence of G6PD deficiency among neonates with hyperbilirubinemia in the western part of Guangdong is higher compared to that in eastern Guangdong and Fujian province.
Multiple reports have demonstrated that G6PD-deficient infants have a significantly higher predisposition to neonatal jaundice and are more susceptible to acute bilirubin encephalopathy (2, 4, 7). In our study, the prevalence of G6PD deficiency was 30% in infants with severe jaundice and 18.18% (70/385) in infants with mild-medium jaundice. Of the neonates with acute bilirubin encephalopathy, 37.5% (3 in 8) were G6PD-deficient. Moreover, the frequency of G6PD deficiency in Yangjiang males was 8.75%, which was higher than that of the entire Guangdong province (13). In the voluntary Kernicterus Registry in the United States, 20.8% of 125 affected newborns were G6PD-deficient, while the male frequency of G6PD deficiency was estimated to be 0.5%–2.9% (14). Our study, along with previous research, indicates that G6PD-deficient infants are predisposed to neonatal jaundice, and even to kernicterus.
In 2004, the American Academy of Pediatrics (AAP) suggested that G6PD deficiency should be considered as a high-risk factor for jaundice in newborns ≥35 weeks old, and should therefore be evaluated in the diagnosis and treatment of jaundiced newborns (15). In clinical practice, given the relatively high prevalence of G6PD deficiency in this population, G6PD screening for all local newborns is required. Furthermore, transcutaneous bilirubin monitoring is strongly recommended when an infant with G6PD deficiency is discharged. Early detection is beneficial for prompt treatment, which is in accordance with the AAP guidelines. These improvements in neonatal care could decrease neonatal morbidity and mortality in this region.
G6PD deficiency is the most prevalent inherited enzyme deficiency disease, yet the mechanism of neonatal hyperbilirubinemia resulting from G6PD deficiency remains incompletely understood (4). Previously, it was believed that jaundice in infants with G6PD deficiency was mainly caused by excessive bilirubin production during hemolysis (16–18). Nevertheless, some studies have found minimal evidence of hemolysis in jaundiced neonates with G6PD deficiency (19–21). In our cohort of infants with hyperbilirubinemia, the hemoglobin levels in the G6PD-deficient group were significantly lower than those in the normal G6PD group (P < 0.001), and slightly higher than those in the ABO hemolysis group (134.33 ± 24.18 g/L) (P = 0.014). Since fetal erythropoiesis in infants with G6PD deficiency was the same as that in controls, and G6PD was dispensable for human erythroid cell differentiation (22, 23). Our findings suggest that decreased hemoglobin levels may be due to hemolytic factors in jaundiced infants with G6PD deficiency. Another possible explanation is the disruption of the oxidant-antioxidant balance and impaired recycling of peroxiredoxin 2, which can impact bilirubin clearance (4). Moreover, co-inheritance of a uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) gene variant is an additional risk factor for neonatal jaundice in G6PD-deficient infants (11).
G6PD is caused by loss-of-function mutations in the G6PD gene and follows an X-linked recessive inheritance pattern. The distribution of G6PD deficiency is predominantly found in the south of the Yangtze River in China, with Guangdong province exhibiting a high incidence of G6PD deficiency (13). Interestingly, common variants among G6PD-deficient individuals in southern China are unique to these populations (13, 24). In our study, Canton (c.1376 G > T) and Kaiping (c.1388 G > A) were the most frequent variants, accounting for over 78% of G6PD-deficient infants with jaundice. This distribution pattern was consistent throughout Guangdong province and the entire country (13, 25).
In summary, neonatal hyperbilirubinemia and acute bilirubin encephalopathy in Yangjiang were primarily caused by G6PD deficiency, infections, and hemolytic disease of the newborn. Compared to other parts of China, the high prevalence of G6PD deficiency in the western region of Guangdong made it the predominant factor for neonatal hyperbilirubinemia. In our cohort of infants with jaundice, the G6PD-deficient group had significantly lower hemoglobin levels than the normal G6PD group, suggesting that hemolytic factors in this group may contribute to reduced hemoglobin and increased bilirubin levels in jaundiced infants. We strongly recommend G6PD screening and transcutaneous bilirubin monitoring for all newborns in this area to improve neonatal healthcare.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of People's Hospital of Yangjiang. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
L-YY conceptualized and designed the study, coordinated and supervised data collection, and reviewed and revised the manuscript. Y-KY collected and analyzed the data, drafted the initial manuscript, and revised the manuscript. Z-KC polished the language, Y-KY and Y-BC did the molecular analysis, FL and Y-WL collected the data, and carried out the initial analysis. C-FL, Y-CH, B-RX, S-HH, Y-MX, and Y-EC participated in the clinical practice and data collection. All authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Guangdong Province (No: 2016A030307035) and the High-Level Development Plan of People's Hospital of Yangjiang (No: G2020007). The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician. (2005) 72(7):1277–82.16225031
2. Subspecialty Group of Neonatology, Society of Pediatrics, Chinese Medical Association, Chinese Multicenter Study Coordination Group for Neonatal Bilirubin Encephalopathy. Clinical characteristics of bilirubin encephalopathy in Chinese newborn infants-a national multicenter survey. Zhonghua Er Ke Za Zhi. (2012) 50(5):331–5.22883033
3. Mei H, Dong X, Wu B, Wang H, Lu Y, Hu L, et al. Clinical and genetic etiologies of neonatal unconjugated hyperbilirubinemia in the China neonatal genomes project. J Pediatr. (2022) 243:53–60.e9. doi: 10.1016/j.jpeds.2021.12.038
4. Lee HY, Ithnin A, Azma RZ, Othman A, Salvador A, Cheah FC. Glucose-6-phosphate dehydrogenase deficiency and neonatal hyperbilirubinemia: insights on pathophysiology, diagnosis, and gene variants in disease heterogeneity. Front Pediatr. (2022) 10:875877. doi: 10.3389/fped.2022.875877
5. Yang H, Wang Q, Zheng L, Lin M, Zheng XB, Lin F, et al. Multiple genetic modifiers of bilirubin metabolism involvement in significant neonatal hyperbilirubinemia in patients of Chinese descent. PLoS One. (2015) 10(7):e0132034. doi: 10.1371/journal.pone.0132034
6. Chinese Multicenter Study Coordination Group for Neonatal Hyperbilirubinemia. Predictive value of hour-specific transcutaneous bilirubin nomogram for neonatal hyperbilirubinemiaa national multicenter study. Zhonghua Er Ke Za Zhi. (2015) 53(11):830–4.26758321
7. Kuzniewicz MW, Li SX, McCulloch CE, Newman TB. Predicting the need for phototherapy after discharge: update for 2022 phototherapy guidelines. Pediatrics. (2022) 150(3):e2022058020. doi: 10.1542/peds.2022-058020
8. Yang H, Lin F, Chen ZK, Zhang L, Xu JX, Wu YH, et al. UGT1A1 mutation association with increased bilirubin levels and severity of unconjugated hyperbilirubinemia in ABO incompatible newborns of China. BMC Pediatr. (2021) 21(1):259. doi: 10.1186/s12887-021-02726-9
9. Tang F, Huang YL, Jiang X, Jia XF, Li B, Feng Y, et al. Evaluations of newborn screening program performance and enzymatic diagnosis of glucose-6-phosphate dehydrogenase deficiency in Guangzhou. Zhonghua Er Ke Za Zhi. (2018) 56(5):359–63. doi: 10.3760/cma.j.issn.0578-1310.2018.05.010
10. Hu R, Lin M, Ye J, Zheng BP, Jiang LX, Zhu JJ, et al. Molecular epidemiological investigation of G6PD deficiency by a gene chip among Chinese Hakka of southern Jiangxi province. Int J Clin Exp Pathol. (2015) 8(11):15013–8.26823837
11. Xu JX, Lin F, Chen ZK, Luo ZY, Zhan XF, Wu JR, et al. Co-inheritance of G6PD deficiency and 211 G to a variation of UGT1A1 in neonates with hyperbilirubinemia in eastern Guangdong. BMC Pediatr. (2021) 21(1):564. doi: 10.1186/s12887-021-03010-6
12. Zhou J, Yang C, Zhu W, Chen S, Zeng Y, Wang J, et al. Identification of genetic risk factors for neonatal hyperbilirubinemia in Fujian province, southeastern China: a case-control study. Biomed Res Int. (2018) 2018:7803175. doi: 10.1155/2018/7803175
13. Lin F, Lou ZY, Xing SY, Zhang L, Yang LY. The gene spectrum of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangdong province, China. Gene. (2018) 678:312–7. doi: 10.1016/j.gene.2018.07.068
14. Bhutani VK, Johnson LH, Jeffrey Maisels M, Newman TB, Phibbs C, Stark AR, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol. (2004) 24(10):650–62. doi: 10.1038/sj.jp.7211152
15. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2004) 114(1):297–316. doi: 10.1542/peds.114.1.297
16. Necheles TF, Rai US, Valaes T. The role of haemolysis in neonatal hyperbilirubinaemia as reflected in carboxyhaemoglobin levels. Acta Paediatr Scand. (1976) 65(3):361–7. doi: 10.1111/j.1651-2227.1976.tb04898.x
17. Slusher TM, Vreman HJ, McLaren DW, Lewison LJ, Brown AK, Stevenson DK. Glucose-6-phosphate dehydrogenase deficiency and carboxyhemoglobin concentrations associated with bilirubin-related morbidity and death in Nigerian infants. J Pediatr. (1995) 126(1):102–8. doi: 10.1016/s0022-3476(95)70510-4
18. Kaplan M, Wong RJ, Stevenson DK. Hemolysis and glucose-6-phosphate dehydrogenase deficiency-related neonatal hyperbilirubinemia. Neonatology. (2018) 114(3):223–5. doi: 10.1159/000489820
19. Mukthapuram S, Dewar D, Maisels MJ. Extreme hyperbilirubinemia and G6PD deficiency with no laboratory evidence of hemolysis. Clin Pediatr. (2016) 55(7):686–8. doi: 10.1177/0009922815610630
20. Jalloh S, Van Rostenberghe H, Yusoff NM, Ghazali S, Nik Ismail NZ, Matsuo M, et al. Poor correlation between hemolysis and jaundice in glucose 6-phosphate dehydrogenase-deficient babies. Pediatr Int. (2005) 47(3):258–61. doi: 10.1111/j.1442-200x.2005.02052.x
21. Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. (2018) 378(1):60–71. doi: 10.1056/nejmra1708111
22. Yeruchimovich M, Shapira B, Mimouni FB, Dollberg S. Neonatal nucleated red blood cells in G6PD deficiency. Am J Perinatol. (2002) 19(4):215–9. doi: 10.1055/s-2002-28485
23. Boonpeng K, Ketprasit N, Palasuwan A, Kulkeaw K, Palasuwan D. Glucose-6-phosphate dehydrogenase is dispensable for human erythroid cell differentiation in vitro. Exp Hematol. (2023) 121:18–29.e2. doi: 10.1016/j.exphem.2023.02.002
24. Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J. (2013) 12:418. doi: 10.1186/1475-2875-12-418
Keywords: neonatal hyperbilirubinemia, neonatal jaundice, etiology, G6PD deficiency, hemolysis
Citation: Yang Y-K, Lin C-F, Lin F, Chen Z-K, Liao Y-W, Huang Y-C, Xiao B-R, Huang S-H, Xu Y-M, Chen Y-E, Cao Y-B and Yang L-Y (2023) Etiology analysis and G6PD deficiency for term infants with jaundice in Yangjiang of western Guangdong. Front. Pediatr. 11:1201940. doi: 10.3389/fped.2023.1201940
Received: 7 April 2023; Accepted: 26 June 2023;
Published: 10 July 2023.
Edited by:
Giovanni Mario Pes, University of Sassari, ItalyReviewed by:
Tina Marye Slusher, University of Minnesota Twin Cities, United StatesRichard Oscar Francis, Columbia University, United States
© 2023 Yang, Lin, Lin, Chen, Liao, Huang, Xiao, Huang, Xu, Chen, Cao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ye Yang eWFuZ2xlZXllZUBzaW5hLmNvbQ==
 Yi-Kang Yang1,2
Yi-Kang Yang1,2 Fen Lin
Fen Lin Li-Ye Yang
Li-Ye Yang