- 1Departments of Medicine, Mizan Tepi University, Mizan, Ethiopia
- 2Departments of Psychatry, Dilla University, Dilla, Ethiopia
- 3Departments of Midwifery, Dilla University, Dilla, Ethiopia
- 4Departments of Anatomy, Jimma University, Jimma, Ethiopia
Background: Neural tube defects are a major public health issue that contributes significantly to morbidity and mortality, particularly in low-income countries such as Ethiopia. In Ethiopia, particularly in the study setting, there is a paucity of data on the prevalence, magnitude, and associated factors of neural tube defects. As a result, the purpose of this study was to evaluate neural tube defects and associated factors in JUMC.
Methods: This study was an institution-based cross-sectional study conducted from June to September 2021. Data was collected using a structured questionnaire adapted from previous literature. Data were analyzed using SPSS version 26 software. Logistic regression analysis was performed to assess the association between dependent and independent variables. Independent variables with a p-value < 0.05 were taken as statistically significant with neural tube defects.
Results: The prevalence of NTDs in this study was 3.6%. Preterm newborns with GA < 34 AOR 2.9(1.2–9.7), newborns with birth weight b/n 1,000–1,499 AOR 5.2(1.1–9.4), born with weight b/n 1,500–2,499 AOR 2.1(1.3–8.7), exposure to smoke AOR 2.1(1.2–8.8), radiation AOR 6.8(1.3–14.5), at least one history of abortion AOR 10.1(7.2–21.0) and mothers with AED intake AOR 5.7(2.3–18.4) were found to be associated significantly.
Conclusion: The results indicated a significant frequency of neural tube abnormalities in newborns. The use of AEDs, abortion, and radiation have all been linked to those NTD cases. Pregnant women are advised to learn more about the need of beginning prenatal care as soon as possible because the aforementioned issues will be addressed during this treatment.
Background
A neural tube defect (NTD) happens when the neural tube fails to close properly, exposing the neurodevelopment or spinal cord to amniotic fluid (1). Spina bifida, anencephaly (absence of brain calvariates, totally or partially), encephalocele (brain shrink and Calvary defect meninges), craniorachischisis (anencephaly associated with permanent spinal and neural tissue exposure), and anencephaly (dysraphism of the occipital region accompanied by retroflexion of the neck and trunk) are included in the spectrum (2).
NTDs are divided into two types: “open” NTDs where Open” NTDs include craniorachischisis, which results from total neurulation failure and leaves most of the brain and spinal cord open, anencephaly, which occurs when a defect occurs in the cranial region, and spina bifida cystica in the lumbosacral region (3).
Neural tube defects are among the top five most severe birth defects worldwide, and they are associated with significant death, morbidity, and disability. Each year, an estimated 300,000 babies are born with NTDs, resulting in approximately 88,000 deaths and 8,6 million disability-adjusted life years (DALYs) (4, 5). NTDs can account for up to 29% of neonatal deaths due to observed birth defects in low-income countries. In Africa, NTD are the most common birth defects. Around 1–3/1,000 births are affected per year. In Ethiopia, 63.3 cases per 6,910,000 children had an estimated pooled prevalence of neural tube defects (6–8).
Multiple medical and socioeconomic impacts are associated with neural tube defects. They could 71 lead to fetal malformation or stillbirth, and about 50 percent optional termination of pregnancies. Around 75% of living births with an NTD resulted in the deaths of children under 5 and disabilities globally (9). In resource-limited countries where preventive measures and long-term 75 care for surviving patients are restricted, the consequences of NTDs are especially obvious (10).
Life-long physical problems from NTD require lifetime medical attention which adds a considerable burden for the patients concerned, their families, national health services, and governments (11). Parents face high distress when their child is diagnosed with defects during pregnancy, and they must choose between the grief of a termination/stillbirth or the financial and emotional challenges of caring for a child with disabilities (12).
Lifetime direct medical and indirect costs are significant for the affected patients, parents, and families. Prevention ensures that such a multi-factorial burden does not occur. In addition to its burden, stigma towards NTDs by the community has been documented elsewhere in Africa, affecting the quality of life of families through social, economic, and emotional distress (13).
Studies conducted across the globe have identified different risk factors associated with NTDs. These include low socioeconomic status, maternal exposure to certain environmental factors (i.e., chemicals and pesticides), tobacco use during pregnancy, genetic factors, pregnancy in the late maternal age, poor intake of folic acid prior to or during pregnancy, sex of the neonate, and lack of antenatal care (14–17). Furthermore, there exists little evidence from Ethiopia, particularly the study area. As a result, the current study was designed to estimate the level of NTD among newborns.
Methods and materials
Study design and period
An institutional-based cross-sectional study design was employed from June 2021 to September 2021, at Jimma university medical center.
Study setting
The study was conducted at Jimma University Medical Center (JUMC). Jimma University medical center (JUMC) is found in Jimma town, Oromia regional state, which is 352 km from Addis Ababa to the southwest, the capital city of Ethiopia. JUMC is one of the oldest governmental hospitals, which was established in 1937 during the Italian occupation for the service of their soldiers. After the withdrawal of the colonial occupiers, it has been running as a public hospital under the Ministry of Health by different names at different times and is currently named “Jimma University Medical Center”.
Population
The inclusion criteria of the study were: all newborns and their mothers discovered during data collection. Those newborns with mothers who were seriously ill or unable to communicate during the data collection period were excluded from the study.
Sample size determination and sampling technique
The sample size was calculated using the single population proportion formula with a 95% confidence interval, a 3% margin of error (because the estimated proportion is small, 20%), the previous study's population proportion of 5.71%, and a 10% non-response rate. The study's final sample size was 253. During the data collection period, study participants were chosen using systematic random sampling.
Study variables
Neural Tube Defects (presence/absence) was the outcome variable and independent variables include Socio demographic factors mother and new born (Maternal Age, Residence, Marital status, Gestational age in weeks, Sex of new born, Birth weight in grams), Perinatal and Behavioral Characteristics of Mothers (Place of antenatal care, Mode of delivery, Passive smoking, Radiation exposure, History of abortion or/and still birth, Amount of coffee per day, AEDs intake during pregnancy, Parity, Spouse relation), and Physical Examination of Newborns (Consciousness, Tone upper left and right, Tone left and right lower, Anal tone, Moro reflex, grasp reflex, suckling reflex, Sensation right and left lower, Sensation right and left upper, Power right and left lower, Deep tendon reflex right and left lower, Cradle maneuver).
Data collection method and instrument
Data was collected using a structured questionnaire adapted from previous literature. The questionnaire was first prepared in English and then translated into local languages (Amharic and Afan Oromo) and back into English for consistency by different language experts. Data was then collected by two neonatal nurses and one General Practitioner using face-to-face interviews with maternally related variables (sociodemographic characteristics, labor and delivery-related factors, obstetric and maternal lifestyle, and clinical symptoms of newborns) and physical examination of the newborn by a GP and a thorough review of medical records to obtain birth weight and condition of the newborn before admission.
Data processing and analysis
The collected data was checked for its completeness and cleaned before entry into the computer. Then, the data was coded, cleaned, edited, and entered into Epi Data version 4.6 and exported to SPSS window version 26 for analysis. Descriptive statistics were presented in frequency, tables, texts, and summary measures. Bivariate and multivariate analyses were done to observe the association between each independent variable and outcome variable by using binary logistic regression. The goodness of fit was checked by the Hosmer-Lemeshow statistic at a P-value of greater than 0.05. All variables with P < 0.25 in the bivariate analysis were included in the final model of multivariate analysis in order to control all possible confounders. The statistical association was measured by odds ratio with 95% CI. Adjusted odds ratio along with 95% CI was estimated to identify the associated factors with Neural tube defects by using multivariate analysis in binary logistic regression. In this study, a P-value < 0.05 was considered statistically significant.
Result
Maternal sociodemographic characteristics
From the total of 253 mother neonate pairs requested, all participated in the study giving a response rate of 100%. The mean age of the mothers was 23 ± 5year with rural residents accounting for 67.99%. Of the mothers who participated in the study, 186 (73.51%) were married with the plurality (104, 41.1%) attending up to primary school and 146 (57.7%) having a low monthly income (<2143brr). The majority of newborns were female, accounting for 139 (54.5%). Regardless of sex, 21 (8.3%) were born <34 weeks, 59 (23.3%) b/n 34–36 wks, and the majority were born between 37 and 42 weeks of gestation respectively. Most newborns (174, 68.82%) weighed b/n 2,500–4,000 grams (Table 1).
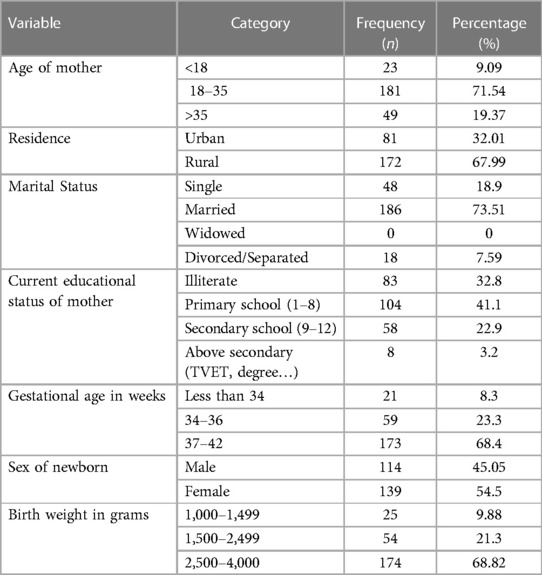
Table 1. Sociodemographic characteristics of mother-newborn pairs at labor ward JUMC, southwestern Ethiopia, in August 2021.
Perinatal and behavioral characteristics
Regarding perinatal and behavioral characteristics, 89.64% had ANC visits and a majority 163 (64.4%) had follow-up at the health center level. Of all the mothers that participated in the study, 10 (3.95%) of them took folate supplements at or before the first trimester of pregnancy. Only 62 (24.49%) of them were exposed to passive smoking and 23 (9.09%) were exposed to radiation during pregnancy. The majority 212 (83.79%) of the mothers had coffee intake, with 169 (66.7%) of drinkinh less than three cups per day. It was found that 13 (5.12%) of the mothers had a history of abortion and/or stillbirth. Most (150, 59.7%) of the mothers are Multiparous and 3 (1.2%) of them were in a consanguineous relationship (Table 2).
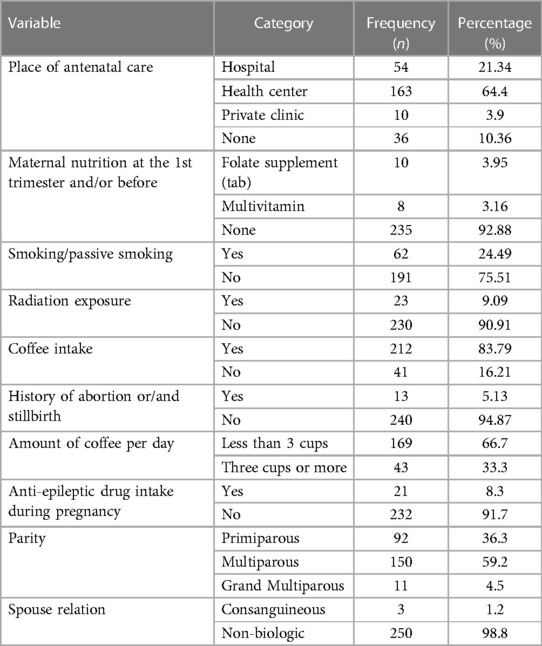
Table 2. Perinatal and behavioral characteristics of mothers at labor ward JUMC southwest Ethiopia, August 2021 EC.
Prevalence of neural tube defect
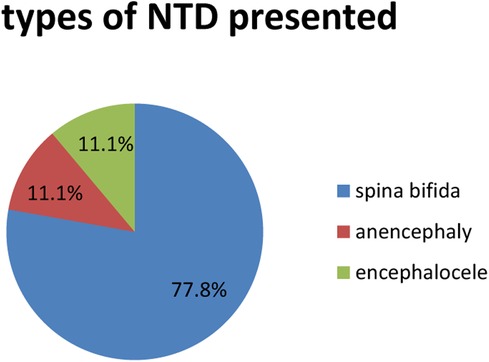
From 253 newborns, only 9 (3.6%) of them were found to have neural tube defects, with the most common (7, 77.7%) attributed to spina bifida followed by anencephaly and encephalocele, each accounting for 1 (11.1%) NTD. The most common area for spina bifida to be located was the thoracolumbar region (4, 57.4%) (Figure 1).
Physical examination of newborns
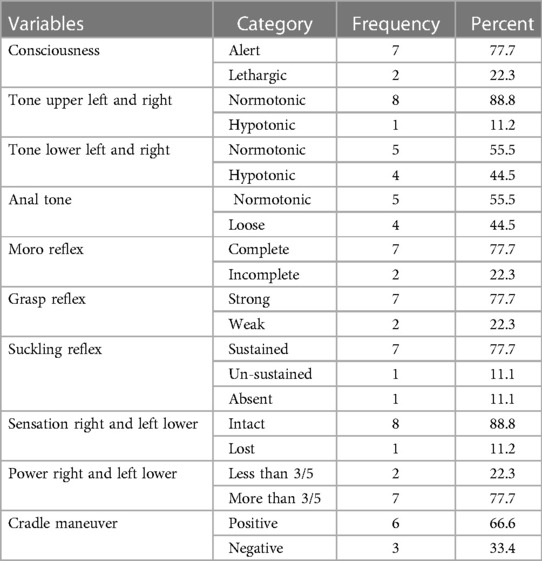
A physical examination done on the affected neonates reveals that consciousness was altered in 2 (22.3%) of them, with an upper limb being hypotonic in 1 (11.2%) and a lower limb being hypotonic in 4 (44.5%). Loose anal tone only affected in 4 (44.5%) of them, with abnormal reflexes, Moro, grasp, and suckling accounting for 2 (22.3%) each (Table 3).
Factors associated with neural tube defects
Bivariate regression analysis was done to see factors associated with neural tube defects. Hence, GA in weeks, Birth weight, maternal nutrition, exposure to smoke, radiation, Amount of coffee intake per day, Residence, history of abortion, and AED intake during pregnancy were found to be associated with neural tube defect and were inserted into multivariate regression analysis; meanwhile, in multivariate regression analysis GA, birth weight, exposure to smoke, radiation, history of abortion and AED intake during pregnancy were found to be significantly associated with neural tube defects. Preterm newborns with GA < 34 have a 3 times greater chance of having an NTD with an AOR of 2.9 (1.2–9.7).
Newborns with birth weight b/n 1,000–1,499 have approximately five times more chance with AOR 5.2 (1.1–9.4) of having neural tube defect while those born with weight n/n 1,500–2,499 have 2 times the chance of NTD with an AOR of 2.1 (1.3–8.7). Exposure to smoke and radiation were found to have the likelihood of association with developing NTD two times with AOR 2.1(1.2–8.8) and 7 times with AOR 6.8 (1.3–14.5) respectively. Furthermore, mothers with a history of at least one abortion had ten times the chance of having a baby with NTD, with AOR 10.1(7.2–21.0), and mothers with AED intake have approximately 6 times more chance of having a baby with NTD, with AOR 5.7(2.3–18.4) than their counterparts (Table 4).
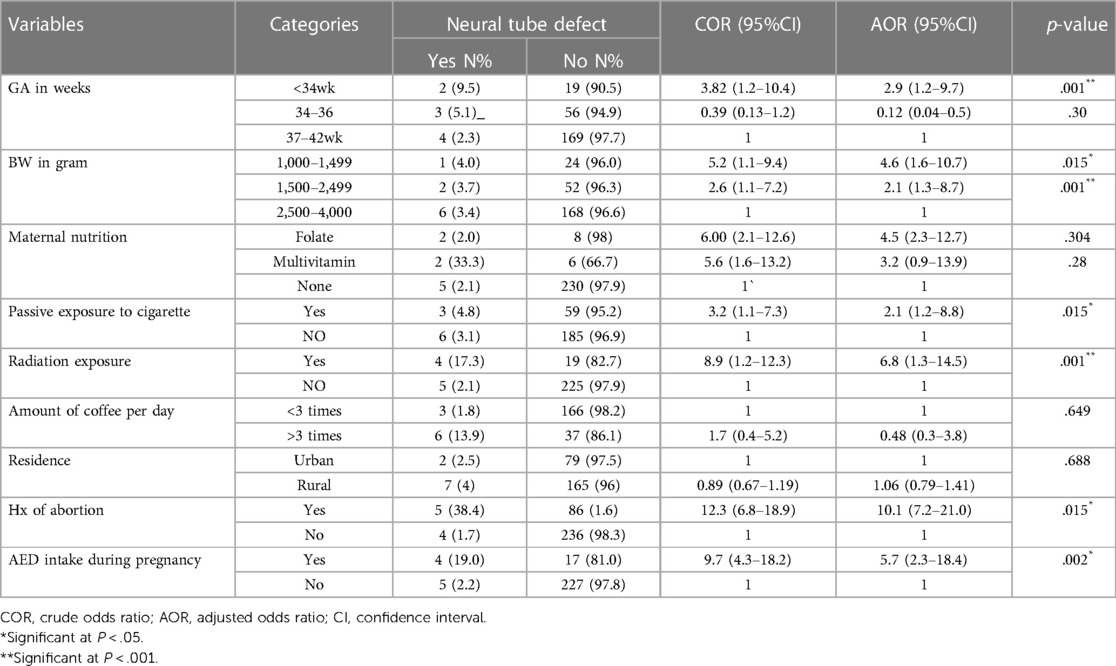
Table 4. Bivariate and multivariable factors associated with NTDs at labor ward JUMC in August, 2021 GC.
Discussion
This study assessed the prevalence of neural tube defects and their associated factors among newborns in Jimma medical center. The overall magnitude of NTD in this study was found to be 3.6% (95%CI: 2.29–5.65) among newborns at Labor Ward JUMC in southwest Ethiopia. Spina bifida accounted for the majority (77.8%) of cases, and anencephaly and encephalocele accounted for 11.1% each. This is comparable to the study done at NICU in HFSUH Eastern Ethiopia having a prevalence of 5.71%; of these, 20 (83.5%) and 4 (16.5%) had spina bifida and encephalocele respectively (18). NTDs were defined as cases of anencephaly, spina bifida, and encephalocele based on ICD-10 criteria. During seven months, they observed 55 cases of NTDs out of 8,677 births after 28 weeks of gestation—birth prevalence of 63.4 per 10,000 births [95% confidence interval (CI), 51–77] (14).
Based on our study and prior studies as noted above, the rate of NTDs is much higher than that reported in Latin America where the prevalence of neural tube defects was 4.73 per 1,000 deliveries (89:18,807). The most common neural tube defects were myelomeningocele (47.2%), anencephaly (26.9%), and encephalocele (16.9%) (19). Additionally, our study finding is higher than the study done on Neural tube defects among neonates delivered in Al-Ramadi Maternity and Children's Hospital, western Iraq, where 33 infants were delivered with NTDs, giving an incidence of 3.3/1,000 births. The most prevelant NTD types were myelomeningocele and anencephaly at thoracolumbar and lumbosacral sites (20).
The possible explanation for the difference might be differences in socio-cultural and environmental factors, availability of health services, and low attention given to NTDs in developing countries including ourown. In addition to this low sample size in this study might be a possible explanation for the difference observed.
Factors associated with NTDs were identified by multivariate logistic regression analysis. According to the study analysis GA, birth weight, exposure to smoke, radiation, history of abortion/stillbirth, and AED intake during pregnancy were factors associated with NTD.
The study reveals that Preterm newborns with GA < 34 have 3 times the chance of having an NTD. Newborns with birth weight b/n 1,000–1,499 have approximately a five times greater chance of having a neural tube defect while those born with weight b/n 1,500–2,499 have a 2 times higher chance of NTD. These factors are supported or consistent with the studies done in Hiwot Fana (18), Ethiopia, and three teaching hospitals in Addis Ababa (14).
Exposure to smoke and radiation were found to have a likelihood of association with developing NTD two and seven times greater than the baseline, respectively. This is supported by studies carried out in China (21) and Turkey (22).
Mothers with AED intake have approximately 6 times more chance of having a baby with NTD than their counterparts. The studies conducted in the Netherlands (23) and western Ethiopia (24) also support this finding.
Furthermore, mothers with history of at least one abortion had ten times the chance of having a baby with NTD. This finding is supported by studies in China (21), Saudi Arabia (25), and Iran (26).
Conclusion
The burden of neural tube defects was 3.6 percent among newborns in Jimma medical center. It is preventable if mandatory fortification of foods with folic acid is initiated before conception. NTDs have been associated with gestational age and birth weight, AED intakes during pregnancy, abortion history or mortality, and exposure to radiation. Our findings recommend that pregnant mothers become more aware of the timely introduction of prenatal care because the above factors will be tackled in the course of this care. More attention is needed to monitor NTDs in southwest Ethiopia. This study highlights the urgent need for further interventional studies to develop innovative solutions to improve outcomes in the study settings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Jimma University ethical review committee (IRB). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All of the listed authors contributed significantly, directly, and intellectually to the work and approved its publication. All authors contributed to the article and approved the submitted version.
Funding
Jimma University College of Medicine and Health Science provided funding for this study.
Acknowledgments
We would like to thank Jimma University's Faculty of Medical Sciences for providing research funding. We would like to express our appreciation to all of the data collectors, supervisors, and study participants for their significant contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO/CDC/ICBDSR. Birth defects surveillance: a manual for programme. Geneva World Heal Organ. (2014). 1:101–23. https://www.knowledgehub.org.za/
2. (WHO) WHO. Congenital anomalies. Facts sheets. World Heal Organ. (2016) 55:1044–6. https://www.who.int/news-room/fact-sheets/detail/birth-defects
3. Sadler TW. Embryology of neural tube development. Am J Med Genet C Semin Med Genet. (2005) 135c:2–8. doi: 10.1002/ajmg.c.30049
4. Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—united States, 1995–2011. MMWR Morb Mortal Wkly Report. (2015) 64:1. PMID: 25590678; PMCID: PMC4584791
5. Morris JK, Springett AL, Greenlee R, Loane M, Addor M-C, Arriola L, et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS One. (2018) 13:2–7. doi: 10.1371/journal.pone.0194986
6. Taye M, Afework M, Fantaye W, Diro E, Worku A. Magnitude of birth defects in central and northwest Ethiopia from 2010 to 2014: a descriptive retrospective study. PLoS One. (2016) 11:10–3. doi: 10.1371/journal.pone.0161998
7. Sorri G, Mesfin E. Patterns of neural tube defects at two teaching hospitals in Addis Ababa, Ethiopia a three years retrospective study. Ethiop Med Journal. (2015) 53:119–26. https://www.researchgate.net/profile/
8. Atlaw D, Worku A, Taye M, Woldeyehonis D, Muche A. Neural tube defect and associated factors in bale zone hospitals, southeast Ethiopia. J Pregnancy Child Heal. (2019) 6:412. https://www.researchgate.net/profile/Demelash-Handiso/
9. WHO. “MCEE-WHO methods and data sources for child causes of death 2000–2016,.” dep evidence, inf res. Geneva: WHO. Matern Child Epidemiol Estim (MCEE). (2016).
10. Seidahmed MZ, Abdelbasit OB, Shaheed MM, Alhussein KA, Miqdad AM, Khalil MI, et al. Epidemiology of neural tube defects. Saudi Med Journal. (2014) 35:S29–35. PMID: 25551108; PMCID: PMC4362102
11. Kondo A, Matsuo T, Morota N, Kondo AS, Okai I, Fukuda H. Neural tube defects: risk factors and preventive measures. Congenit Anom (Kyoto). (2017) 57:150–6. doi: 10.1111/cga.12227
12. Flores A, Valencia D, Sekkari A, Hillard CL, Williams J, Groisman B, et al. Building capacity for birth defects surveillance in Africa: implementation of an intermediate birth defects surveillance workshop. J Glob Heal Perspect. (2015) 11:10–4. PMID: 26753106; PMCID: PMC4706176
13. Flores AL, Vellozzi C, Valencia D, Sniezek J. Global burden of neural tube defects, risk factors, and prevention. Indian J Community Heal. (2014) 26:3–5. PMID: 26120254; PMCID: PMC4480200
14. Gedefaw A, Teklu S, Tadesse BT. Magnitude of neural tube defects and associated risk factors at three teaching hospitals in Addis Ababa, Ethiopia. Biomed Res Int. (2018) 2018:1–8. doi: 10.1155/2018/4829023
15. Salih MA, Murshid WR, Seidahmed MZ. Epidemiology, prenatal management, and prevention of neural tube defects. Saudi Med Journal. (2014) 35:S15–28. PMID: 25551106; PMCID: PMC4362104
16. Dessie MA, Zeleke EG, Workie SB, Berihun AW. Folic acid usage and associated factors in the prevention of neural tube defects among pregnant women in Ethiopia: cross-sectional study. BMC Pregnancy Childbirth. (2017) 17(313):12–5. doi: 10.1186/s12884-017-1506-2
17. Liu J, Xie J, Li Z, Greene NDE, Ren A. Sex differences in the prevalence of neural tube defects and preventive effects of folic acid (FA) supplementation among five counties in northern China: results from a population-based birth defect surveillance programme. BMJ Open. (2018) 8:11. doi: 10.1136/bmjopen-2018-022565
18. Edris Y, Abdurahman H, Desalew A, Weldegebreal F. Neural tube defects and associated factors among neonates admitted to the neonatal intensive care units in hiwot fana specialized university hospital, harar, Ethiopia. Glob Pediatr Heal. (2020) 7:7–11. doi: 10.1177/2333794X20974218
19. Rosenthal J, Casas J, Taren D, Alverson CJ, Flores A, Frias J. Review article neural tube defects in Latin America and the impact of fortification : a literature review. Public Heal Nutr. (2013) 17(3):537–50. doi: 10.1017/S1368980013000256
20. Al-Ani ZR, Al-Hiali SJ, Al-Mehimdi SM. Neural tube defects among neonates delivered in Al-Ramadi maternity and children’s hospital, western Iraq. Saudi Med J. (2010) 31(2):163–9. https://www.researchgate.net/profile/Zaid-Al-Ani/20174732
21. Li Z, Ren A, Zhang L, Ye R, Li S, Zheng J, et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res Part A—Clin Mol Teratol. (2006) 76(4):237–40. doi: 10.1002/bdra.20248
22. Altun E, Atik A, Adilay HU, Kahraman A, Öztürk K, Güçlü B, et al. Effects of irradiation on neural tube development. Turk Neurosurg. (2020) 30(3):371–6. doi: 10.5137/1019-5149.JTN.27015-19.1
23. Blatter BM, van der Star M, Roeleveld N. Review of neural tube defects : risk factors in parental occupation and the environment. Environ Health Perspect. (1994) 102(2):140–5. doi: 10.1289/ehp.94102140
24. Sorri G, Mesfin E. Patterns of neural tube defects at two teaching hospitals in Addis Ababa, Ethiopia a three years retrospective study. Ethiop Med J. (2015) 53(3):119–26. https://www.researchgate.net/profile/Eyasu-Kassa-2/26677521
25. Al-Mendalawi MD. Epidemiology of neural tube defects. Saudi Med J. (2015) 36(3):373–4. doi: 10.15537/smj.2015.3.11087
Keywords: prevalence, neural tube defect, newborn, jimma, Ethiopia
Citation: Kidane M, Sime Y, Gashaw A and Chane G (2023) Neural tube defects among new borns: a cross-sectional study. Front. Pediatr. 11:1191556. doi: 10.3389/fped.2023.1191556
Received: 27 March 2023; Accepted: 3 May 2023;
Published: 22 May 2023.
Edited by:
Theodora Boutsikou, National and Kapodistrian University of Athens, GreeceReviewed by:
Adaobi Solarin, Lagos State University Teaching Hospital, NigeriaJeffrey Thomas White, University of Louisville, United States
© 2023 Kidane, Sime, Gashaw and Chane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohanes Sime am9obnNpbWU0MDBAZ21haWwuY29t
 Mahder Kidane1
Mahder Kidane1 Yohanes Sime
Yohanes Sime Anteneh Gashaw
Anteneh Gashaw Getachew Chane
Getachew Chane