
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 28 July 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1189648
 Chao Gong1
Chao Gong1 Annan Liu1
Annan Liu1 Beibei Lian1
Beibei Lian1 Xixi Wu1
Xixi Wu1 Pei Zeng1
Pei Zeng1 Chaoli Hao1
Chaoli Hao1 Bobo Wang1
Bobo Wang1 Zhimei Jiang1,2
Zhimei Jiang1,2 Wei Pang1,2
Wei Pang1,2 Jin Guo1,2*
Jin Guo1,2* Shaobo Zhou3*
Shaobo Zhou3*
Objective: To study the worldwide prevalence and associated factors of epilepsy in children and adolescents with Cerebral Palsy (CP) and to analyze the differences between various subgroups.
Method: We identified all potential studies on the prevalence of epilepsy in children and adolescents with CP from PubMed, Web of Science, and Embase. The search time was from the establishment of the database to November 2022. Randomized effects meta-analysis models were used to calculate the prevalence of epilepsy in CP. Subgroup analysis and meta-regression were utilized to further explore heterogeneity between articles and prevalence disparities between subgroups. The funnel plot and Egger's test were used to investigate potential publication bias.
Results: Seventy-two articles, comprising 53,969 children and adolescents with CP, were included in this study. The results indicated a total epilepsy prevalence of 38.0% (95% CI: 34.8%–41.2%) in CP. The prevalence of epilepsy was 46.4% (95% CI: 41.4%–51.5%) in clinical sample-based studies and 31.6% (95% CI: 28.7%–34.5%) in population-based studies. Meta-regression demonstrated that the sample source, neonatal seizure, family history of epilepsy, EEG or cranial imaging abnormalities, intellectual/cognitive impairment, and topographical types of CP were heterogeneous contributors to the epilepsy prevalence in CP.
Conclusion: Approximately one-third of children and adolescents with CP have epilepsy, and the sample source can significantly impact the total prevalence of epilepsy. Neonatal seizures, family history of epilepsy, EEG abnormalities, cranial imaging abnormalities, severe intellectual disability, and quadriplegia may be contributing factors to epilepsy comorbid in CP. Further study is required to verify the strength of these associations with epilepsy. This study aids in identifying the clinical characteristics of young people with CP at risk of developing epilepsy, which may assist clinicians in the early prevention and diagnosis of epilepsy within this population.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=367766, identifier CRD42022367766.
Cerebral palsy (CP) is a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain (1). The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, behaviour, epilepsy, and secondary musculoskeletal problems (1). According to population-based studies, the global prevalence of CP ranges between 0.1% and 0.4% (2), making it a common disorder threatening the early survival of children.
Epilepsy is an independent, significant, and common clinical problem in children with CP and is often regarded as an indicator of CP severity. The prevalence of epilepsy in the general population ranges from 0.4% to 0.8% (3). However it is significantly more prevalent in children with CP. Literature on epilepsy incidence in children with CP varies from 15% to 90%, with most estimates falling between 35% and 41%, making it five times higher than that of able-bodied children (4). This discrepancy may be attributed to CP and epilepsy sharing physiopathologic mechanisms caused by common etiology and risk factors. Many prenatal, peripartum, and postpartum injury, such as hypoxic-ischemic encephalopathy, infection, and congenital brain malformations, can cause CP and epilepsy (5). Epilepsy can be severe in some young people with CP, or it may be self-limiting and similar to other childhood epilepsies (6, 7). Nevertheless, epilepsy poses a significant threat to the early survival of young people with CP due to a variety of injuries and dysfunctions. The onset of epilepsy often indicates a more severe brain injury, affecting all functional aspects (8). Studies have shown that CP with epilepsy are often associated with severe intellectual disabilities, movement disorders, psychological and behavioral problems, and a lower quality of life, with less autonomy (9).
Many studies have shown that CP and epilepsy share a common etiologies and risk factors, and their co-occurrence rate is high. However, no systematic review and meta-analysis have yet been conducted on the prevalence of epilepsy in CP. Therefore, this systematic review aims to retrieve all published articles on the prevalence of epilepsy in children and adolescents with CP, estimate the epilepsy prevalence in CP using a meta-analysis, and analyze factors leading to epilepsy in CP, to provide evidence-based medical support.
The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42022367766).
Three databases (PubMed, Embase, and Web of Science) were systematically searched to determine the prevalence of epilepsy in children and adolescents with CP for all potentially relevant studies from database establishment to November 2022. The following search terms were used: cerebral pals*, CP, Little Disease, Spastic Diplegia, epileps*, seizure*, Aura, Convulsion, prevalence, epidemic, epidemiology, rate, morbidity, searched by combining MeSH Terms and Title/Abstract, combined according to Boolean logic principles (using AND, OR, or NOT). We used Endnote 20 to manage and remove repetitive articles. The reference lists of selected articles were also manually searched. In addition, articles on either CP or the prevalence of comorbidities with CP were searched in this systematic review. The search terms were presented in Supplementary S1.
(1) Articles that directly or indirectly reported the prevalence of CP with epilepsy. (2) English articles. (3) Articles containing original data: a cross-sectional study, a case-control study, and a cohort study. (4) Children and adolescents under the age of 19 with CP were included, in line with World Health Organization (WHO) criteria for the age of children and adolescents (10).
(1) Reviews, systematic reviews, meta-analyses, meeting summaries, student papers, comments, or letters. (2) To ensure that estimates reflect the expected population, articles with fewer than 50 participants were excluded. (3) The full text could not be obtained. (4) Article data were missing, repeated, or unavailable. (5) Articles on studying a certain type of CP population.
Two reviewers (CG and BBL) screened the retrieved articles by reading titles and abstracts according to the above criteria to exclude unrelated and repetitive articles, then read the full texts of the remaining publications independently. Finally, they discussed differences with a third expert (JG) to reach a consensus. The following data were extracted separately from each article: first author, year of publication, time of case collection, type of study, the total number of individuals with CP, number with epilepsy, prevalence, age, diagnostic criteria for epilepsy developed by the International League Against Epilepsy (ILAE), and proportion of females. If necessary, the study authors were contacted for more data or clarification. After discussion, the disagreement was resolved by consensus with the third senior reviewer (ZMJ).
We used the Hoy et al. (11) tool to assess the risk of bias in studies measuring the prevalence of epilepsy in CP. The tool included ten items to judge the risk of bias: (1) selection-related bias, (2) bias associated with non-response, (3) measurement-related bias, and (4) bias associated with analysis. Each item included two options: (1) high or low risk. The high-risk option was chosen if the study lacked basic information to make the judgment. The overall assessment of the risk of bias was rated as low (three or fewer high-risk items), moderate (four to five high-risk items), and high (six or more high-risk items) risk bias. Two reviewers (XXW and PZ) individually reviewed each study. The disagreement was resolved by consensus and in consultation with the senior reviewer (WP). The details of Hoy were presented in Supplementary S2.
Stata 17.0 (Stata Corp, College Station, TX, USA) was used to analyze the prevalence of epilepsy in children and adolescents with CP and provided its 95% confidence interval (CI). The Q test analyzed the heterogeneity among the included studies, and I2 quantitatively judged the heterogeneity. If the heterogeneity was high (I2 ≥ 50%), the source of heterogeneity was further analyzed. After excluding the influence of articles with obvious heterogeneity, the random effect model (REM) was used for Meta-analysis. Otherwise, the fixed effect model (FEM) was used. Subgroup analysis and Meta-regression analysis were used to explore the sources of heterogeneity and the differences in the prevalence of epilepsy among subgroups.
According to the sample source in the articles, we divided the articles into two groups: a clinical sample-based study group and a population-based study group. Clinical sample-based studies considered individuals with CP from hospitals, clinics, or rehabilitation institutions. Population-based studies were considered individuals with CP in the entire population or a random sampling population. Subgroup analysis was performed on the gender, ethnic group, study type, national income level, diagnostic criteria for epilepsy developed by the International League Against Epilepsy (ILAE), and publication time of the two groups to explore the sources of heterogeneity and the differences in the prevalence of epilepsy between different groups.
In addition, we performed subgroup analysis and meta-regression analysis based on the characteristics of children with CP to explore the differences in the prevalence of epilepsy between different groups. The related factors included gestational age, birth weight, birth asphyxia, neonatal seizures (NS), family history of epilepsy, central nervous system (CNS) abnormalities based on MRICS (Magnetic Resonance Imaging Classification System), electroencephalography (EEG) abnormalities, cranial imaging abnormalities, cognitive/intellectual status, clinical type of CP and topographical type of CP.
3,020 articles were retrieved: PubMed: 605, Embase: 904, Web of Science: 1,511. After removing duplicates, 1,641 articles remained. After title and abstract screening, 204 full-texts were evaluated for eligibility according to inclusion and exclusion criteria, 69 articles were included. In addition, 3 articles were included by other search methods. Therefore, a total of 72 articles were included in this study. The article selection process is shown in Figure 1.
The 72 articles included in this meta-analysis were published from 1998 to 2022, involving 38 countries and regions worldwide, and the data were collected from 1988 to 2019. There were 32 articles based on clinical samples and 40 articles based on population. According to the main ethnic composition of the country, the ethnic groups included are Europa (43 articles), Mongolian (8 articles), Black (11 articles), and multi-ethnic mixed (10 articles). The number of children with CP included in the article ranged from 62 to 9,654, totaling 53,969 people. The quality scores of the articles ranged from 1 to 5 points, of which 52 were low and 20 were moderate. A detailed description of the articles is shown in Table 1. The detailed quality assessment results are shown in Supplementary S3.
As shown in Figure 2, the total prevalence of epilepsy in CP was 38.0% (95% CI: 34.8%–41.2%), and significant heterogeneity was found in the study I2 = 94.8%, P < 0.001). Table 2 shows a statistically significant difference between clinical sample-based and population-based studies (P < 0.001). As shown in Figure 2 and Table 2, 32 articles were clinical sample-based studies. The prevalence of epilepsy was 46.4% (95% CI: 41.4%–51.5%), with significant heterogeneity (I2 = 88.3%, P < 0.001). As shown in Figure 2, 40 articles were population-based studies. The prevalence of epilepsy was 31.6% (95% CI: 28.7%–34.5%), with significant heterogeneity (I2 = 91.9%, P < 0.001).
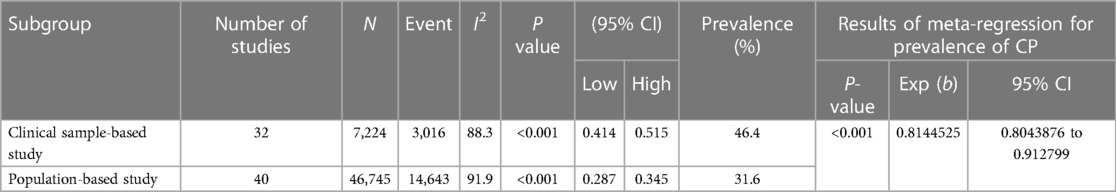
Table 2. According to the sample source in the articles, the estimated prevalence of epilepsy in CP.
As shown in Table 3, the prevalence of epilepsy in males with CP was 47.4%, and the prevalence of epilepsy in females with CP was 49.2%. The prevalence of epilepsy in 1981, 1989, and 1993 ILAE was 44.3%, and the majority of epilepsy in 2014 and 2017 ILAE was 44.9%. According to the ethnic group, the prevalence of epilepsy in the European ethnic group was 46.6%, the prevalence of epilepsy in the Mongolian ethnic group was 32.8%, the prevalence of epilepsy in the Black ethnic group was 48.6%, and the prevalence of epilepsy in the multi-ethnic group was 64.4%. According to the national income level, the prevalence of epilepsy in low-and middle-income countries was 47.0%, and the prevalence of epilepsy in high-income countries was 43.0%. The prevalence of epilepsy in CP was 39.9% from 1998 to 2003, 50.2% from 2011 to 2016, and 46.7% from 2017 to 2022.
As shown in Table 4, the prevalence of epilepsy in males with CP was 36.2%, and the prevalence of epilepsy in females with CP was 31.4%. The prevalence of epilepsy in 1981, 1989, and 1993 ILAE was 33.2%, and the majority of epilepsy in 2014 and 2017 ILAE was 32.4%. According to the ethnic group, the prevalence of epilepsy in the European ethnic group was 31.6%, the prevalence of epilepsy in the Mongolian ethnic group was 29.6%, the prevalence of epilepsy in the Black ethnic group was 38.1%, and the prevalence of epilepsy in the multi-ethnic group was 29.6%. According to the national income level, the prevalence of epilepsy in low- and middle-income countries was 30.7%, and that in high-income countries was 32.1%. The prevalence of epilepsy in CP was 26.4% from 1998 to 2003, 34.0% from 2004 to 2010, 32.7% from 2011 to 2016, and 31.5% from 2017 to 2022. The distribution of epilepsy prevalence is shown in Figure 3.
As shown in Table 5, in terms of gestational age, the prevalence of epilepsy in preterm CP (36.4%, 95% CI: 28.4%–44.4%) was lower than in term birth (45.7%, 95% CI: 38.9%–45.7%). Regarding birth weight, the prevalence of epilepsy in low birth weight CP (44.9%, 95% CI: 30.4%–59.4%) was lower than that in normal weight children (53.4%, 95% CI: 38.7%–68.0%). Regarding birth asphyxia, the prevalence of epilepsy in CP with birth asphyxia (46.6%, 95% CI: 33.4%–59.8%) was higher than that without birth asphyxia (41.7%, 95% CI: 38.1%–52.0%). Regarding NE, the prevalence of epilepsy in CP with NE (66.9%, 95% CI: 56.6%–77.2%) was significantly higher than that without NE (42.1%, 95% CI: 35.3%–48.8%) (P < 0.001). Regarding a family history of epilepsy, the prevalence of epilepsy in CP with a family history of epilepsy (89.2%, 95% CI: 81.1%–97.3%) was significantly higher than that without a family history of epilepsy (49.0%, 95% CI: 44.4%–53.7%) (P < 0.001). Regarding EEG abnormalities, the prevalence of epilepsy in CP with EEG abnormalities (60.8%, 95% CI: 45.3%–76.3%) was significantly higher than that without EEG abnormalities (24.0%, 95% CI: 6%–42.0%) (P = 0.009). Regarding cranial imaging abnormalities, the prevalence of epilepsy in CP with cranial imaging abnormalities (60.8%, 95% CI: 57.8%–37.2%) was significantly higher than that without cranial imaging abnormalities (36.3%, 95% CI: 24.3%–48.3%) (P = 0.009).
Regarding CNS abnormalities based on MRICS, the prevalence from high to low was predominantly gray matter injuries (55.9%, 95% CI: 42.8%–69.0%), brain malformation (52.8%, 95% CI: 47.9%–57.8%), miscellaneous (43.8%, 95% CI: 47.9%–57.8%), normal (40.6%, 30.8%–50.4%) and predominantly white matter injuries (36.5%, 95% CI: 15.3%–57.7%). Regarding cognition/intelligence, the prevalence significantly increased with the degree of cognition/intelligence, followed by normal (17.9%, 95% CI: 12.1%–23.8%), mild (38.5%, 95% CI: 21.5%–51.5%), moderate (67.7%, 95% CI: 56.8%–78.6%), severe (75.2%, 95% CI: 68.8%–81.5%) (P < 0.001). Regarding the clinical type of CP, the prevalence from high to low was the dyskinetic type (52.9%, 95% CI: 44.7%–61.1%), mixed type (45.9%, 95% CI: 36.4%–64.3%), spastic type (42.7%, 95% CI: 37.4%–48.0%) and ataxia type (39.1%, 95% CI: 29.0%–49.1%). Regarding the topographical type of CP, the prevalence from high to low was quadriplegia (61.8%, 95% CI: 57.0%–66.5%), hemiplegia (40.2%, 95% CI: 31.8%–48.7%), and diplegia (26.3%, 95% CI: 21.6%–30.9%) (P < 0.001).
The funnel plot was used to assess the presence of publication bias, revealing an evident asymmetry in the overall prevalence of epilepsy. However, we conducted separate analyses by sample source, resulting in symmetrical funnel plots for both clinical case-based and population-based studies (see Figure 4). The application of Egger's test indicated a significant publication bias in the meta-analysis (t = 2.39, P < 0.05). Further analysis utilizing Egger's test based on sample source revealed no significant publication bias in the clinical sample-based study (t = 0.34, P = 0.733) and population-based study (t = −0.50, P = 0.623), as depicted in Figure 5.
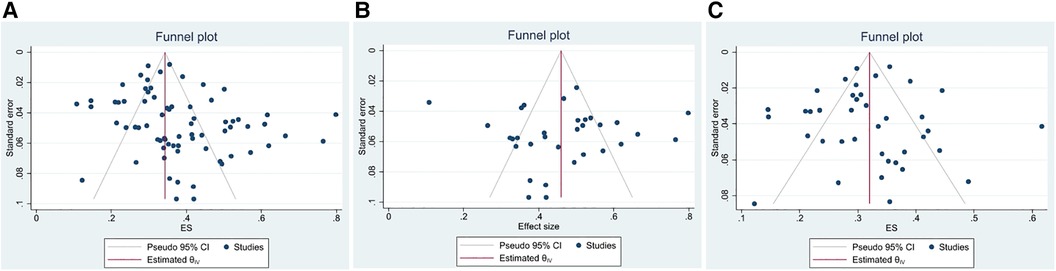
Figure 4. The funnel plot of the total prevalence of epilepsy in CP. (A) All studies (B) Clinical sample-based study (C) Population-based study.
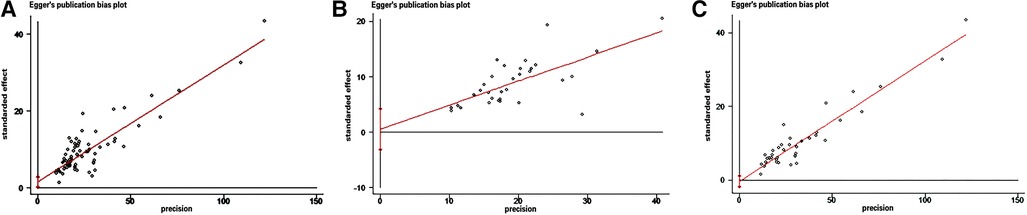
Figure 5. Egger's test of the prevalence of epilepsy in CP. (A) All studies (B) Clinical sample-based studies (C) Population-based studies.
A sensitivity analysis was conducted using the single-article removal method, and no studies were found to affect the overall results significantly. Meta-regression analysis was then performed on the included articles, with the prevalence of epilepsy in CP as the dependent variable. Independent variables for the meta-regression analysis included the sample source of the articles, gestational age, birth weight, birth asphyxia, NE, family history of epilepsy, CNS abnormalities based on MRICS, EEG abnormalities, cranial imaging abnormalities, cognitive/intellectual status, clinical type of CP, and topographical type of CP. The results revealed significant heterogeneity among the sample source of articles, NE, family history of epilepsy, EEG abnormalities, imaging abnormalities, intellectual/cognitive impairment, and topographical type of CP (P < 0.05), as illustrated in Tables 2, 5.
The total prevalence of epilepsy in CP in this study was 38.0%, and epilepsy in children with CP was 46.4% in a clinical case-based study and 31.6% in a population-based study. The number of individuals with CP in included articles was enough to represent the prevalence of epilepsy in CP, which can reflect the prevalence of epilepsy in CP to some extent. In clinical sample-based studies, the prevalence of epilepsy in CP was generally high, which might be caused by the individuals were mainly from medical institutions, or the individuals admitted were generally more severe. They are more likely to have comorbidities such as intellectual disability, epilepsy, and speech disorders, leading to overestimating the prevalence of epilepsy in CP (83). In contrast, CP with milder symptoms might not go to hospitals or rehabilitation facilities for rehabilitation, thus missing some individuals with mild to moderate CP that were less likely to develop epilepsy. In population-based studies, the prevalence of CP at the national level was unknown because some countries or regions need a specific population-based national registry (48). Therefore the studies only covered part of the region and represented the prevalence of epilepsy in that region or country. However, even so, population-based studies can cover a wider area than clinical sample-based studies and provide a more precise and scientific prevalence of epilepsy in children with CP.
In terms of ethnic group, the prevalence of epilepsy in CP was significantly higher in Black-majority countries (48.6% and 38.1%) than in other-majority countries in both clinical sample-based and population-based studies, which may be associated with poor economics. In the clinical sample-based study, the prevalence of epilepsy was higher in low- and middle-income countries than in high-income countries (47.0% vs. 43.0%). Firstly, due to the low level of parental education, lack of CP-related professional knowledge, poor perinatal and postpartum management, and poor early diagnosis and intervention, CP is generally more severe in motor and cognitive impairment, and the possibility of secondary complications such as epilepsy increases (84–86). Secondly, compared to high-income countries, children from low- and middle-income countries were more often admitted to hospitals for treatment of severe CP, and this segment of the CP population had a high prevalence of epilepsy (87). In contrast, in population-based studies, the prevalence of epilepsy in low- and middle-income countries and high-income countries was very close (30.7% vs. 32.1%), which might be responsible for filling that missing segment of children with mild to moderate CP. The number of articles we included (n = 43) and individuals (n = 31,098) were highest in countries of predominantly European origin, which may give a more accurate prevalence of epilepsy in this segment of the CP population (46.6% and 31.2%). The low prevalence of epilepsy in Mongolian-majority countries (32.8% and 29.6%) might be because we restricted the search language to English and missed some articles published in non-English languages. Moreover, the number of articles included in this study was generally low (n = 8), and there might be some publication bias. The veracity of the results remains to be verified by a large number of original studies.
Based on the time of publication of the article, it was found that the prevalence of epilepsy in CP was low in 1998–2002 (39.9% and 26.4%) compared with other periods, and the prevalence stabilized in 2011–2022, both in clinical sample-based studies and population-based studies. Our interpretation of the results was that it might not be that the prevalence of epilepsy in CP has increased in the last decade. The diagnostic sensitivity or specificity of epilepsy had increased with the updating of epilepsy diagnostic criteria and the promotion of improved assessment techniques, resulting in more children with CP being accurately diagnosed with epilepsy. For example, epilepsy diagnosis in the articles from 1998 to 2002 relied more on the clinical history and symptoms for diagnosis, whereas articles from 2011 to 2022 used clinical symptoms combined with EEG more often. Secondly, it also correlated with the number of articles included. Our inclusion was mainly after 2010 (n = 57) and generally less before 2010 (n = 15), which might have some publication bias. Regarding gender, the prevalence of CP in males was significantly higher than in females. However, there was no significant difference between groups in the prevalence of epilepsy in the clinic-based and population-based studies, suggesting that gender had a negligible effect on the prevalence of epilepsy.
Several articles suggested that a history of NS was associated with a high risk of epilepsy in CP and was a strong predictor of epilepsy in children with CP. Levene et al. (88) reported that NS adversely affected the developmental progression of CNS and can lead to intrinsic brain lesions that might predispose to cognitive, behavioral, or epileptic problems later in life. It was postulated that NS, especially those starting within the first 72 h, was a significant risk factor for developing epilepsy (31). In addition, Mert et al. (20) showed that children with CP with a history of NS were 3.3 times more likely to have a poor prognosis for epilepsy than those without a history of neonatal seizures. Therefore, a history of NS in CP was a significant related factor for the development and poor prognosis of epilepsy.
Our study found a high prevalence of epilepsy in CP with a family history of epilepsy (89.2%) compared to those without a family history of epilepsy (49.0%). Mert et al. (20) showed that the prevalence of epilepsy in children with CP with a family history of epilepsy was 5.5 times higher than that in children without a family history of epilepsy. Therefore, it can be hypothesized that genetic factors play an essential role in the pathogenesis of epilepsy development and can be a significant predictor of seizures in children with CP.
It is well known that intellectual disability and CP often occur together (89). The prevalence of epilepsy in CP with cognitive/intellectual disability increased with the degree of intellectual disability, probably because CP with epilepsy have more severe brain injury. Thus the coexistence of intellectual disability and epilepsy was high, and each seizure might aggravate the brain injury in children with CP and have some effect on their intelligence (12, 47). According to the study conducted by Karatoprak et al. (31), the prevalence of epilepsy in children with severe intellectual disabilities was found to be 8.9 times higher compared to children with average intelligence. El-tallowy et al. (47) showed that children with CP might suffer from extensive brain injury to various parts of the brain, such as brain gray matter, deep brain white matter, and the central nucleus. Therefore, they were vulnerable to intellectual disability and epilepsy. Cognitive/intellectual disability was uncommon in diplegia because it generally did not damage brain gray matter. In contrast, epilepsy and cognitive/intellectual disability were more common and severe in quadriplegia because of extensive brain injury (36). Although cognitive/intellectual disability is more common in children with CP with severe brain injury, and the possibility of seizures is higher, some children with severe brain injury have normal intelligence and do not often accompany by epilepsy. This may be related to the site of injury. For example, more than half of the intelligence of dyskinetic CP with deep gray matter injury is normal and may not be accompanied by epilepsy (90).
Further study of the possible risk of epilepsy development and its relationship with EEG and cranial imaging might help to find the risk factors associated with epilepsy affecting CP (16). In this study, the cranial imaging applied in the included articles included CT and MRI. Cranial MRI was the most recommended neuroimaging modality. Widely-adopted MRICS could be used for brain abnormalities—malformations, predominantly white matter injuries, mostly gray matter injuries, and miscellaneous and normal imaging. It helps to provide further insights into the nature of the abnormalities to aid the diagnosis, treatment planning, and monitoring of patients with CP. The epilepsy diagnosis was based on family and personal history and symptoms, and EEG was a gold standard for diagnosis (91). Hanci et al. (32) showed that widespread or focal epileptiform abnormalities might be essential in developing CP epilepsy. The data showed that the likelihood of co-morbid epilepsy would be higher in CP with the presence of EEG abnormalities (60.8%) and cranial imaging abnormalities (57.8%) and was statistically different between groups of children with CP without epilepsy (P < 0.05). That suggests the importance of early and effective EEG and cranial imaging combined with clinical history for detecting epilepsy in CP.
Many studies have shown that gray matter injury was a significant cause of seizures compared to white matter injury. For example, temporal and frontal lobe injury was highly epileptogenic (7, 46, 73). This study also validated the higher odds of seizures in CP with predominantly gray matter injury (55.9%) compared to predominantly white matter injuries (36.5%) in the present study. However, if the brain white matter was affected or the damage was limited to the basal ganglia and thalamus, the likelihood of epilepsy became low (33). In addition, this study showed a 52.8% prevalence of epilepsy in brain malformations. Carlsson et al. (46) found an increased frequency of seizures in young people with CP with CNS infections and malformations with the injury to the brain gyrus.
Due to the heterogeneity of symptoms and complications of CP, there was no clear global consensus on the type of CP (1, 92). Therefore, in this study, we analyzed CP according to its clinical type (spastic, dyskinetic, ataxic, and mixed) and topographical type (hemiplegia, diplegia, and quadriplegia) based on the typing characteristics of the included articles to explore the differences. This study showed that clinical type was not responsible for the prevalence of epilepsy in CP, but the topographical type mainly affected seizures in children with CP (P < 0.001). This study showed that quadriplegia was the leading cause of seizures affecting children with CP (61.8%), followed by hemiplegia (40.2%) and diplegia (26.3%). Because quadriplegia was characterized by extensive brain injury or brain softening secondary to ischemic-hypoxic events with a higher degree of injury and a high prevalence of epilepsy compared to hemiplegia and diplegia (33). Most hemiplegia will have focal cortical injury or infarction due to perinatal arterial ischemic infarction and a higher prevalence of epilepsy. In contrast, diplegic injury tended to be predominantly periventricular leukomalacia, not involving cortical gray matter, and had a lower prevalence of epilepsy (13, 93, 94).
In terms of clinical type, our study included 12,711 young people with CP, 82.5% of them were spastic CP, the most clinical type of CP. And 42.7% of them had epilepsy, the epilepsy prevalence rate was lower than that of the dyskinetic and mixed types. The reason for this was that in addition to the spastic CP only described in some articles, we also included the description of spastic diplegia, spastic hemiplegia, and spastic quadriplegia in a unified category as spastic CP, with a higher proportion of young people with spastic diplegia (34%), resulting in a lower overall prevalence of epilepsy in spastic CP than that in the other clinical types. In this study, children with dyskinetic type had the highest prevalence in studies with clinical as a typing criterion. However, children with simple dyskinetic CP with only basal ganglia lesions were less likely to have epilepsy. There are some reasons for that. First, the dyskinetic type had a higher incidence of neonatal seizures (53). Second, brain injuries in the temporal and frontal lobes were high risk of suffering seizure (53). Third, the more dif ficulty distinguishing between complex partial seizures and the dyskinetic pattern caused an overdiagnosis of epilepsy (53). Fourth, the number of dyskinetic-type individuals in each article was generally few, which had a certain bias from the results. In addition, the ataxic type had the lowest prevalence of epilepsy, but even then, there was a significant incidence of epilepsy (39.1%). We interpreted the reason for this to be that although the prevalence of epilepsy due to cerebellar injury was less likely, various high-risk factor injury were not limited to the cerebellum because the diagnosis of CP was based on clinical symptoms (14). The type of diagnosis of CP might be limited only to the most significant clinical symptoms. Therefore, our interpretation of the results preferred that the severity of the injury and the area of brain injury in CP may be the main factors affecting the onset of epilepsy rather than clinical typing.
The high prevalence of epilepsy in CP might be due to common etiology and risk factors. It was unclear why the same lesions cause CP with or without epilepsy, and we speculated that it might be due to genetic susceptibility. In this study, the prevalence of epilepsy in children with CP born prematurely with low birthweight was lower than that in children born at term and average weight, suggesting that preterm birth and low birth weight were not significant risk factors contributing to seizures in children with CP. Prematurity is caused predominantly periventricular leukomalacia the leading cause of diplegia in preterm infants. In contrast, due to brain gray matter injury, CP was more common in full-term infants (12, 16, 95, 96). Since epilepsy was uncommon in children with spastic diplegia and white matter injury, epilepsy was rarely seen in preterm infants (95).
The advantages of this study include several key points. Firstly, it represents the first and most comprehensive systematic review and meta-analysis conducted on the prevalence of epilepsy in individuals with CP. This study encompasses a larger number of included articles and individuals, resulting in more robust prevalence estimates and increased confidence in the findings. Secondly, a meticulous approach was taken to categorize the articles into clinical sample-based and population-based studies based on the source of samples. This division allows for a more refined analysis and comparison of prevalence rates. Additionally, subgroup analyses were conducted considering factors such as gender, ethnic group, and national income level. This approach helps to mitigate the heterogeneity among studies and provides a more scientifically sound method to assess the prevalence of epilepsy in different subgroups. Thirdly, the study delves into a comprehensive analysis of various confounding factors associated with CP, such as gestational age, birth weight, history of NE, cognitive/intellectual status, and the specific type of CP. By considering these factors, the prevalence of epilepsy was examined from a more comprehensive perspective. Moreover, potential sources of heterogeneity and factors influencing the prevalence of epilepsy were identified. This aids in better understanding the variations observed in the prevalence estimation and provides insights into the associated factors contributing to the occurrence of epilepsy in individuals with CP. Overall, the advantages of this study lie in its comprehensive nature, meticulous categorization and analysis of factors, and the identification of potential sources of heterogeneity. These aspects contribute to a more thorough understanding of the prevalence of epilepsy in individuals with CP.
There are several limitations to consider in this study. Firstly, we did not conduct an age-based meta-analysis of epilepsy prevalence due to the wide age ranges covered in the included studies, which made age group comparisons unfeasible. However, it is worth noting that previous research has shown a higher occurrence of epilepsy in the early years of individuals with CP, with most seizures occurring within the first year of life (14, 17, 22, 30, 33). Furthermore, some population-based studies on adolescents have an age range of up to 19 years, exceeding the commonly accepted age of 18 (45, 58, 62, 68). This necessitated our decision to divide the age range accordingly. Moreover, the single combined estimate of prevalence should be interpreted with caution due to several limitations. Firstly, heterogeneity exists even in subgroup analyses, which is often difficult to avoid in epidemiological studies (97). Secondly, variations in prevalence between studies may stem from factors such as sample source, different definitions of CP and epilepsy, representativeness of samples, and the specificity of diagnostic tools. Many studies relied on clinical history and symptoms to confirm epilepsy, which can result in both under- and over-diagnosis. Thirdly, the data of some articles are not comprehensive enough, such as the failure to reflect the time range of data collection, diagnostic criteria of epilepsy, age range and female ratio on the Table 1. Some articles were excluded from subgroup analysis due to the lack of CP characteristics data, and the number of articles included in subgroup analysis may have a certain bias on the results. Additionally, the high prevalence of epilepsy in CP observed in clinical sample-based studies may be due to an over-representation of more severe individuals admitted to medical institutions for intellectual disability, epilepsy, motor disorders, and speech disorders. Furthermore, it is essential to note that this study excluded unpublished articles and articles published in non-English languages, which could have resulted in an underestimation or overestimation of the true prevalence of epilepsy in CP. Lastly, differences in the characteristics of the individuals in the study may have influenced the results. While this study provides valuable insights, these limitations should be considered when interpreting the findings.
In summary, our findings indicate that more than one-third of young people with CP experience epilepsy, with an overall prevalence of 38.0%. Prevalence rates differ based on the study type, with clinic-based studies showing a prevalence of 46.4% and population-based studies showing a prevalence of 31.6%. The source of CP cases in the articles emerged as a significant factor contributing to the heterogeneity in epilepsy prevalence. Our analysis suggests NE, a family history of epilepsy, abnormal EEG findings, abnormal cranial imaging, severe intellectual disability, and quadriplegia may be associated with comorbid epilepsy in CP. Given the high prevalence of epilepsy in CP, it is recommended that children with these related factors be followed up regularly for a long time. Cranial imaging and EEG should be performed on young people with CP suspected of having a risk of seizures, and appropriately use anticonvulsant medication to reduce seizure frequency in individuals with CP. Furthermore, systematic investigations into the prevalence of epilepsy in individuals with CP and exploring factors contributing to seizures may also shed light on potential shared genetic mechanisms, providing new insights into the genetic patterns and possible overlaps between these disorders.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
CG and JG are responsible for the conception and design of the article, the revision of the paper, and the overall responsibility of the study; CG, PZ, and XW are responsible for the implementation of the study and feasibility analysis, statistical processing; CG, BL and JG were responsible for literature retrieval, data collection, and collation; XW, PZ and WP were responsible for quality assessment. CG, SZ, ZJ, and WP were responsible for the analysis and interpretation of the results. CG, JG, AL and BW wrote and revised the paper. All authors contributed to the article and approved the submitted version.
The Heilongjiang Provincial Natural Science Foundation Project (No. LH2020H006) and fund Basic scientific research operating expenses of provincial institutions of higher learning in Heilongjiang Province (NO.2019-KYYWF-1366 and No.2022-KYYWF-0653) supported this study.
We thank all the authors, editors, and reviewers who contributed to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1189648/full#supplementary-material
1. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of CP April 2006. Dev Med Child Neurol Suppl. (2007) 109:8–14.17370477
2. Centers for disease control and prevention. Data and statistics for CP (2021). Available at: https://www.cdc.gov/ncbddd/cp/data.html (Accessed May, 2021).
3. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55(4):475–82. doi: 10.1111/epi.12550
4. Depositario-Cabacar DF, Zelleke TG. Treatment of epilepsy in children with developmental disabilities. Dev Disabil Res Rev. (2010) 16(3):239–47. doi: 10.1002/ddrr.116
5. The definition and classification of cerebral palsy. Dev Med Child Neurol. (2007) 49(s109):1–44. doi: 10.1111/j.1469-8749.2007.00001.x
6. Cooper MS, Mackay MT, Dagia C, Fahey MC, Howell KB, Reddihough D, et al. Epilepsy syndromes in cerebral palsy: varied, evolving and mostly self-limited. Brain. (2023) 146(2):587–99. doi: 10.1093/brain/awac274
7. Cooper MS, Mackay MT, Fahey M, Reddihough D, Reid SM, Williams K, et al. Seizures in children with cerebral palsy and white matter injury. Pediatrics. (2017) 139(3):e20162975. doi: 10.1542/peds.2016-2975
8. Prasad R, Verma N, Srivastava A, Das BK, Mishra OP. Magnetic resonance imaging, risk factors and co-morbidities in children with cerebral palsy. J Neurol. (2011) 258(3):471–8. doi: 10.1007/s00415-010-5782-2
9. Aicardi J. Epilepsy in brain-injured children. Dev Med Child Neurol. (1990) 32(3):191–202. doi: 10.1111/j.1469-8749.1990.tb16925.x
10. WHO. Global accelerated action for the health of adolescents (AA-HA!): guidance to support country implementation. Geneva. (2017).
11. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65(9):934–9. doi: 10.1016/j.jclinepi.2011.11.014
12. Kwong KL, Wong SN, So KT. Epilepsy in children with cerebral palsy. Pediatr Neurol. (1998) 19(1):31–6. doi: 10.1016/s0887-8994(98)00011-3
13. Zafeiriou DI, Kontopoulos EE, Tsikoulas I. Characteristics and prognosis of epilepsy in children with cerebral palsy. J Child Neurol. (1999) 14(5):289–94. doi: 10.1177/088307389901400504
14. Bruck I, Antoniuk SA, Spessatto A, Bem RS, Hausberger R, Pacheco CG. Epilepsy in children with cerebral palsy. Arq Neuropsiquiatr. (2001) 59(1):35–9. doi: 10.1590/s0004-282(2001000100008
15. Sianturi P, Syarifuddin A, Saing B. Incidence of epilepsy among patients with cerebral palsy (CP) in yayasan pemeliharaan anak cacat (YPAC) â medan. Med J Indones. (2002) 11(3):158–63. doi: 10.13181/mji.v11i3.69
16. Senbil N, Sonel B, Aydin OF, Gürer YK. Epileptic and non-epileptic cerebral palsy: EEG and cranial imaging findings. Brain Dev. (2002) 24(3):166–9. doi: 10.1016/s0387-7604(02)00025-6
17. Singhi P, Jagirdar S, Khandelwal N, Malhi P. Epilepsy in children with cerebral palsy. J Child Neurol. (2003) 18(3):174–9. doi: 10.1177/08830738030180030601
18. Kułak W, Sobaniec W. Risk factors and prognosis of epilepsy in children with cerebral palsy in north-eastern Poland. Brain Dev. (2003) 25(7):499–506. doi: 10.1016/s0387-7604(03)00040-8
19. Zelnik N, Konopnicki M, Bennett-Back O, Castel-Deutsch T, Tirosh E. Risk factors for epilepsy in children with cerebral palsy. Eur J Paediatr Neurol. (2010) 14(1):67–72. doi: 10.1016/j.ejpn.2009.06.002
20. Mert GG, Incecik F, Altunbasak S, Herguner O, Mert MK, Kiris N, et al. Factors affecting epilepsy development and epilepsy prognosis in cerebral palsy. Pediatr Neurol. (2011) 45(2):89–94. doi: 10.1016/j.pediatrneurol.2011.03.001
21. Frank-Briggs AI, Alikor EAD. Sociocultural issues and causes of cerebral palsy in port harcourt, Nigeria. Niger J Paediatr. (2011) 38(3):115–9. doi: 10.1111/j.1469-8749.2012.04283.x
22. Aronu AE, Ibekwe RC, Ojinnaka NC. Epilepsy in Nigerian children with cerebral palsy in enugu. J Pediatr Neurol. (2013) 11(01):023–7. doi: 10.3233/JPN-120592
23. Kakooza-Mwesige A, Forssberg H, Eliasson AC, Tumwine JK. Cerebral palsy in children in Kampala, Uganda: clinical subtypes, motor function and co-morbidities. BMC Res Notes. (2015) 8:166. doi: 10.1186/s13104-015-1125-9
24. Keeratisiroj O, Thawinchai N, Siritaratiwat W, Buntragulpoontawee M. Prognostic predictors for ambulation in Thai children with cerebral palsy aged 2 to 18 years. J Child Neurol. (2015) 30(13):1812–8. doi: 10.1177/0883073815582267
25. Bearden DR, Monokwane B, Khurana E, Baier J, Baranov E, Westmoreland K, et al. Pediatric cerebral palsy in Botswana: etiology, outcomes, and comorbidities. Pediatr Neurol. (2016) 59:23–9. doi: 10.1016/j.pediatrneurol.2016.03.002
26. Minocha P, Sitaraman S, Sachdeva P. Clinical spectrum, comorbidities, and risk factor profile of cerebral palsy children: a prospective study. J Pediatr Neurosci. (2017) 12(1):15–8. doi: 10.4103/1817-1745.205622
27. Gincota Bufteac E, Andersen GL, Torstein V, Jahnsen R. Cerebral palsy in moldova: subtypes, severity and associated impairments. BMC Pediatr. (2018) 18(1):332. doi: 10.1186/s12887-018-1305-6
28. Gürkan F, Gökben S, Serin HM, Yılmaz S, Aktan G, Tekgül H, et al. Determining risk factors of epilepsy in children with cerebral palsy: a retrospective study. J Pediatr Res. (2018) 5(2):76. doi: 10.4274/jpr.24471
29. Sucuoğlu H. Demographic and clinical characteristics of patients with cerebral palsy. İstanbul Med J. (2017) 19(3):219–24. doi: 10.5152/imj.2018.88310
30. Aydın K, Kartal A, Keleş Alp E. High rates of malnutrition and epilepsy: two common comorbidities in children with cerebral palsy. Turk J Med Sci. (2019) 49(1):33–7. doi: 10.3906/sag-1803-79
31. Karatoprak E, Sözen G, Saltık S. Risk factors associated with epilepsy development in children with cerebral palsy. Childs Nerv Syst. (2019) 35(7):1181–7. doi: 10.1007/s00381-019-04152-w
32. Hanci F, Türay S, Dilek M, Kabakuş N. Epilepsy and drug-resistant epilepsy in children with cerebral palsy: a retrospective observational study. Epilepsy Behav. (2020) 112:107357. doi: 10.1016/j.yebeh.2020.107357
33. Pavone P, Gulizia C, Le Pira A, Greco F, Parisi P, Di Cara G, et al. Cerebral palsy and epilepsy in children: clinical perspectives on a common comorbidity. Children (Basel). (2020) 8(1):16. doi: 10.3390/children8010016
34. Sadowska M, Sarecka-Hujar B, Kopyta I. Evaluation of risk factors for epilepsy in pediatric patients with cerebral palsy. Brain Sci. (2020) 10(8):481. doi: 10.3390/brainsci10080481
35. Al-Blowi A, Al-Mutairi R, Ghabbany RM, Manaa AM, Aloufi MM, Ternati GK, et al. The prevalence of malnutrition and the nutritional status in children with CP and its causes in madinah maternity and children hospital. Curr Pediatr Res. (2020) 24(7):273–80.
36. Şık N, Sarıoğlu FC, Öztekin Ö, Sarıoğlu B. Evaluation of the relationship between cranial magnetic resonance imaging findings and clinical status in children with cerebral palsy. Turk J Med Sci. (2021) 51(3):1296–301. doi: 10.3906/sag-2010-187
37. Tsige S, Moges A, Mekasha A, Abebe W, Forssberg H. Cerebral palsy in children: subtypes, motor function and associated impairments in Addis Ababa, Ethiopia. BMC Pediatr. (2021) 21(1):544. doi: 10.1186/s12887-021-03026-y
38. Jibril YN, Shamsu KA, Muhammad NB, Hasheem MG, Tukur AR, Salisu AD. Determinants of hearing loss in children with cerebral palsy in kano, Nigeria. Niger J Clin Pract. (2021) 24(6):802–7. doi: 10.4103/njcp.njcp_480_20
39. Chaudhary S, Bhatta NK, Poudel P, Agrawal J, Kalawar RPS, Jayswal JP. Profile of children with cerebral palsy at a tertiary hospital in eastern Nepal. BMC Pediatr. (2022) 22(1):415. doi: 10.1186/s12887-022-03477-x
40. Mangamba DCK, Enyama D, Foko LPK, Tankou J, Njinkui DN, Essome H, et al. Epidemiological, clinical, and treatment-related features of children with cerebral palsy in Cameroon: a hospital-based study. Arch Pediatr. (2022) 29(3):219–24. doi: 10.1016/j.arcped.2022.01.006
41. Archana K, Saini L, Gunasekaran PK, Singh P, Sahu JK, Sankhyan N, et al. The profile of epilepsy and its characteristics in children with cerebral palsy. Seizure. (2022) 101:190–6. doi: 10.1016/j.seizure.2022.08.009
42. Karim T, Jahan I, Dossetor R, Giang NTH, Van Anh NT, Dung TQ, et al. Nutritional Status of children with cerebral palsy-findings from prospective hospital-based surveillance in Vietnam indicate a need for action. Nutrients. (2019) 11(9):21–32. doi: 10.3390/nu11092132
43. Aneja S, Ahuja B, Taluja V, Bhatia VK. Epilepsy in children with cerebral palsy. Indian J Pediatr. (2001) 68(2):111–5. doi: 10.1007/BF02722024
44. Nordmark E, Hägglund G, Lagergren J. Cerebral palsy in southern Sweden I. Prevalence and clinical features. Acta Paediatr. (2001) 90(11):1271–6. doi: 10.1080/080352501317130317
45. Parkes J, Dolk H, Hill N, Pattenden S. Cerebral palsy in northern Ireland: 1981–93. Paediatr Perinat Epidemiol. (2001) 15(3):278–86. doi: 10.1046/j.1365-3016.2001.00349.x
46. Carlsson M, Hagberg G, Olsson I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev Med Child Neurol. (2003) 45(6):371–6. doi: 10.1017/s0012162203000719
47. El-Tallawy HN, Farghaly WM, Shehata GA, Badry R, Rageh TA. Epileptic and cognitive changes in children with cerebral palsy: an Egyptian study. Neuropsychiatr Dis Treat. (2014) 10:971–5. doi: 10.2147/NDT.S59600
48. Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. (2002) 44(5):309–16. doi: 10.1017/s0012162201002134
49. Andersen GL, Irgens LM, Haagaas I, Skranes JS, Meberg AE, Vik T. Cerebral palsy in Norway: prevalence, subtypes and severity. Eur J Paediatr Neurol. (2008) 12(1):4–13. doi: 10.1016/j.ejpn.2007.05.001
50. Sigurdardóttir S, Thórkelsson T, Halldórsdóttir M, Thorarensen O, Vik T. Trends in prevalence and characteristics of cerebral palsy among Icelandic children born 1990 to 2003. Dev Med Child Neurol. (2009) 51(5):356–63. doi: 10.1111/j.1469-8749.2009.03303.x
51. Kirby RS, Wingate MS, Van Naarden Braun K, Doernberg NS, Arneson CL, Benedict RE, et al. Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the autism and developmental disabilities monitoring network. Res Dev Disabil. (2011) 32(2):462–9. doi: 10.1016/j.ridd.2010.12.042
52. Himmelmann K, Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev Med Child Neurol. (2011) 53(6):516–21. doi: 10.1111/j.1469-8749.2011.03932.x
53. Sellier E, Uldall P, Calado E, Sigurdardottir S, Torrioli MG, Platt MJ, et al. Epilepsy and cerebral palsy: characteristics and trends in children born in 1976-1998. Eur J Paediatr Neurol. (2012) 16(1):48–55. doi: 10.1016/j.ejpn.2011.10.003
54. Singhi P, Saini AG. Changes in the clinical spectrum of cerebral palsy over two decades in north India–an analysis of 1212 cases. J Trop Pediatr. (2013) 59(6):434–40. doi: 10.1093/tropej/fmt035
55. Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—autism and developmental disabilities monitoring network, USA, 2008. Dev Med Child Neurol. (2014) 56(1):59–65. doi: 10.1111/dmcn.12268
56. Yalcinkaya EY, Huner B, Dincer U, Diracoglu D, Aydin R, Icagasioglu A, et al.. Demographic and clinical findings of cerebral palsy patients in Istanbul: a multicenter study. Türkiye Fiziksel Tip ve Rehabilitasyon Dergisi. (2014) 60(2):134–8. doi: 10.5152/tftrd.2014.42402
57. Granild-Jensen JB, Rackauskaite G, Flachs EM, Uldall P. Predictors for early diagnosis of cerebral palsy from national registry data. Dev Med Child Neurol. (2015) 57(10):931–5. doi: 10.1111/dmcn.12760
58. Meehan E, Freed GL, Reid SM, Williams K, Sewell JR, Rawicki B, et al. Tertiary paediatric hospital admissions in children and young people with cerebral palsy. Child Care Health Dev. (2015) 41(6):928–37. doi: 10.1111/cch.12263
59. Delacy MJ, Reid SM, Australian Cerebral Palsy Register Group. Profile of associated impairments at age 5 years in Australia by cerebral palsy subtype and gross motor function classification system level for birth years 1996 to 2005. Dev Med Child Neurol. (2016) 58(Suppl 2):50–6. doi: 10.1111/dmcn.13012
60. Tan SS, van der Slot WM, Ketelaar M, Becher JG, Dallmeijer AJ, Smits DW, et al. Factors contributing to the longitudinal development of social participation in individuals with cerebral palsy. Res Dev Disabil. (2016) 57:125–35. doi: 10.1016/j.ridd.2016.03.015
61. Khandaker G, Smithers-Sheedy H, Islam J, Alam M, Jung J, Novak I, et al. Bangladesh Cerebral Palsy Register (BCPR): a pilot study to develop a national cerebral palsy (CP) register with surveillance of children for CP. BMC Neurol. (2015) 15:173. doi: 10.1186/s12883-015-0427-9
62. Delobel-Ayoub M, Klapouszczak D, van Bakel MME, Horridge K, Sigurdardottir S, Himmelmann K, et al. Prevalence and characteristics of autism spectrum disorders in children with cerebral palsy. Dev Med Child Neurol. (2017) 59(7):738–42. doi: 10.1111/dmcn.13436
63. Abas O, Abdelaziem F, Kilany A. Clinical spectrum of cerebral palsy and associated disability in south Egypt: a local survey study. Open Access Maced J Med Sci. (2017) 5(1):37–41. doi: 10.3889/oamjms.2017.020
64. Kakooza-Mwesige A, Andrews C, Peterson S, Wabwire Mangen F, Eliasson AC, Forssberg H. Prevalence of cerebral palsy in Uganda: a population-based study. Lancet Glob Health. (2017) 5(12):e1275–82. doi: 10.1016/S2214-109X(17)30374-1
65. Yim SY, Yang CY, Park JH, Kim MY, Shin YB, Kang EY, et al. Society of pediatric rehabilitation and developmental medicine, Korea. Korean database of cerebral palsy: a report on characteristics of cerebral palsy in South Korea. Ann Rehabil Med. (2017) 41(4):638–49. doi: 10.5535/arm.2017.41.4.638
66. Hollung SJ, Vik T, Lydersen S, Bakken IJ, Andersen GL. Decreasing prevalence and severity of cerebral palsy in Norway among children born 1999 to 2010 concomitant with improvements in perinatal health. Eur J Paediatr Neurol. (2018) 22(5):814–21. doi: 10.1016/j.ejpn.2018.05.001
67. Reid SM, Meehan EM, Arnup SJ, Reddihough DS. Intellectual disability in cerebral palsy: a population-based retrospective study. Dev Med Child Neurol. (2018) 60(7):687–94. doi: 10.1111/dmcn.13773
68. Chiang KL, Kuo FC, Cheng CY, Chang KP. Prevalence and demographic characteristics of comorbid epilepsy in children and adolescents with cerebral palsy: a nationwide population-based study. Childs Nerv Syst. (2019) 35(1):149–56. doi: 10.1007/s00381-018-3920-9
69. Jonsson U, Eek MN, Sunnerhagen KS, Himmelmann K. Cerebral palsy prevalence, subtypes, and associated impairments: a population-based comparison study of adults and children. Dev Med Child Neurol. (2019) 61(10):1162–7. doi: 10.1111/dmcn.14229
70. Khandaker G, Muhit M, Karim T, Smithers-Sheedy H, Novak I, Jones C, et al. Epidemiology of cerebral palsy in Bangladesh: a population-based surveillance study. Dev Med Child Neurol. (2019) 61(5):601–9. doi: 10.1111/dmcn.14013
71. Påhlman M, Gillberg C, Himmelmann K. One-third of school-aged children with cerebral palsy have neuropsychiatric impairments in a population-based study. Acta Paediatr. (2019) 108(11):2048–55. doi: 10.1111/apa.14844
72. Andrews C, Kakooza-Mwesige A, Almeida R, Swartling Peterson S, Wabwire-Mangen F, Eliasson AC, et al. Impairments, functional limitations, and access to services and education for children with cerebral palsy in Uganda: a population-based study. Dev Med Child Neurol. (2020) 62(4):454–62. doi: 10.1111/dmcn.14401
73. Rafique A, Naz H. A survey-based report on the occurrence of cerebral palsy in Urban areas of Karachi. J Pak Med Assoc. (2020) 70(8):1442–4. doi: 10.5455/JPMA.28135
74. Jahan I, Al Imam MH, Karim T, Muhit M, Hardianto D, Das MC, et al. Epidemiology of cerebral palsy in Sumba Island, Indonesia. Dev Med Child Neurol. (2020) 62(12):1414–22. doi: 10.1111/dmcn.14616
75. Hollung SJ, Bakken IJ, Vik T, Lydersen S, Wiik R, Aaberg KM, et al. Comorbidities in cerebral palsy: a patient registry study. Dev Med Child Neurol. (2020) 62(1):97–103. doi: 10.1111/dmcn.14307
76. Yamagishi H, Osaka H, Toyokawa S, Kobayashi Y, Shimoizumi H. Survey on children with cerebral palsy in tochigi prefecture, Japan. Pediatr Int. (2021) 63(8):951–7. doi: 10.1111/ped.14536
77. Duke RE, Torty C, Okorie U, Kim MJ, Eneli N, Edadi U, et al. Pattern of comorbidities in school-aged children with cerebral palsy in cross river state, Nigeria. BMC Pediatr. (2021) 21(1):165. doi: 10.1186/s12887-021-02637-9
78. Al-Garni S, Derbala S, Saad H, Maaty AI. Developmental anomalies and associated impairments in Saudi children with cerebral palsy: a registry-based, multicenter study. Egyptian Rheumatology and Rehabilitation. (2021) 48(1):1–9. doi: 10.1186/s43166-021-00057-2
79. Bambi EN, Mwesige AK, Lekuya HM, Kasirye P, Idro R. Chronic pain among children with cerebral palsy attending a Ugandan tertiary hospital: a cross-sectional study. BMC Pediatr. (2021) 21(1):456. doi: 10.1186/s12887-021-02928-1
80. Karim T, Das MC, Muhit M, Badawi N, Khandaker G, Mohammad SS. Improving epilepsy control among children with cerebral palsy in rural Bangladesh: a prospective cohort-based study. BMJ Open. (2022) 12(4):e052578. doi: 10.1136/bmjopen-2021-052578
81. Szpindel A, Myers KA, Ng P, Dorais M, Koclas L, Pigeon N, et al. Epilepsy in children with cerebral palsy: a data linkage study. Dev Med Child Neurol. (2022) 64(2):259–65. doi: 10.1111/dmcn.15028
82. Linton G, Hägglund G, Czuba T, Alriksson-Schmidt AI. Epidemiology of fractures in children with cerebral palsy: a Swedish population-based registry study. BMC Musculoskelet Disord. (2022) 23(1):862. doi: 10.1186/s12891-022-05813-9
83. Paget S, Ostojic K, Goldsmith S, Nassar N, McIntyre S. Determinants of hospital-based health service utilization in cerebral palsy: a systematic review. Arch Phys Med Rehabil. (2022) 103(8):1628–37. doi: 10.1016/j.apmr.2021.12.003
84. Leite HR, Jindal P, Malek SA, Rosenbaum P. Research on children with cerebral palsy in low- and middle-income countries. Pediatr Phys Ther. (2022) 34(4):551–5. doi: 10.1097/PEP.0000000000000949
85. Sogbossi ES, Houekpetodji D, Kpadonou TG, Bleyenheuft Y. Mothers’ perception of cerebral palsy in a low-income country of West Africa: a cross-sectional study. Disabil Rehabil. (2022) 44(17):4767–74. doi: 10.1080/09638288.2021.1919765
86. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. (2017) 171(9):897–907; Erratum in: JAMA Pediatr. 2017 Sep 1;171(9):919. doi: 10.1001/jamapediatrics.2017.1689
87. Karim T, Dossetor R, Huong Giang NT, Dung TQ, Son TV, Hoa NX, et al. Data on cerebral palsy in Vietnam will inform clinical practice and policy in low and middle-income countries. Disabil Rehabil. (2022) 44(13):3081–8. doi: 10.1080/09638288.2020.1854872
88. Levene M. The clinical conundrum of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. (2002) 86:F75–7. doi: 10.1136/fn.86.2.f75
89. Jan MMS. Cerebral palsy: comprehensive review and update. Ann Saudi Med. (2006) 26(2):123–32. doi: 10.5144/0256-4947.2006.123
90. Fluss J, Lidzba K. Cognitive and academic profiles in children with cerebral palsy: a narrative review. Ann Phys Rehabil Med. (2020) 63(5):447–56. doi: 10.1016/j.rehab.2020.01.005
91. Scheffer IE, Berkovic S, Meletti S, Connolly MB, French J, Guilhoto L, et al. ILAE Classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
92. Ogoke CC. Clinical classification of cerebral palsy. In: Clinical and therapeutic aspects. (2018). p. 21. doi: 10.5772/intechopen.79246
93. Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol. (2007) 49(2):144–51. doi: 10.1111/j.1469-8749.2007.00144.x
94. Shevell MI, Majnemer A, Morin I. Etiologic yield of cerebral palsy: a contemporary case series. Pediatr Neurol. (2003) 28(5):352–9. doi: 10.1016/s0887-8994(03)00006-7
95. Wallace SJ. Epilepsy in cerebral palsy. Dev Med Child Neurol. (2001) 43(10):713–7. doi: 10.1017/s0012162201001281
96. Towsley K, Shevell MI, Dagenais L. REPACQ Consortium. Population-based study of neuroimaging findings in children with cerebral palsy. Eur J Paediatr Neurol. (2011) 15(1):29–35. doi: 10.1016/j.ejpn.2010.07.005
Keywords: cerebral palsy, epilepsy, prevalence, children, meta-analyses
Citation: Gong C, Liu A, Lian B, Wu X, Zeng P, Hao C, Wang B, Jiang Z, Pang W, Guo J and Zhou S (2023) Prevalence and related factors of epilepsy in children and adolescents with cerebral palsy: a systematic review and meta-analysis. Front. Pediatr. 11:1189648. doi: 10.3389/fped.2023.1189648
Received: 19 March 2023; Accepted: 10 July 2023;
Published: 28 July 2023.
Edited by:
Pasquale Parisi, Sapienza University of Rome, ItalyReviewed by:
Kuo-Liang Chiang, Kuang Tien General Hospital, Taiwan© 2023 Gong, Liu, Lian, Wu, Zeng, Hao, Wang, Jiang, Pang, Guo and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Guo Z3VvamluODAwMkAxNjMuY29t Shaobo Zhou cy56aG91QGdyZWVud2ljaC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.