
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 15 June 2023
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1188704
Objectives: To explore the effects of tuberculosis (TB) infection at different sites on anthropometric indicators, malnutrition and anemia incidence in children in Southwest China.
Methods: From January 2012 to December 2021, a total of 368 children aged 1 month to 16 years were enrolled. According to the sites of TB infection, they were divided into three groups: tuberculous meningitis (T group), tuberculous meningitis complicated withpulmonary tuberculosis (TP group), and tuberculous meningitis complicated with pulmonary tuberculosis and abdominal tuberculosis (TPA group). Data on weight, height, nutritional risk, blood biochemical indicators and basic descriptions were collected within 48 h after admission.
Results: The body mass index-for-age z score (BAZ), height-for-age z score (HAZ), and concentrations of hemoglobin (Hb) and albumin (ALB) decreased in the following order: T group, TP group, and TPA group. The prevalence of malnutrition was the highest in the TPA group (69.5%, 82/118) and 10-to 16-year-old group (72.4%, 63/87). Children aged 0.5–2 years exhibited the highest anemia prevalence of 70.6% (48/68) among the four age groups.The TPA group had the highest incidence of anemia (70.5%, 67/95) compared to T group and TP group.Compared with the treatment group, the abandonment group had a lower BAZ, HAZ and levels of HB and ALB, a higher rate of severe malnutrition, and higher nutritional risk scores. Children who had a low BAZ [odds ratio (OR) = 1.98], nutritional risk (OR = 0.56) and anemia (OR = 1.02) were less likely to obtain treatment with their guardians' support.
Conclusions: Children with tuberculous meningitis were at risk for growth disorders and anemia, especially when complicated with pulmonary tuberculosis and abdominal tuberculosis. The prevalence of anemia and malnutrition was the highest among patients aged 1 month to 2 years and 10–16 years, respectively. Nutritional status was one of the causes of abandoning treatment.
Tuberculosis (TB) is a chronic infectious disease that causes death worldwide. Childhood TB is considered a global pediatric emergency prevention disease (1). Approximately 9.9 million people suffered from TB and 1.3 million died from TB worldwide in 2020. Children under 15 years of age account for 11% of the total TB population but 15% of TB deaths (2). Although there are differences between countries, the burden of malnutrition and TB among children is the highest in Africa and Asia (3). China has the second largest number of TB cases in the world, with an estimated 0.84 million new TB cases in 2020, mainly distributed in poor areas in Southwest China (2). TBM is the most common and serious disease among children with extrapulmonary TB, followed by abdominal tuberculosis (ATB) (4, 5).
Malnutrition is common among children with TB. According to reports in the last 5 years, the incidence rates of malnutrition and severe malnutrition [weight-for-age z score (WAZ) < −3] were 49.1%−75.2% and 10.7–20.8%, respectively (6–9), in childhood TB. TB is closely related to nutrition (10). Malnutrition is increasingly recognized as a key risk factor for childhood TB (11). The World Health Organization (WHO) emphasized the association between malnutrition and TB in its roadmap for the diagnosis and treatment of childhood and adolescent TB (12). TB and malnutrition are mutually reinforcing and increase the prevalence and mortality of each other (13). Malnutrition affects the prognosis of TB and influences the growth and development of children. TB easily invades the intestine when ATB occurs, which directly affects the digestion and absorption of nutrients. Nutritional problems can be caused and exacerbated by inadequate dietary intake and intestinal absorption disorders in children with TB.
Nevertheless, the levels of growth and anemia among children with tuberculous meningitis (TBM) with or without pulmonary tuberculosis (PTB) and ATB have not been reported in Southwest China. Therefore, we conducted a cross-sectional survey in a large specialty children's hospital to understand the nutritional status of the patients mentioned above in Southwest China.
This was a cross-sectional survey that was carried out between January 2012 and December 2021 in the Children's Hospital of Chongqing Medical University, which is a national Grade A children's hospital and approved as the national Children's Regional Medical Center. This hospital has the largest diagnosis and treatment center for infectious diseases and undertakes the diagnosis and treatment of infectious diseases in children in Southwest China, including Yunnan, Guizhou, Sichuan, Chongqing and Tibet. This study was approved by the Ethics Committee of the Children's Hospital of Chongqing Medical University in Chongqing, China.
The inclusion criteria were as follows: (a) children aged 1 month to 16 years; (b) children hospitalized for the first time; (c) children whose guardians signed a written informed consent form; Children with genetic metabolic diseases, congenital malformations of important organs, malignant tumors or other diseases with a known influence on physical growth were excluded from this study.
TBM was defined as follows: “Definite TBM” if AFB seen on culture from Cerebrospinal fluid (CSF) microscopy or mycobacterium tuberculosis (MTB) detected by culture from CSF; “Probable TBM” if the total score of ≥10 when neuroimaging was not available and a total score of ≥12 when neuroimaging was available (14). Children who fulfilled either of the two definitions of “Definite TBM” or “Probable TBM” as given above were included in the T group.
The diagnose of PTB included two types: “smear-positive PTB” if at least two sputum smear examinations positive for AFB or one sputum smear examination positive for AFB plus abnormal chest radiograph consistent with active PTB;“smear-negative PTB” if the clinical symptoms with ineffective broad-spectrum antibiotics treatment, effective anti-TB treatment, radiological abnormalities consistent with active PTB, and close contact with TB patients (15). Children who suffered from both TBM and PTB were included in the TP group.
Children diagnosed with ATB were divided into two types. “Confirmed case of ATB”—diagnosis based on the bacteriological identification of MTB through acid-fast stain and/or culture and/or polymerase chain reaction (PCR)—based assays or the presence of caseating granulomas on histology. “Clinically diagnosed ATB”—diagnosis based on exclusion of other diseases, with suggestive features on imaging(abdominal ultrasonogram and/or computed tomography), histology and biochemistry, and with effective response to anti-TB treatment (16). Children who suffered from TBM complicated with PTB and ATB were included in the TPA group.
According to the reasons for discharge (abandonment or recovery from illness), they were divided into treatment group and abandonment group.
Weight was measured with undergarments by an electronic scale to the nearest 0.01 kg. Height was measured by a nonstretch measuring tape or height rod and marked to the nearest 0.1 cm. BMI was calculated according to the weight and height data, and the formula was as follows: BMI = weight (kg)/height (m)2. Anthropometric data were assessed as height-for-age z scores (HAZs) and BMI-for-age z scores (BAZs) using Anthro 2005, based on the WHO growth standards (http://www.who.int/childgrowth/software/en/). Cutoff points of <−1 standard deviation (SD) were used to define a low BAZ (malnutrition); −2 ≤ BAZ < −1 was used to define mild malnutrition; −3 ≤ BAZ < −2 was moderate malnutrition; and BAZ < −3 was severe malnutrition (17). BAZ < −2 indicated wasting.
Blood biochemical indicators were collected within 48 h after admission, including hemoglobin (Hb), total serum protein (TP), serum albumin (ALB), blood urea nitrogen (BUN), creatinine (Cr) and serum alkaline phosphatase (ALP). Hb levels were determined by the sodium dodecyl sulfate hemoglobin (SDS-Hb) determination method. TP,ALB, BUN and Cr concentrations were measured by biuret method,bromocresol green process,enzymatic method,and colorimetric method respectively. We used the kinetic rate approach that measured ALP activity by utilizing 2-amino-2-methyl-1-propanol (AMP) bufer.Anemia was defined based on the WHO guidelines (18) as follows: (1) children aged 6–59 months: Hb < 110 g/L; (2) children aged 5–11 years: Hb < 115 g/L; and (3) children aged 12–16 years: Hb < 120 g/L.
We used the Screening Tool for Risk on Nutritional status and Growth (STRONG) (19) to screen children for nutritional risk within 48 h after admission. This nutritional risk screening questionnaire consisted of 4 items: (1) nutritional intake and losses (1 point), including excessive diarrhea (≥5 times/day) and/or vomiting (>3 times per day) and reduced food intake during the last few days before admission; (2) subjective clinical assessment (1 point), including diminished subcutaneous fat and/or muscle mass and/or hollow face; (3) high-risk disease (2 points), including diseases with a risk of malnutrition or expected major surgery; and (4) weight loss or poor weight gain (1 point). According to the nutritional risk score, there were three levels of nutritional risk: low risk (0 points), moderate risk (1–3 points), and high risk (4–5 points).
Data were analyzed by SAS 9.4. The significance level was set at 5%. The Kolmogorov–Smirnov test was used to investigate whether the concentrations of Hb, TP, ALB, Cr, BUN, ALP, and the anthropometric indicators were normally distributed prior to analysis. Data are presented as the means, SDs and medians (interquartile ranges, IQRs). The means of 2 groups of continuous normally distributed variables were compared by independent sample t tests, while Tukey‒Kramer tests were used to compare the differences among the 3 groups of data in pairs. The Wilcoxon signed-rank test was used for nonnormally distributed data. Differences in prevalence were tested with a chi-square test. In the logistic regression analysis, continued treatment was the dependent variable (0 = no, 1 = yes). The final correction factors included in the logistic regression model were age, BAZ, anemia, degree of nutritional risk and diagnosis. The independent variables of each grade were assigned as follows: age (1 = <2 years old, 2 = 2–5 years old, 3 = 5–10 years old, 4 = 10–16 years old); BAZ (1 = BAZ < −3, 2 = −3 ≤ BAZ < −2, 3 = −2 ≤ BAZ < −1, 4 = BAZ ≥ −1); anemia (0 = yes, 1 = no); degree of nutritional risk (0 = low risk, 1 = moderate risk, 2 = high risk); and diagnosis (1 = TBM, 2 = TBM + PTB, 3 = TBM + PTB + ATB).
In total, 368 children met the eligibility criteria for this survey. The children were from different regions in Southwest China, with 322 (87.5%) living in rural areas and 46 (12.5%) living in urban areas; 208 (56.5%) were boys, while 160 (43.5%) were girls. There were 99 (26.9%) cases in the T group, 151 (41%) in the TP group, and 118 (32.1%) in the TPA group. As Table 1 shows, there was no significant difference among the three groups in terms of age. The average age was 3.83 ± 1.14 years, with 113 (30.7%) between 1 month–2 years, 73 (19.8%) between 2–5 years, 95 (25.8%) between 5–10 years, and 87 (23.6%) between 10–16 years (Table 2). All of the children had nutritional risk (nutritional risk score ≥ 1 point), 125 (34%) had high nutritional risk (4–5 points), and 243 (66%) had moderate nutritional risk (1–3 points) (data not shown).The education level of guardians of children with tuberculosis in this study was generally poor [Junior middle school or below: 83% (306/368)]. The guardian reported that the food intake of the child was only 30%–50% of the usual amount.
As shown in Table 1, the BAZ, HAZ, and concentrations of Hb and ALB decreased in the following order: T group, TP group, and TPA group. These were lower in the TPA group than in the other two groups, especially among children aged 10–16 years (Table 2, Figures 1, 2). Nevertheless, there were no significant differences in BAZs, HAZs or concentrations of Hb or ALB between the T group and TP group (P < 0.05). However, the ages of the children among the three groups were not significantly different. Furthermore, the concentrations of TP, Cr, BUN and ALP also showed no significant differences among the 3 groups.
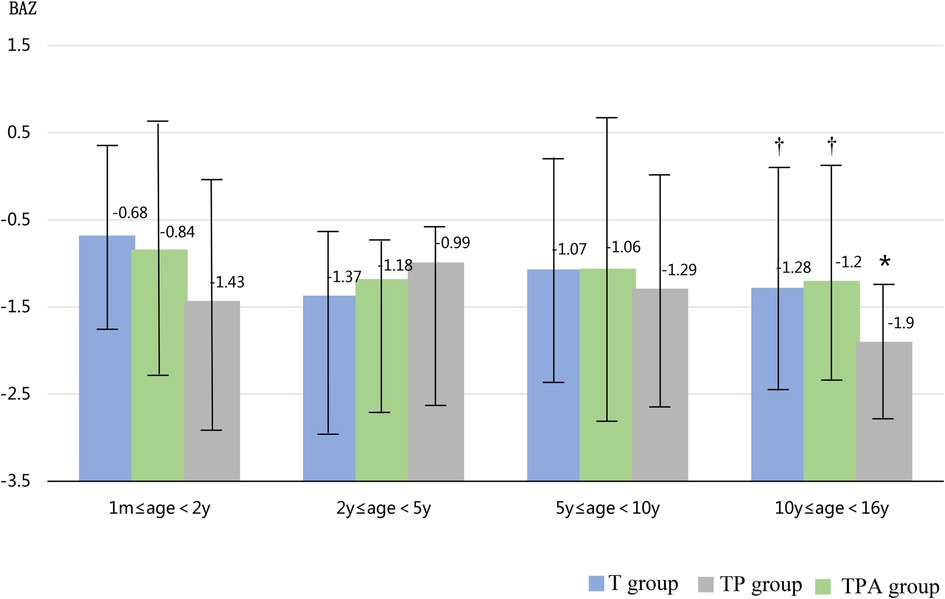
Figure 1. Comparison of BAZ levels in three groups at different ages. BAZ,body mass index-for-age z score. *Significant difference from other groups (p < 0.05).
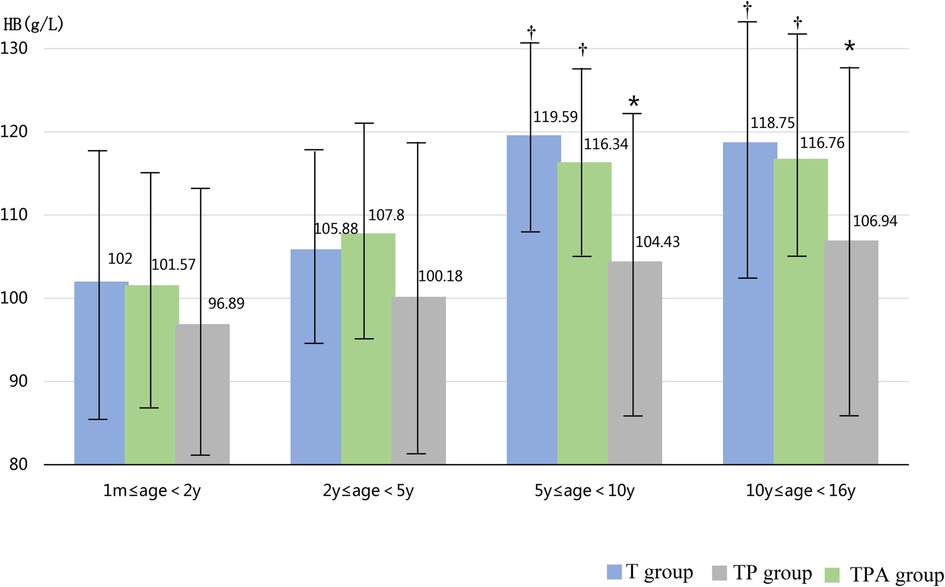
Figure 2. Comparison of HB levels in three groups at different ages. Hb,hemoglobin. *Significant difference from other groups (p < 0.05).
As shown in Table 3, the prevalence of malnutrition in the TPA group (82/118, 69.5%) was significantly higher than that in the T group and TP group (P < 0.05). Similarly, the rate of malnutrition among children in the 10–16 age group (63/87, 72.4%) was higher than that in the other three age groups (P < 0.05). However, there was no significant difference in the incidence rate of different degrees of malnutrition among the three groups and four age groups (P > 0.05).
Figure 3 showed the incidence of anemia among children over 6 months of age. Children aged 0.5–2 years exhibited the highest anemia prevalence of 70.6% (48/68) among the four age groups (P < 0.05). The TPA group had the highest incidence of anemia (70.5%, 67/95) compared to T group and TP group (P < 0.05).
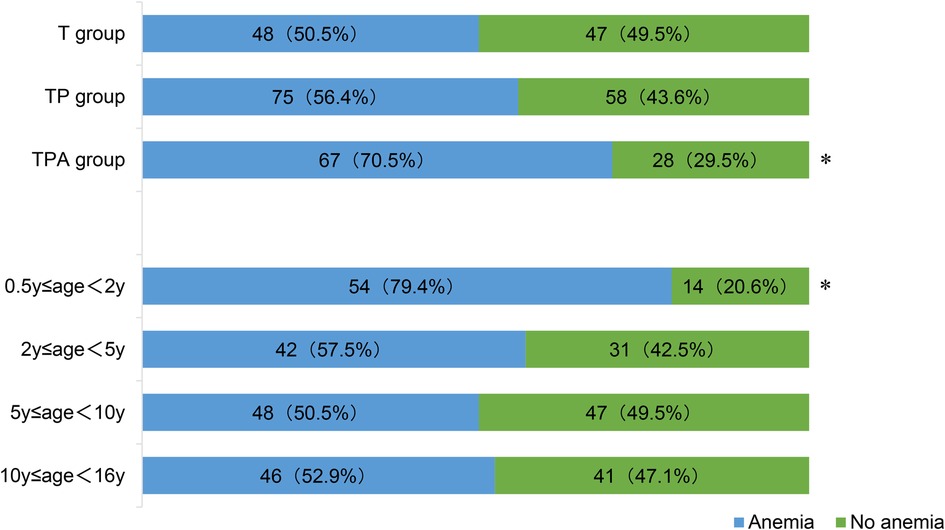
Figure 3. Anemia in children over 6 months of age. *Significant difference from other groups (p < 0.05). Anemia was defined as Hb concentration: (1) children aged 6–59 months: Hb<110 g/L; (2) children aged 5–11 years: Hb<115 g/L; and (3) children aged 12–16 years: Hb<120 g/L.
As shown in Table 4, the basic characteristics of the children, including region, sex, diagnosis and guardians' education were not significantly different between the 2 groups (P > 0.05). However, compared with the treatment group, children in the abandonment group were younger and had higher nutritional risk scores (P < 0.05). Furthermore, the BAZs, HAZs and concentrations of Hb and ALB in the abandonment group were lower than those in the treatment group, and the differences were statistically significant (P < 0.05, Table 4). Compared with the treatment group, the rate of wasting (50%) and the prevalence of anemia (68.2%) were higher among children over 6 months of age in the abandonment group (P = 0.0002, 0.005, respectively) (data not shown). The prevalence of severe malnutrition in the abandonment group (15/55, 27.3%) was higher than that in the treatment group (24/313, 7.7%) (P = 0.0002). No significant differences were observed in the prevalence of malnutrition between the two groups (P > 0.05).
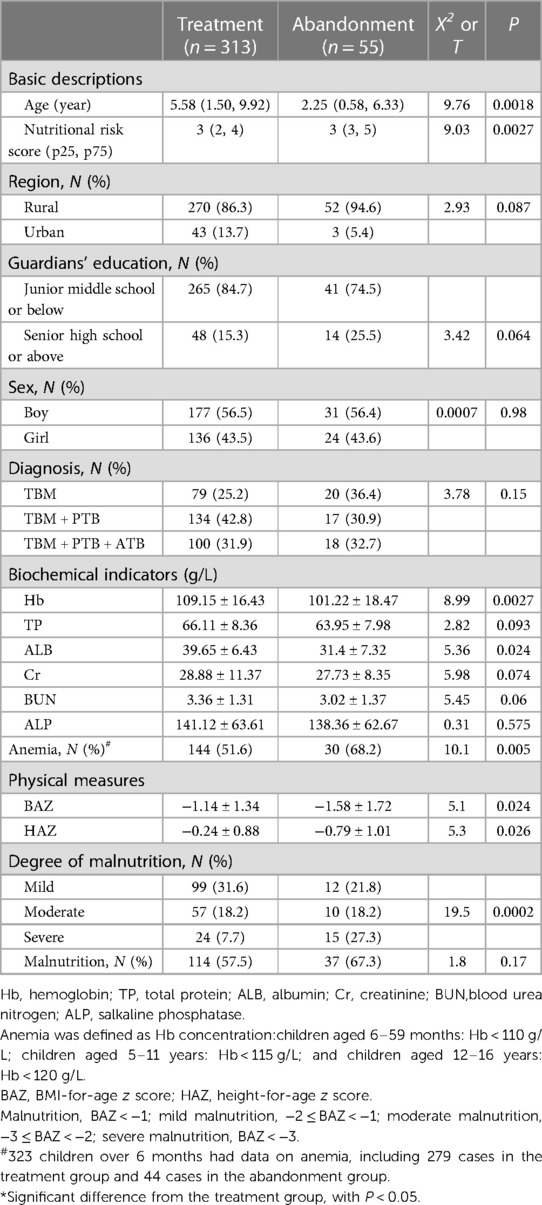
Table 4. Basic descriptions, biochemical indicators and physical characteristics of children in the treatment group and abandonment group.
According to the results of the univariate analysis, parameters with statistical significance were included in the multiple logistic regression analysis. The results showed (Table 5) that age, malnutrition, anemia, nutritional risk degree, and diagnosis were significantly associated with guardians' decision for continued treatment, with a P < 0.05. In particular, children who were older (OR = 1.1), had a higher BAZ (OR = 1.98), and had no anemia (OR = 1.02) were more likely to obtain treatment with their guardians' support than those who were younger, had a lower BAZ, and suffered from anemia (P < 0.05). Children who had nutritional risk (OR = 0.56) were less likely to obtain treatment with their guardians' support than children who did not have nutritional risk (P < 0.05). TBM patients with PTB and/or ATB (OR = 0.65) were less likely to obtain treatment with their guardians' support than children with TBM (P < 0.05).
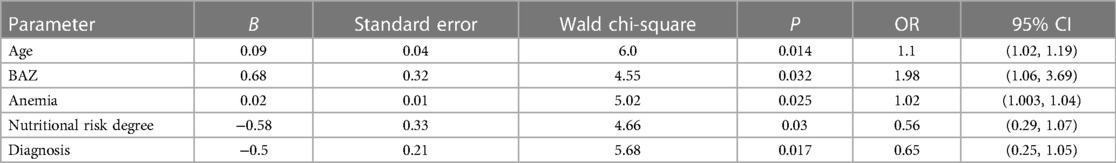
Table 5. The relationship between significant predictor variables and guardians’ treatment decisions.
As is known, malnutrition and anemia are common among children with TB.It has been reported that poor nutritional status was associated with increased extrathoracic TB (20). The aim of this survey was to explore the effects of TB infection at different sites on anthropometric indicators, malnutrition and anemia incidence in children in Southwest China.In this survey,the BAZs, HAZs, and concentrations of Hb among children with TB were decreased, the prevalence of malnutrition and anemia was 58.7% (216/368)and 58.8% (190/323), respectively.These findings suggest that nutritional status is a serious public health problem among children with TBM in Southwest China. Furthermore, this survey was the first compared the nutritional status of children with TB infection at different sites.
Existing data demonstrate that malnutrition is common among children with TB (6, 7, 9, 21) and vice versa (22, 23), with mortality tending to increase when these two diseases co-occur (24). In our survey, children in the TPA group had a higher incidence of malnutrition, reaching 69.5% (82/118). This suggests that children were more likely to suffer from malnutrition when these three diseases (TBM, PTB and ATB) co-existed. Intestinal TB involving the ileocecal region and ulcerative type mainly presents with malabsorption syndrome.Poor nutrition could be caused by decreased intake, malabsorption and increased loss (due to vomiting, abdominal pain, diarrhea, etc.).
Infancy (children aged 1 month to 2 years) and adolescence (children aged 10–16 years) are the two growth and TB infection peaks (24–26). Therefore, it is likely that infants and adolescents are more susceptible to stunted growth when TB occurs. However, contrary to other reports (3, 13, 27, 28), the prevalence of malnutrition was not the highest in children under 2 years of age in our survey. We speculate that they may have benefited from national policies. In 2017, the General Office of the State Council of the People's Republic of China issued the National Nutrition Plan of Action (2017–2030) (29), which explicitly proposed attaching importance to the nutrition of children in the first 1,000 days of life (within 2 years after birth) and formulated a series of measures. The nutritional status of children aged 10–16 years was the worst, with the malnutrition rate reaching 72.4% (63/87), which were higher than children at other ages. Adolescence is the second growth peak. they have a rapid growth in height and weight, and need more nutrition than children of other ages.Compared with healthy children of the same age, children in the 16 year-olds received fewer nutrients but consume more due to disease factors.There was no difference in the incidence of malnutrition between children at 2–10 years and those under 2 years. Some research showed that children at 2–10 years old,especially younger school age children (5–10 years old) seem to be relatively protected against TB, prior to a second peak in incidence during adolescence (24, 30). TB infection between 5 and 10 years of age rarely progressed to serious disease, and such progression was associated with significant clinical symptoms.The interactions between human host and the mycobacterium tuberculosis are extremely complex and dynami, and the true correlates of protective paediatric immunity remain unknown.
Anemia is a major health concern especially in developing countries. Children and women of reproductive age are especially susceptible.The main causes of anemia are infection, nutrition (iron deficiency disorder), bleeding and so on.TB and anaemia are mutually reinforcing and increase the prevalence of each other (31, 32).Children with TB may have a higher incidence of anaemia than healthy children due to infection and reduced nutrient intake (33–35). Anemia is an important factor affecting the growth and health of children, as well as the prognosis of diseases. However, there are few reports about anemia in children with TBM—the most serious type of TB. Our results showed that the incidence of anemia in the TPA group and 0.5∼ to 2-year-old group was the highest, reaching 70.5% and 79.4%, respectively. TB can induce systemic inflammation, and anemia of inflammation may worsen with an increase in the number of infected sites. Compared with adolescents, infants and young children with TBM have an acute onset and rapid progression (36), which may prompt them to seek medical treatment earlier. Malnutrition often takes a certain amount of time to occur, while anemia may occur at any time.
In this survey,it was showed that some TB patients eventually abandoned treatment. Some researchers reported that low income and education were the reasons of abandoning treatment (37). Unfortunately, most of the guardians in this survey were unwilling to provide annual household income due to privacy issues, it was collected the regional distribution (including rural and urban) to reflect the economics in this survey. There was no statistical difference in regional distribution and education level between the abandonment group and the treatment group (P > 0.05, Table 4). This results were inconsistent with others’ (37), which might be attributed to the fact that the patients’ guardians were mainly from poor areas in Southwest China, who were at a low socioeconomic level and low education level. Furthermore, it had not been reported that whether nutritional status was a factor that affected guardians'decision to treat (abandonment or treatment).This survey was the first reported the nutritional status of children in the abandonment group and the treatment group. It showed that nutritional status was one of the causes of abandoning treatment in children with TB infection in Southwest China.
This cross-sectional survey showed that the nutritional status of children with TB at different sites in Southwest China was seriously affected. Malnutrition and anemia were common among TBM patients, especially those combined with PTB and ATB. The prevalence rates of anemia and malnutrition were the highest among children aged 0.5–2 years and 10–16 years, respectively. Therefore, in clinical practice, we should focus on monitoring anthropometric indicators and biochemical indicators to detect growth deviation and anemia as early as possible, and carry out standardized nutrition management.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were approved by Ethical approval was obtained from the Ethics committee of the Children's Hospital of Chongqing Medical University. Written informed consent was obtained from the individual, and minor' legal guardians/next of kin, for the publication of any potentially identifiable images or data included in this article.
ZG, QL and YL conceived, designed the study, and collected the data. ZG and QD wrote the manuscript. LK modified the chart of the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1188704/full#supplementary-material
1. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global Tuberculosis report 2020—reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113(1):S7–S12. doi: 10.1016/j.ijid.2021.02.107
2. World Health Organization. Global tuberculosis report 2021. (2021). Available at: https://www.who.int/publications/i/item/9789240037021_eng.pdf (Accessed October 14, 2021).
3. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health. (2017) 5(9):e898–906. doi: 10.1016/S2214-109X(17)30289-9
4. Yang LY, Huang YF, Yu Y, Liu Y. Clinical epidemiological analysis of 920 cases of pulmonary tuberculosis in children. J Clin Pediatr. (2019) 37(06):413–7.
5. Mezochow A, Thakur K, Vinnard C. Tuberculous meningitis in children and adults: new insights for an ancient foe. Curr Neurol Neurosci Rep. (2017) 17(11):85. doi: 10.1007/s11910-017-0796-0
6. Aygun D, Akcakaya N, Cokugras H, Camcıoglu Y. Evaluation of clinical and laboratory characteristics of children with pulmonary and extrapulmonary Tuberculosis. Medicina (Kaunas). (2019) 55(8):428. doi: 10.3390/medicina55080428
7. Laghari M, Sulaiman SAS, Khan AH, Memon N. Epidemiology of tuberculosis and treatment outcomes among children in Pakistan: a 5 year retrospective study. PeerJ. (2018) 6:e5253. doi: 10.7717/peerj.5253
8. Nansumba M, Kumbakumba E, Orikiriza P, Bastard M, Mwanga JA, Boum Y, et al. Treatment outcomes and tolerability of the revised WHO anti-tuberculosis drug dosages for children. Int J Tuberc Lung Dis. (2018) 22(2):151–7. doi: 10.5588/ijtld.17.0535
9. Wobudeya E, Jaganath D, Sekadde MP, Nsangi B, Haq H, Cattamanchi A. Outcomes of empiric treatment for pediatric tuberculosis, Kampala, Uganda, 2010–2015. BMC Public Health. (2019) 19(1):446. doi: 10.1186/s12889-019-6821-2
10. Munthali T, Chabala C, Chama E, Mugode R, Kapata N, Musonda P, et al. Tuberculosis caseload in children with severe acute malnutrition related with high hospital based mortality in Lusaka, Zambia. BMC Res Notes. (2017) 10(1):206. doi: 10.1186/s13104-017-2529-5
11. Reuter A, Hughes J, Furin J. Challenges and controversies in childhood tuberculosis. Lancet. (2019) 394(10202):967–78. doi: 10.1016/S0140-6736(19)32045-8
12. World Health Organization. Roadmap towards ending TB in children and adolescents. 2nd ed. Geneva: World Health Organization (2018). Available at: http://www.who.int/tb/publications/ 2018/tb-childhood roadmap/en/ (Accessed October 11, 2021).
13. Vonasek BJ, Radtke KK, Vaz P, Buck WC, Chabala C, McCollum ED, et al. Tuberculosis in children with severe acute malnutrition. Expert Rev Respir Med. (2022) 16(3):273–84; Erratum in: Expert Rev Respir Med. (2022) 16(5):i. doi: 10.1080/17476348.2022.2043747
14. Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. (2010) 10(11):803–12. doi: 10.1016/S1473-3099(10)70138-9
15. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization (2014).
16. Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. (2017) 145(4):448–63. doi: 10.4103/ijmr.IJMR_1950_16
17. Becker P, Carney LN, Corkins MR, Monczka J, Smith E, Smith SE, et al. Consensus statement of the academy of nutrition and dietetics/American society for parenteral and enteral nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract. (2015) 30(1):147–61. doi: 10.1177/0884533614557642
18. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (2019). Available at: https://www.who.int/vmnis/indicators/haemoglobin.pdf (Accessed March 10, 2019).
19. Hulst JM, Zwart H, Hop WC, Joosten KF. Dutch National survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr. (2010) 29(1):106–11. doi: 10.1016/j.clnu.2009.07.006
20. Blount RJ, Tran B, Jarlsberg LG, Phan H, Thanh Hoang V, Nguyen NV, et al. Childhood tuberculosis in Northern Viet Nam: a review of 103 cases. PLoS One. (2014) 9(5):e97267. doi: 10.1371/journal.pone.0097267
21. du Preez K, du Plessis L, O'Connell N, Hesseling AC. Burden, spectrum and outcomes of children with tuberculosis diagnosed at a district-level hospital in South Africa. Int J Tuberc Lung Dis. (2018) 22(9):1037–43. doi: 10.5588/ijtld.17.0893
22. Wagnew F, Worku W, Dejenu G, Alebel A, Eshetie S. An overview of the case fatality of inpatient severe acute malnutrition in Ethiopia and its association with human immunodeficiency virus/tuberculosis comorbidity-a systematic review and meta-analysis. Int Health. (2018) 10(6):405–11. doi: 10.1093/inthealth/ihy043
23. Osório DV, Munyangaju I, Muhiwa A, Nacarapa E, Nhangave AV, Ramos JM. Lipoarabinomannan antigen assay (TB-LAM) for diagnosing pulmonary Tuberculosis in children with severe acute malnutrition in Mozambique. J Trop Pediatr. (2021) 67(3):fmaa072. doi: 10.1093/tropej/fmaa072
24. Roy RB, Whittaker E, Seddon JA, Kampmann B. Tuberculosis susceptibility and protection in children. Lancet Infect Dis. (2019) 19(3):e96–108. doi: 10.1016/S1473-3099(18)30157-9
25. Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, the Society of Pediatrics, Chinese Medical Association; Subspecialty Group of Child Health Care, the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. Expert consensus on clinical practice of assessment and management of childhood physical development. Zhonghua Er Ke Za Zhi. (2021) 59(3):169–74. (Chinese). doi: 10.3760/cma.j.cn112140-20210116-00050
26. Stein CM, Zalwango S, Malone LL, Thiel B, Mupere E, Nsereko M, et al. Resistance and susceptibility to Mycobacterium tuberculosis infection and disease in Tuberculosis households in Kampala, Uganda. Am J Epidemiol. (2018) 187(7):1477–89. doi: 10.1093/aje/kwx380
27. Girum T, Kote M, Tariku B, Bekele H. Survival status and predictors of mortality among severely acute malnourished children <5 years of age admitted to stabilization centers in gedeo zone: a retrospective cohort study. Ther Clin Risk Manag. (2017) 13:101–10. doi: 10.2147/TCRM.S119826
28. World Bank, United Nations Children’s Fund, World Health Organization. Levels and trends in child malnutrition: key findings of the 2019 Edition of the Joint Child Malnutrition Estimates. (2021). Available at: http://www.unicef.org/media/files/JME_2015_edition_Sept_2015. pdf (Accessed June 10, 2021).
29. Cai L, Hu X, Liu S, Wang L, Wang X, Tu H, et al. China Is implementing the national nutrition plan of action. Front Nutr. (2022) 9:983484. doi: 10.3389/fnut.2022.983484
30. Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. (2004) 8(4):392–402.15141729
31. Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. (2019) 1450(1):15–31. doi: 10.1111/nyas.14092
32. Gelaw Y, Getaneh Z, Melku M. Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ Health Prev Med. (2021) 26(1):13. doi: 10.1186/s12199-020-00931-z
33. Gil-Santana L, Cruz LAB, Arriaga MB, Miranda PFC, Fukutani KF, Silveira-Mattos PS, et al. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of antitubercular therapy. Sci Rep. (2019) 9(1):1381. doi: 10.1038/s41598-018-37860-5
34. Chhabra S, Kashyap A, Bhagat M, Mahajan R, Sethi S. Anemia and nutritional Status in Tuberculosis patients. Int J Appl Basic Med Res. (2021) 11(4):226–30. doi: 10.4103/ijabmr.ijabmr_76_21
35. Saboor M, Zehra A, Qamar K, Moinuddin . Disorders associated with malabsorption of iron: a critical review. Pak J Med Sci. (2015) 31(6):1549–53. doi: 10.12669/pjms.316.8125
36. Pediatric Tuberculosis Committee, Chinese Association of Tuberculosis Science, Chinese Association of Research Hospitals, National Clinical Medical Research Center for Respiratory Diseases, Beijing Key Laboratory of Pediatric Respiratory Infection Diseases. Expert consensus on diagnosis of tuberculous meningitis in children. Chin Clin J Pract Pediatr. (2022) 37(7):497–501. doi: 10.3760/cma.j.cn101070-20211207-01437
Keywords: nutritional status, biochemical indexes, tuberculosis, children, China
Citation: Gao Z, Liu Q, Deng Q, Kong L and Liu Y (2023) Growth and anemia among children with tuberculosis infection at different sites in Southwest China. Front. Pediatr. 11:1188704. doi: 10.3389/fped.2023.1188704
Received: 17 March 2023; Accepted: 30 May 2023;
Published: 15 June 2023.
Edited by:
Eduardo Daniel Rosas-Blum, Pediatric GI of El Paso, PLLC, United StatesReviewed by:
María B. Arriaga, Vanderbilt University Medical Center, United States© 2023 Gao, Liu, Deng, Kong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongfang Liu bGl1eW9uZ2Zhbmc4MTFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.