- 1Department of Pediatrics, University of Medicine Pharmacy Science and Technology George Emil Palade of Targu Mures, Targu Mures, Romania
- 2Department of Neonatology, University of Rochester Medical Center Golisano Children’s Hospital at Strong, Rochester, NY, United States
- 3Department of Pediatrics, University of Iowa Stead Family Children’s Hospital, Iowa City, IA, United States
Recent research has increased focus and interest in characterizing the physiology of the transition circulation using echocardiography. Critique of published normative neonatal echocardiography data among healthy term neonates has not been performed. We have performed a comprehensive literature review using the key terms: cardiac adaptation, hemodynamics, neonatal transition, term newborns. Studies were included if they had reported echocardiography indices of cardiovascular function in the presence of maternal diabetes, intrauterine growth restricted newborns and prematurity and had a comparison group of healthy term newborns within first seven postnatal days. Sixteen published studies evaluating transitional circulation in healthy newborns were included. There was marked heterogeneity in the methodologies used; specifically, inconsistency in time of evaluation and imaging techniques used makes it challenging to determine specific trends of expected physiologic changes. Some studies revealed nomograms for echocardiography indices, though limitations persist in terms of sample size, number of reported parameters and consistency of measurement technique. A comprehensive standardized echocardiography framework which includes consistent techniques for assessment dimensions, function, blood flow, pulmonary/systemic vascular resistance, and shunts pattern is warranted to ensure consistency in the use of echocardiography to guide care of healthy and sick newborns.
Introduction
Transition to extrauterine life is one of the most challenging processes for the human body. Under normal conditions, the switch from the parallel fetal to serial neonatal circulation should happen immediately after birth triggered by lung aeration and umbilical cord clamping. First, a 10-fold increase in pulmonary blood flow (PBF) occurs, as pulmonary vascular resistance (PVR) decreases (1), augmenting left ventricle (LV) preload. Second, systemic vascular resistance (SVR) and arterial blood pressure (BP) rapidly increase by removing the connection between fetal and placental circulation. Third, the shunt at the patent ductus arteriosus (PDA) level switches from pure right-to-left to predominantly left-to-right further increasing PBF and LV preload. The vulnerability of transitional newborns relates to myocardial immaturity. Although the neonatal right ventricle (RV) is primed to work against a relatively high resistance circulation in utero, recent study suggest that RV appears to be more vulnerable among intrauterine growth restricted (IUGR) affected fetuses (2). In addition, impaired RV function is associated with adverse outcomes in newborns with neonatal encephalopathy undergoing therapeutic hypothermia (3). The LV myocardium, rich in non-compliant tissue, has a great vulnerability to increase his stroke volume for a given increase in afterload. Impaired myocardial performance is seen in many disorders of the neonatal period specifically, birth asphyxia, newborns of diabetic mother (IDM), sepsis, IUGR, and preterm birth. For example, fetuses compromised by acute and severe asphyxia are at risk of sustained elevation of PVR, low cardiac output (CO) secondary to impaired systolic performance (4) and low filling of the LV after birth (5). The adaptive response of the IUGR fetus, experiencing chronic mild hypoxia secondary to placental insufficiency, is characterized by redistribution of blood flow to the brain and heart (6). After birth, IUGR are at a great risk for hemodynamic instability due to low LV stroke volume (7), and higher systemic and PVR (8). The IDMs with cardiac hypertrophy are susceptible to decreased LV function and left outflow tract obstruction (9), increased PVR, and acute pulmonary hypertension (10). Further, their risk of cardiocirculatory instability is aggravated by greater myocardial vulnerability to coronary artery hypoperfusion (11).
Rationale and purpose of the review
The primary aim was to review published literature related to echocardiography measurements of heart function, systemic and pulmonary hemodynamics, during the postnatal transition. Knowledge of the normal postnatal cardiovascular adaptive changes in healthy babies is vital to enable recognition of deviations from normal, and to aid understanding of mechanisms of disease and thresholds for intervention. Echocardiography is increasingly used for assessment of dysregulation of transitional physiology. The establishment of standardized imaging and measurement frameworks to characterize normative changes during the postnatal transition is essential to streamline future research efforts.
Methods
Publications were identified from a systematic search of PubMed, conducted in November 2022. The search strategy included the following text terms: “transition”, “cardiocirculatory adaptation”, “echocardiography”, “normal reference values”, and “neonate”. The search was further refined by adding the terms' “systolic function”, “myocardial velocities”, and “functional indices”. The search time period was up to March 2023. The focus of the study was to characterize the cardiovascular transitional changes of the healthy term newborns.
Inclusion criteria for selecting studies were those that used echocardiography in the transitional period to describe: (i) longitudinal cardiovascular changes among healthy term newborns from low-risk pregnancies; (ii) comparative changes between healthy term versus preterm newborns; (iii) comparative changes between term IUGR or IDM patients and healthy term controls. Exclusion criteria were studies that focused on: (i) preterm newborns receiving respiratory support or treatment for PDA; (ii) newborns with congenital heart disease; (iii) newborns with arterial cord blood pH less than 7 or required admission to the neonatal intensive care unit; (iv) non-English language reports.
Two independent reviewers (LMS, IP) assessed all studies until consensus was reached. Four studies were excluded based on the aforementioned criteria leaving 16 studies for analyses, the oldest published in 1988 and the newest in 2018. Echocardiography indices were categorized as dimensional/structural, functional, resistance, flow, and shunt measurements. Details of the specific echocardiographic view [apical two-chamber (2C), four-chamber (4C), five chamber (5C), parasternal long axis, parasternal short axis, high parasternal, subcostal], measurement technique [M-mode, two-dimensional (2D), Color Doppler (CWD), pulse-wave Doppler (PWD), PWTD, tissue Doppler imaging (TDI), speckle tracking echocardiography (STE)], and compliance with American Society of Echocardiography recommendations. Additional variables included year of publication, record of investigators echo training, inter- and intra- observer variability data, sample size, and number of evaluation (time points).
Results
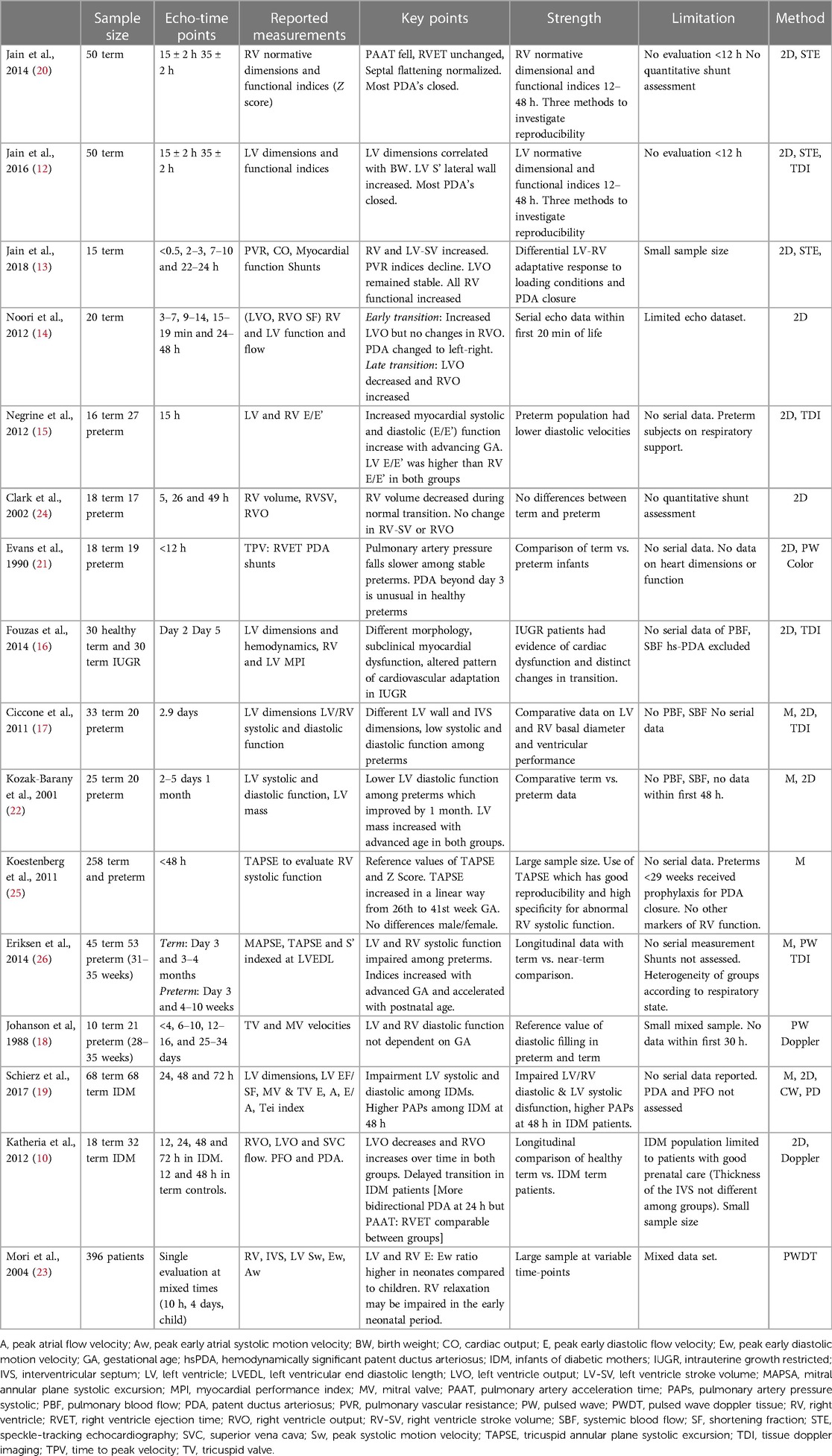
Overall, 150 echocardiographic indices were reported in the selected studies. Specific cardiac parameters assessed in the selected reports included: RV and LV systolic and diastolic functional indices (10, 12–19); isolated RV dimensions and normative z scores (20); LV normative dimensional correlated with birth weight and functional indices (12); PVR indices (10, 13, 14, 19, 21); isolated LV systolic and diastolic function (22, 23); isolated RV systolic function (24); tricuspid annular peak systolic excursion (TAPSE) norms for birth weight and gestational age (25); RV and LV systolic function, mitral annular peak systolic excursion (MAPSE), TAPSE indexed to LV end-diastolic length (LVEDL) (26). A summary of study details including main findings, methods, strengths and limitations are presented in Table 1.
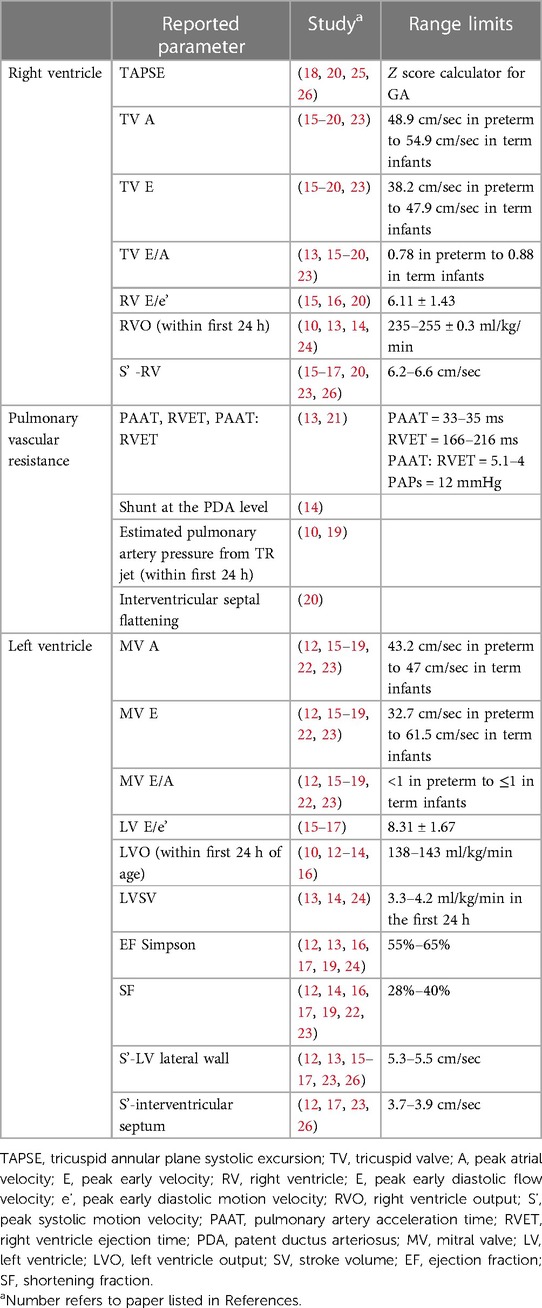
The most commonly reported conventional echocardiography measurements, which are used in clinical practice, were TAPSE, mitral valve inflow (MV) E/A, shortening fraction (SF), ejection fraction (EF) by Simpson's biplane method, and ventricular outflow (VO) for the right and left ventricle. Additional measurements reported, but less commonly used in routine clinical practice, included TV E, TV A, TV E/A, RV S', RV E/E', and MV E, MV A, LV E/e', LV S' of lateral wall and interventricular septum, and LV stroke volume (SV) Table 2. Time of echocardiography evaluation varied among the publications; specifically, four studies reported measurements only in the first 24 h (13–15, 21), one study at 48 h only (25), six studies provided serial evaluation (<120 h) between early and late transition (10–12, 17, 19, 20, 24) and four studies compared measurements of neonates in early and/or late transition to older children (18, 22, 23, 26). The frequency of echocardiography assessment also was variable: single (n = 4) time-point evaluation (15, 17, 22, 23); paired (n = 5) time-points (12, 16, 20, 22, 26), three (n = 2) time-points (19, 24) or four (n = 4) time-points (10, 13, 14, 18). More than half of selected studies reported inter- and intra-observer variability rates (10, 12, 13, 16, 20–23, 25), while one quarter were performed by a single sonographer to minimize operator dependency error (14, 15, 24, 26). The sample size of healthy term newborns varied from ≤20 (13–15, 18, 21, 24) to ≥50 cases (12, 19, 20, 23, 25). Echocardiography methods included conventional measurements only in eight studies (10, 14, 18, 19, 21, 22, 24, 25), conventional and color/tissue Doppler echocardiography (cTDI) in five studies (15–17, 23, 26) and advanced myocardial evaluation using STE in three studies (12, 13, 20).
Dimensions and structure
Echocardiography dimensions were adjusted for birth weight, sex, and gestational age (12), indexed for body surface area (22), or adjusted for heart size (i.e., LV end-diastolic length) (26). LV linear dimensions were obtained from apical 4Ch, long or short axis parasternal view or both, either using M-mode (17, 22), by joining straight lines between basal segments of the LV walls (20), or as the distance from the apical epicardium to the level of the septal attachment of the mitral valve (26). LV dimensions were measured at end-diastole (20), or at both end-diastole and end-systole (17); of note, some studies do not report the specific phase of acquisition in the cardiac cycle (26). Most RV linear dimensions were obtained from multiple views; specifically, the RV focused apical 4-chamber view (20, 24) or parasternal long axis (20), or RV focused 3-chamber view (13) were used most. Overall, among healthy term newborns, LV dimensions remain unchanged and RV volume decreased during the transition; of note, a positive linear relationship between newborns' weight and longitudinal systolic function (TAPSE and MAPSE) was found (20, 26). Among selected studies, IUGR newborns were found to have more dilated LV and interventricular septum hypertrophy (16) and IDM were characterized by LV stiffness and myocardial hypertrophy (19).
Heart function
Ejection fraction using Simpson biplane method and shortening fraction from the long axis parasternal view were the most frequently reported indices of LV systolic performance. Overall, conventional indices of LV systolic performance were unchanged during the transition; however, a marginal increase in indices of LV diastolic function (mitral E/A or systole': diastole' ratios >1.0) was noted during the first 48 postnatal hours. Studies using TDI showed that LV myocardial velocities were unchanged during first 48 h (Figure 1A); however, marginal reductions in LV lateral wall s' were noted. Some authors reported higher RV fractional area change measured from RV-3C than RV-4C (20) views, which has prompted use of global FAC. Studies using TDI demonstrated higher RV myocardial velocities compared to LV (15), and higher trans-tricuspid velocities increase with advancing chronological age, but E/A ratios were unchanged. A positive linear relationship between RV myocardial velocities (RV-S') and longitudinal systolic function (TAPSE) with the advancing postnatal age was found among term newborns during physiological transition (Figure 1B).
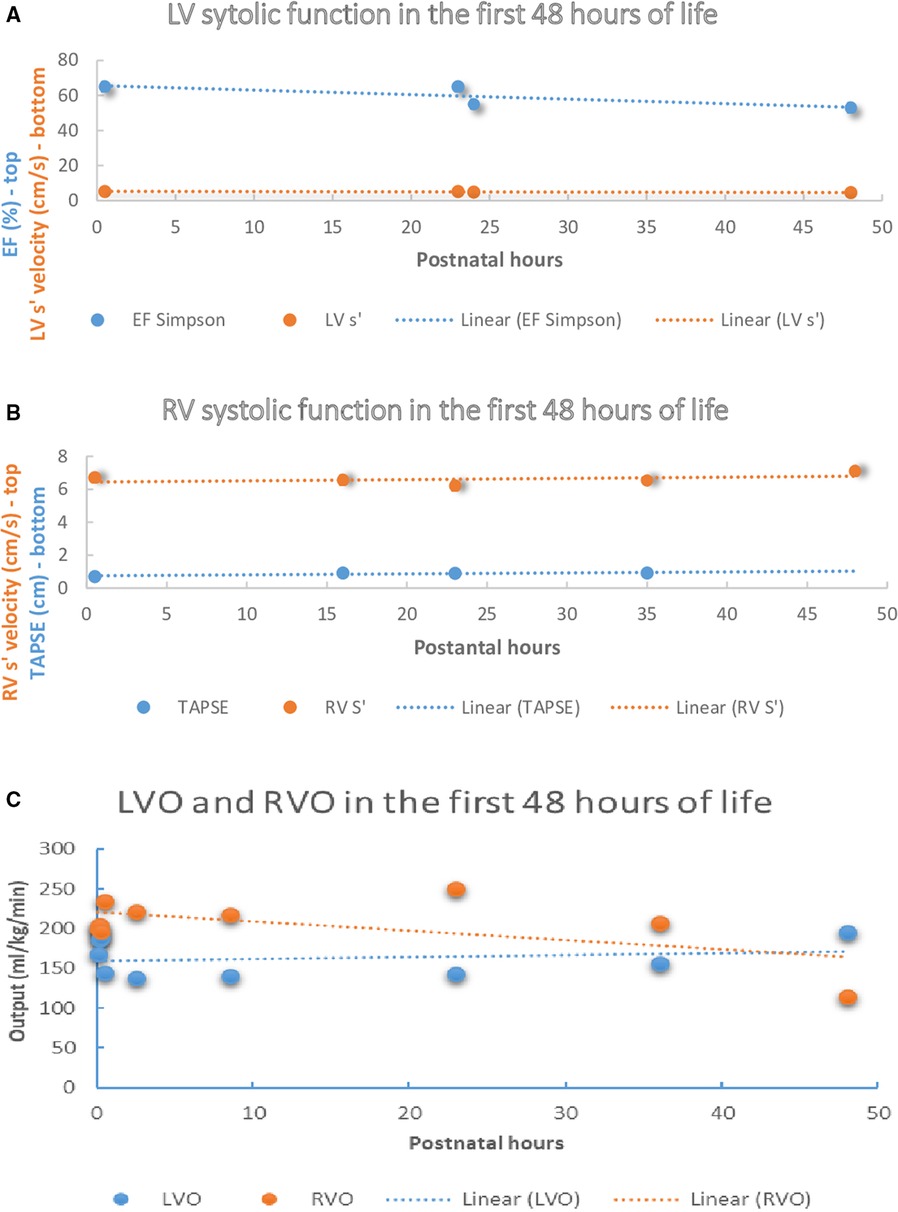
Figure 1. Left and right ventricle systolic function and output in the first 48 h of life amongst healthy term newborns. (A) LV systolic function in the first 48 h of life among healthy term newborns in selected studies (12, 13). (B) RV systolic function in the first 48 h of life among healthy term newborns in selected studies (13, 16, 20). (C) RVO and LVO in the first 48 h of life among healthy term newborns in selected studies (12–14, 16, 24). LV, left ventricle, LV S’, peak systolic motion velocity, EF, ejection fraction, TAPSE, tricuspid annular plane systolic excursion, RV, right ventricle, RV-S’, Peak systolic motion velocity, LVO, left ventricle output, RVO, right ventricle output.
Flow
Some authors report the velocity time integral (VTI), which is a trace of the pulse wave Doppler waveform acquired at the level of the hinge point of the aortic or pulmonary valves. LV-VTI and RV-VTI are most frequently performed in apical 5C and PLAX views respectively. Although mean LVO evaluated at 4 different time points within first 24 h after birth remained unchanged, RVO increased during the same period. In addition, the RVO: LVO ratio was >1.5 reflecting ongoing RV dominance after birth amongst healthy term newborns (13, 20). After the first 72 h RVO and LVO values were comparable among studies reporting these indices during the late transition (14, 16, 24) (Figure 1C). Some authors used regression analyses to test the effect of the heart rate on the selected parameters (26), whereas other studies used linear regression to assess relationship between LVO and RVO with cerebral blood flow and cerebral regional saturations (14), or to evaluate the relation of the LVO, RVO and superior vena caval (SVC) flow with infant characteristics (10).
Resistance
Serial evaluation of pulmonary arterial pressure and PVR were performed using indirect measures [pulmonary artery acceleration time (PAAT), right ventricular ejection time (RVET), PAATi (indexed to total cardiac cycle duration) and indexed PVR (13, 21), interventricular septal flattening (20), direction of PDA flow (21), or estimated pulmonary pressure from tricuspid regurgitation (TR) jet (10, 19)]. The mean value of PAAT and RVET, measured at 4 different time points, increased while PVRi ratio (RVET: PAAT) decreased term newborns within 24 h of birth (13). PAAT and RVET are time dependent indices and, therefore, influenced by heart rate; however, changes in both indices were detected during first 24 h.
Shunts
The most common view for PDA interrogation was the high parasternal view, and flow was evaluated by PWD, CWD, or according to the pulmonary artery flow pattern (22). The PDA shunt became predominantly left-to-right within minutes of birth and closed or small with restrictive flow by 24–48 h in 100% cases (12, 13, 20, 21). In addition, a persistent bidirectional PDA shunt beyond 10–15 h was considered “atypical” (13). Atrial level shunts were exclusively left-to-right in healthy term neonates, irrespective of chronological age (20), with higher incidence of atrial level shunts as chronological age advances (12).
Discussion
Aside from known physiological concepts of neonatal transition to extrauterine life, it is challenging to determine any novel trends and refine our knowledge of early physiologic changes in newborns due to the lack of normative data. Although notable, the available literature remains limited due to small sample size (20–50 newborns) and inconsistent study design. Currently only 4 studies have reported longitudinal cardio circulatory adaptation to extra uterine life among healthy term neonates (12–14, 20). Most studies have focused on characterizing differences in adaptative cardiovascular mechanisms between term and preterm myocardium. Timing and frequency of echocardiography evaluation also varied among publications, with some studies reporting isolated measurements in the first 24–48 h, while others reported serial data between early and late transition. More than half of selected studies reported inter and intra variability rates, while few had images performed by a single sonographer to minimize operator dependency error (14, 15, 24, 26).
Some variability in imaging protocols is expected, given the lack of unified training programs, but the magnitude of differences in current reports are substantial. Most studies (n = 8) included only conventional echocardiography measurements, with 5 studies providing color tissue Doppler imaging (cTDI) and 3 reporting STE derived measurements. While some studies were designed to provide reference range and z score for certain parameters for diagnostic/predictive value (12, 20, 25), there are insufficient data to produce true normative data. Sample size is sufficiently small that it is not possible to appraise the influence of racial, sex or ethnicity on normative datasets. None of the selected studies report the prognostic value of the echocardiography parameters on short- or long-term outcomes.
Consistency, in how images are acquired, and measurements are performed, is essential when interpreting echocardiography data. Half of studies were performed by neonatologists (10, 12–15, 19, 20, 24) with the remaining by cardiologists (16–18, 21–23, 25, 26). Little information was provided on sonographer credentials or training, nor the steps taken to minimize operator-dependent variability. The most commonly reported measurements were LV and RV output, the presence of the PDA level shunts, TAPSE, PAAT and RVET, tricuspid and mitral inflow, and RV systolic myocardial velocity (RV-S'). Importantly, due to persistence of fetal channels in the transitional period, many of the reported measurements are influenced by flow; however, the confounding effects of shunts and flow patterns/direction were not reported among some studies (18, 19, 26) while others excluded newborns with hemodynamically significant PDA (16, 22, 25). In addition, specific measurement techniques were inconsistently reported making it difficult to combine datasets.
As highlighted previously, the postnatal period is characterized by important physiologic changes which are likely to have a major impact of cardiovascular adaptation. Unfortunately, few studies have performed longitudinal evaluation which makes interpretation of hemodynamic datasets in the context of disease states difficult. Where data exists, it is interesting to note that in the immediate transitional period (<30 min) left ventricular output increases, likely due to a combination of increased heart rate, changes in PDA flow, and increased LV preload (Figure 1C). Immediate changes in LV function after birth could be explained by the relative increase in LV preload after birth due to an increase in left to right shunt at the level of FO, increased LV afterload after elimination of the placental circulation, high likelihood of early PDA closure, and augmented postnatal diuresis. One study revealed that RV myocardial velocities were greater than LV (15) and concluded it aligned with the concept of persistence of RV dominance in the early postnatal period; however, this can also be explained by the mechanics of the LV contraction that is only partially affected by the longitudinal shortening (27). Notably, the compiled results in this review cannot be extrapolated to newborns of diabetic mothers, growth restricted newborns, or other populations with abnormal cardiovascular adaptations.
We acknowledge several limitations of this present analysis. First, only reports written in English were included. Therefore, normative data in these studies may not be globally applicable due to racial or ethnicity differences. Second, we are unable to obtain information about the specific training of individuals performing the echocardiography studies. By recognizing this limitation, we aim to promote transparency and encourage documentation of the expertise for the clinicians involved. Third, the lack of available information regarding delayed cord clamping could have potentially influenced the reported measurements since healthy term neonates with delayed cord clamping are reported to undergo a more physiological transition (28). Fourth, there remain gaps in the provision of normative data for some measurements. For example, there are little data on indices of LV afterload including systemic vascular resistance. In addition, some time-dependent indices like the isovolumic relaxation time would benefit from indexing to heart rate or some other comparable time-dependent index. Fifth, it is not clear how often these measurements are used in routine clinical practice; in particular, usage patterns for novel measurements such as PAAT, RVET, or advanced indices of heart function, in guiding treatment is an important gap in the literature. Finally, we were unable to verify the quality of imaging studies and steps taken to ensure consistency in measurement techniques between studies.
Conclusions
This review of the neonatal transition literature highlights major gaps in normative echocardiography data. There remains a great need for standardized echocardiography measurement techniques, protocols, and training to advance our ability to recognize and intervene in the care of a baby with an abnormal transition. Thus, we propose the need to involve relevant stakeholders and develop standardized echocardiography framework for any future research in this domain, with consistent time-points for image acquisition [e.g., immediate (first hour), early (1–24 h), and late transition (48–72 h)], agreed standards for both imaging and measurement techniques, and which target diverse perspectives to ensure racial and ethnic variance is considered.
Author contributions
LS, IP, AF, RG, and PM contributed to conception and design of the study. LS and IP conducted the systematic literature search, study selection, and extracted the data. LS, IP wrote the initial draft of the manuscript. LS, AF, IP, PM wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the University of Medicine, Pharmacy, Science and Technology George Emil Palade of Targu Mures research grant number 10127/10/17.12.2020.
Acknowledgments
The authors are grateful to nurses and families of children included in studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heymann MA. Control of the pulmonary circulation in the fetus and during the transitional period to air breathing. Eur J Obstet Gynecol Reprod Biol. (1999) 84(2):127–32. doi: 10.1016/S0301-2115(98)00321-2
2. Suciu LM, Giesinger RE, Mărginean C, Muntean M, Cucerea M, Făgărășan A, et al. Comparative evaluation of echocardiography indices during the transition to extrauterine life between small and appropriate for gestational age infants. Front Pediatr. (2023) 10:2431. doi: 10.3389/fped.2022.1045242
3. Giesinger RE, El Shahed AI, Castaldo MP, Breatnach CR, Chau V, Whyte HE, et al. Impaired right ventricular performance is associated with adverse outcome after hypoxic ischemic encephalopathy. Am J Respir Crit Care Med. (2019) 200(10):1294–305. doi: 10.1164/rccm.201903-0583OC
4. Costa S, Zecca E, De Rosa G, De Luca D, Barbato G, Pardeo M, et al. Is serum troponin T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr. (2007) 96(2):181–4. doi: 10.1111/j.1651-2227.2007.00104.x
5. Gebauer CM, Knuepfer M, Robel-Tillig E, Pulzer F, Vogtmann C. Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics. (2006) 117(3):843–50. doi: 10.1542/peds.2004-1587
6. Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol. (2016) 594(5):1215–30. doi: 10.1113/JP271099
7. Polglase GR, Allison BJ, Coia E, Li A, Jenkin G, Malhotra A, et al. Altered cardiovascular function at birth in growth-restricted preterm lambs. Pediatr Res. (2016) 80(4):538–46. doi: 10.1038/pr.2016.104
8. Allison BJ, Hooper SB, Coia E, Zahra VA, Jenkin G, Malhotra A, et al. Ventilation-induced lung injury is not exacerbated by growth restriction in preterm lambs. Am J Physiol Lung Cell Mol Physiol. (2016) 310(3):L213–23. doi: 10.1152/ajplung.00328.2015
9. El-Ganzoury MM, El-Masry SA, El-Farrash RA, Anwar A, Ellatife ZA. Infants of diabetic mothers: echocardiographic measurements and cord blood IGF-I and IGFBP-1. Pediatr Diabetes. (2011) 13(2):189–96. doi: 10.1111/j.1399-5448.2011.00811.x
10. Katheria A, Leone T. Altered transitional circulation in infants of diabetic mothers with strict antenatal obstetric management: a functional echocardiography study. J Perinatol. (2012) 32(7):508–13. doi: 10.1038/jp.2011.135
11. VanLoozen DH, Murdison KA, Polimenakos AC. Neonatal myocardial perfusion in right ventricle dependent coronary circulation: clinical surrogates and role of troponin-I in postoperative management following systemic-to-pulmonary shunt physiology. Pediatr Cardiol. (2018) 39:1496–9. doi: 10.1007/s00246-018-1940-6
12. Jain A, El-Khuffash AF, Kuipers BC, Mohamed A, Connelly KA, McNamara PJ, et al. Left ventricular function in healthy term neonates during the transitional period. J Pediatr. (2017) 182:197–203. doi: 10.1016/j.jpeds.2016.11.003
13. Jain A, Mohamed A, Kavanagh B, Shah PS, Kuipers BC, Afif EK, et al. Cardiopulmonary adaptation during first day of life in human neonates. J Pediatr. (2018) 200:50–7. doi: 10.1016/j.jpeds.2018.04.051
14. Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr. (2012) 160(6):943–8. doi: 10.1016/j.jpeds.2011.12.008
15. Negrine RJS, Chikermane A, Wright JGC, Ewer AK. Assessment of myocardial function in neonates using tissue Doppler imaging. Arch Dis Child Fetal Neonatal Ed. (2012) 97(4):F304–6. doi: 10.1136/adc.2009.175109
16. Fouzas S, Karatza AA, Davlouros PA, Chrysis D, Alexopoulos D, Mantagos S, et al. Neonatal cardiac dysfunction in intrauterine growth restriction. Pediatr Res. (2014) 75(5):651–7. doi: 10.1038/pr.2014.22
17. Ciccone MM, Scicchitano P, Zito A, Gesualdo M, Sassara M, Calderoni G, et al. Different functional cardiac characteristics observed in term/preterm neonates by echocardiography and tissue Doppler imaging. Early Hum Dev. (2011) 87(8):555–8. doi: 10.1016/j.earlhumdev.2011.04.012
18. Johnson GL, Moffett CB, Noonan JA. Doppler echocardiographic studies of diastolic ventricular filling patterns in premature infants. Am Heart J. (1988) 116(6):1568–74. doi: 10.1016/0002-8703(88)90745-4
19. Schierz IAM, Pinello G, Piro E, Giuffre M, La Placa S, Corsello G. Transitional hemodynamics in infants of diabetic mothers by targeted neonatal echocardiography, electrocardiography and peripheral flow study. J Matern Fetal Neonatal Med. (2018) 31(12):1578–85. doi: 10.1080/14767058.2017.1320544
20. Jain A, Mohamed A, El-Khuffash A, Connelly KA, Dallaire F, Jankov RP, et al. A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: normative data and z scores. J Am Soc Echocardiogr. (2014) 27(12):1293–304. doi: 10.1016/j.echo.2014.08.018
21. Evans NJ, Archer LN. Postnatal circulatory adaptation in healthy term and preterm neonates. Arch Dis Child. (1990) 65(1 Spec No):24–6. doi: 10.1136/adc.65.1_spec_no.24
22. Kozák-Bárány A, Jokinen E, Saraste M, Tuominen J, Välimäki I. Development of left ventricular systolic and diastolic function in preterm infants during the first month of life: a prospective follow-up study. J Pediatr. (2001) 139(4):539–45. doi: 10.1067/mpd.2001.118199
23. Mori K, Nakagawa R, Nii M, Edagawa T, Takehara Y, Inoue M, et al. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart. (2004) 90(2):175–80. doi: 10.1136/hrt.2002.008110
24. Clark SJ, Yoxall CW, Subhedar NV. Measurement of right ventricular volume in healthy term and preterm neonates. Arch Dis Child Fetal Neonatal Ed. (2002) 87(2):F89–93. doi: 10.1136/fn.87.2.f89
25. Koestenberger M, Nagel B, Ravekes W, Urlesberger B, Raith W, Avian A, et al. Systolic right ventricular function in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology. (2011) 100(1):85–92. doi: 10.1159/000322006
26. Eriksen BH, Nestaas E, Hole T, Liestøl K, Støylen A, Fugelseth D. Myocardial function in term and preterm infants. Influence of heart size, gestational age and postnatal maturation. Early Hum Dev. (2014) 90(7):359–64. doi: 10.1016/j.earlhumdev.2014.04.010
27. Voigt JU, Cvijic M. 2- And 3-dimensional myocardial strain in cardiac health and disease. J Am Coll Cardiovascular Imaging. (2019) 12(9):1849–63. doi: 10.1016/j.jcmg.2019.01.044
Keywords: transition, cardiocirculatory adaptation, ecocardiography, normal reference, newborns
Citation: Suciu LM, Prelipcean I, Făgărășan A, Giesinger RE and McNamara PJ (2023) Normative echocardiography data of myocardial adaptation to extrauterine life: a review of prospective studies. Front. Pediatr. 11:1192618. doi: 10.3389/fped.2023.1192618
Received: 23 March 2023; Accepted: 6 June 2023;
Published: 16 June 2023.
Edited by:
Yogen Singh, Cambridge University Hospitals, United KingdomReviewed by:
Belinda Chan, The University of Utah, United StatesSajeev Job, Cambridge University Hospitals, United Kingdom
© 2023 Suciu, Prelipcean, Făgărășan, Giesinger and McNamara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mihaela Suciu bGF1cmFtaWhhZWxhc3VjaXVAeWFob28uY29t
 Laura Mihaela Suciu
Laura Mihaela Suciu Irina Prelipcean2
Irina Prelipcean2 Amalia Făgărășan
Amalia Făgărășan Regan E. Giesinger
Regan E. Giesinger Patrick J. McNamara
Patrick J. McNamara