- 1Senior Department of Pediatrics, Medical School of Chinese PLA, Chinese PLA General Hospital, Beijing, China
- 2Department of Pediatrics, Guangzhou Women and Children's Medical Center, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou, China
- 3Department of Pediatrics, Xuanwu Hospital Capital Medical University, Beijing, China
- 4Department of Neurology, Beijing Jingdu Children's Hospital, Beijing, China
- 5Department of Radiology, First Medical Center, Chinese PLA General Hospital, Beijing, China
Objectives: X-linked adrenoleukodystrophy (ALD) is a peroxisomal disease caused by mutations in the ABCD1 gene. Childhood cerebral ALD (CCALD) is characterized by inflammatory demyelination, rapidly progressing, often fatal. Hematopoietic stem cell transplant only delays disease progression in patients with early-stage cerebral ALD. Based on emergency humanitarianism, this study aims to investigate the safety and efficacy of sirolimus in the treatment of patients with CCALD.
Methods: This was a prospective, single-center, one-arm clinical trial. We enrolled patients with CCALD, and all enrolled patients received sirolimus treatment for three months. Adverse events were monitored and recorded to evaluate the safety. The efficacy was evaluated using the neurologic function scale (NFS), Loes score, and white matter hyperintensities.
Results: A total of 12 patients were included and all presented with CCALD. Four patients dropped out and a total of eight patients in the advanced stage completed a 3-month follow-up. There were no serious adverse events, and the common adverse events were hypertonia and oral ulcers. After sirolimus treatment, three of the four patients with an initial NFS > 10 showed improvements in their clinical symptoms. Loes scores decreased by 0.5–1 point in two of eight patients and remained unchanged in one patient. Analysis of white matter hyperintensities revealed a significant decrease in signal intensity (n = 7, p = 0.0156).
Conclusions: Our study suggested that autophagy inducer sirolimus is safe for CCALD. Sirolimus did not improve clinical symptoms of patients with advanced CCALD significantly. Further study with larger sample size and longer follow-up is needed to confirm the drug efficacy.
Clinical Trial registration: https://www.chictr.org.cn/historyversionpuben.aspx, identifier ChiCTR1900021288.
Introduction
Autophagy is a tightly regulated intracellular lysosomal pathway involved in the breakdown and removal of damaged cellular components and debris. Autophagic deficiency may be one of the important pathological features underlying many neurodegenerative diseases in patients (1). In recent years, findings from a large number of studies have demonstrated a strong link between disruptions in autophagic pathways and various neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Huntington's disease, and even some leukoencephalopathies (2–6).
X-linked adrenoleukodystrophy (ALD) is a rare neurodegenerative disease that results in the rapid inflammatory demyelination and axonal degeneration, and pathogenic mutations in ABCD1 (ALD) gene located on chromosome Xq28 has been identified as the etiology (7, 8). Despite this, the exact pathogenic mechanism of ALD is still not known. Previous research suggested that a variety of functional disorders caused by the excess of very long chain fatty acids (VLCFA), such as defective mitochondrial biosynthesis targeting the SIRT1/PGC-1α axis, the ubiquitin-proteasome system malfunction, peroxisomes malfunction, and the aberrant overactivation of the mTOR pathway that inhibits the process of autophagy, may be the primary culprits (9). Childhood cerebral adrenoleukodystrophy (CCALD) is the most serious type, and if not timely treated, cerebral inflammatory demyelination can lead to loss of neurological and cognitive function in early childhood (10, 11).
Hematopoietic stem cell transplantation (HSCT) and hematopoietic stem cell gene therapy are effective only in the early stage of CCALD. For patients with advanced-stage cerebral ALD, no therapeutic interventions are available. Launay et al. reported the key role of autophagy in the pathogenesis of ALD. They found that elevated mammalian target of rapamycin (mTOR) signaling, which is a key regulator of cellular metabolism, growth and survival, led to impaired autophagy. Moreover, they have shown that the mTOR pathway inhibitor and autophagy inducer, temsirolimus, a sirolimus derivative, can restore autophagic flow and inhibits axonal degeneration and associated motor function impairments in the mouse model, playing a protective role by preventing the induction of neuronal apoptosis (12). Other studies have also confirmed the role of sirolimus in anti-oxidative stress and neuroprotection by inducing autophagy (13, 14). By regulating autophagy to rescue neuronal apoptosis may be a new targeted therapy for neurodegenerative diseases. Autophagy inducer sirolimus has been reported to be safe for children. In the study of sirolimus in the treatment of tuberous sclerosis in children, the main adverse events associated with sirolimus were oral ulcers, upper respiratory tract infections, fever, and no serious adverse events (SAE) occurred, which demonstrated the safety of sirolimus (15, 16).
Although the efficacy of sirolimus has been confirmed in the mouse model presenting as adrenomyeloneuropathy, it also provides a basis for the study of cerebral ALD because they are all based on the mechanism of abnormal VLCFA accumulation. Based on emergency humanitarianism, we conducted the prospective, one-arm clinical study in patients with advanced CCALD to evaluate the safety of sirolimus and whether it would be effective in reducing the signs and symptoms.
Material and methods
Study design and participants
This was a prospective, single-center, one-arm clinical trial based on emergency humanitarianism. This study was approved by the institutional ethics committee and registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR1900021288). We enrolled patients with CCALD who were treated at Beijing Jingdu Children's Hospital from February 2019 to October 2019. Each patient's diagnosis was confirmed by biochemical, brain MRI, and genetic testing. Criteria for inclusion were as follows: (1) Males between 6 and 18 years of age (2) Genetic test confirmed ABCD1 gene variation (3) Elevated VLCFA levels (4) Central nervous system disease established by radiological examination of anatomical brain MRI findings (5) A score on neurologic function scale (NFS) > 1 points. We acquired written informed consent from the parents/guardians of all enrolled patients.
The early stage of the CCALD is defined as NFS ≤ 1, Loes ≥ 0.5–≤9, and the advanced stage is defined as NFS > 1, Loes > 9.
For patients who were taking other medications orally, such as hydrocortisone, anti-seizure medications, etc., we did not adjust the dosage of all drugs taken at the time of enrollment, and based on the principle of maximizing the benefits of patients, we did not ask the patients to withdraw the medication they were taking.
Study procedures
All enrolled patients received sirolimus (Hangzhou Zhongmei Huadong Pharmaceutical Co. Ltd., 50 mg/50 ml) treatment with an initial dose of 1 mg/m2/day. Blood concentration levels were monitored during the medication and the target trough blood concentration was 5–10 ng/ml (16, 17). The drug dose would be increased to reach the target concentration. Sirolimus treatment lasted for a total of three months. Patients may request a reduction or withdrawal from the study if treatment-related SAE occurs.
Safety was assessed by the reports of adverse events, physical and neurological examinations. The enrolled patients were taken blood samples to monitor blood routine and blood biochemistry before receiving sirolimus, and were regularly reviewed during treatment. SAE and deaths, regardless of whether the events were considered to be related to sirolimus were collected by the investigator.
Efficacy was evaluated using the primary outcome measures that included the score on NFS, Loes score, and white matter hyperintensities (WMH).
The NFS is evaluated according to the ALD neurologic severity scale ranging from 0 to 25 and is based on the appearance and severity of neurological symptoms (Supplementary Table S1). The higher the score, the more severe the neurological symptoms (11). The NFS evaluation was performed by clinicians unrelated to the study to eliminate subjective bias of the investigator. At each evaluation of the NFS, more than two clinicians were involved, and the same clinicians were used to make assessments at the beginning and end of the study.
Brain MRI were acquired using a Siemens 1.5 T scanner for conventional 12 channels of head coil, and collect patient image information on T1WI, T2WI and Flair sequence. The Loes score is based on an evaluator's ratings of the affected location and severity of a lesion, and whether there is focal and/or general brain atrophy (Supplementary Table S2). A normal score is equivalent to 0 for each area, 0.5 for unilateral involvement, with 1 for bilateral lesions or atrophy. Loes score ranges from 0 to 34, with higher scores indicating an increased extent of lesions on brain MRI (18). The brain MRI assessment was done by two independent radiologists. For the Loes score with inconsistent results, a third radiologist will be invited to evaluate and finally come to an agreed result. The same radiologists were used to make assessments at the beginning and end of the study. In addition, all radiologists who participated in the evaluation were not aware of the prior Loes score of the enrolled patients or whether they were involved in the intervention study.
We have used the Loes rating system to evaluate the severity of MRI lesions. However, it is a subjective measure and cannot reliably detect changes in the volume of WMH in the brain. To compare the difference of WMH before and after treatment, we linearly aligned the MRI enhancement with Gadolinium (Gd) pre- and post-treatment for each participant using FLIRT (19, 20) from FSL v6.0 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). For each patient, the WMH region of interest (WMH-ROI) of white-matter was extracted through a manual thresholding method. Specifically, an experienced radiological technician manually set individual-specific threshold for the WMH-ROI by visual inspection. The thresholds were adapted for each patient to include possibly all WMH voxels and exclude voxels of other high-intensity types, such as blood vessels. However, parts of vascular voxels were inevitably included in the WMH-ROIs due to partial overlapping of signal intensities. Although each WMH-ROI inevitably contained less or more vascular signals, the impact on the following analysis could be limited taking into account of the relatively small proportions of vascular voxels compared to WMH-ROIs.
Statistical analysis
All statistical analyses were performed by using statistical software SPSS v. 22.0. Continuous variables were described by median, maximum and minimum values. Descriptive statistics were used to describe the Loes score and NFS score. In order to quantify the changes of WMH, we firstly combined the WMH-ROIs extracted from the images before and after treatment of each patient. For each patient, we then calculated the mean and standard deviation of signals within the conjunct ROI for images pre-and post-treatment, separately. Next, a non-parametric paired test (Wilcoxon signed rank test) was used to investigate whether the WMH in conjunct ROIs was significantly different before and after treatment.
Results
Baseline data
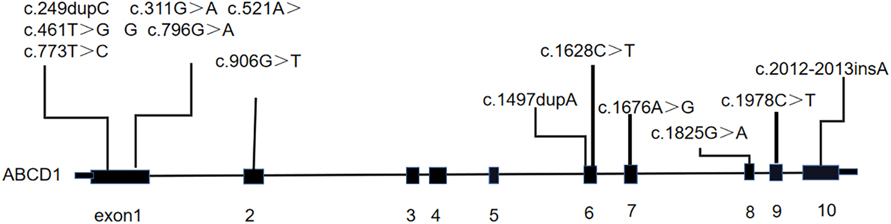
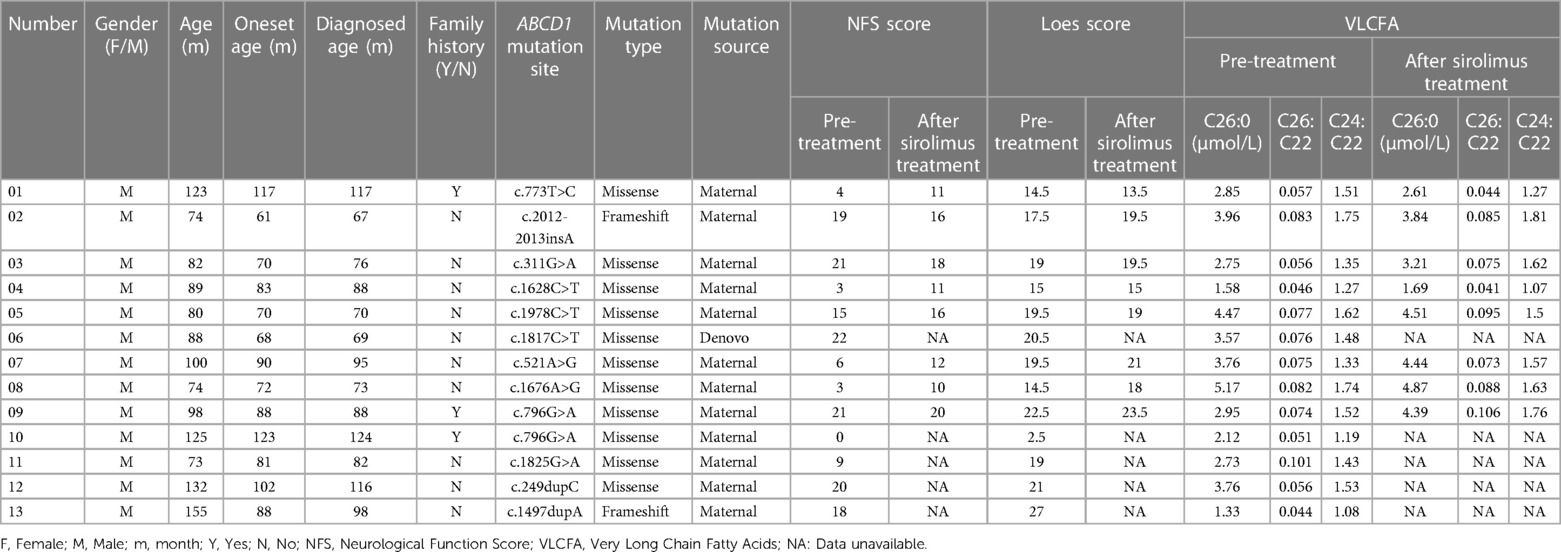
A total of 12 male patients with CCALD were enrolled (Table 1). The median age of disease onset and enrollment was 82 months (61–117 months) and 88.5months (74–155 months), respectively. All patients were found to carry ABCD1 gene mutations via genetic testing, of which nine were missense mutations and three were frameshift mutations. The locations of the gene mutation sites are shown in Figure 1. The mutation sites were maternal in 11 patients, and only one was de novo (Table 1). Two of the 12 patients had a known family history of CCALD. At baseline, each patient underwent neurological functional assessment using the NFS and Loes score. The NFS scores ranged from 3 to 22 points with the median of 16.5, and the Loes scores ranged from 14.5 to 27 points with the median of 19. All patients were in the advanced stage.
Safety
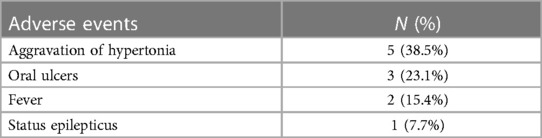
We continuously monitored all adverse events that occurred during the treatment, whether they were related to sirolimus or not (Table 2). Five of 12 patients experienced aggravation of hypertonia after oral sirolimus, of which three patients withdrew. Three patients had oral ulcers during medication. Two patients showed signs of a fever during the course of treatment, and soon returned to normal after active symptomatic anti-infection treatment. Two patients developed status epilepticus during the treatment and seizures were controlled soon after sedation and anticonvulsant therapy. The other patients had no significant adverse events during the treatment.
The occurrence of oral ulcers was related to sirolimus, and through drinking more water and gargling to reduce the drug residues in the mouth, the occurrence of oral ulcers reduced. The occurrence of status epilepticus was considered as one of the clinical symptoms in the progression of ALD. The aggravation of hypertonia has the highest incidence of all adverse events, and when oral ulcers became severe, the hypertonia aggravated because of the pain, so it may be related to the use of sirolimus. No SAE or deaths were observed.
Efficacy evaluation
Of all the 12 enrolled patients, eight patients in the advanced stage received sirolimus treatment and completed the 3-month follow-up. Four patients dropped out, including one (patient 13) who did not complete follow-up, three (patient 6, 11, 12) who discontinued the treatment due to severe hypertonia after oral sirolimus treatment.
Among the eight patients, there were four patients with the NFS score > 10 and another four patients < 10. In the research, we found that after sirolimus treatment, three of the four patients (NFS score > 10) achieved improvement in the clinical symptoms, with NFS scores decreasing by 1–3 points and one patient increased 1 point, while the other four patients (NFS score < 10) were still progressing, with NFS scores increasing by 1–8 points (Figure 2).
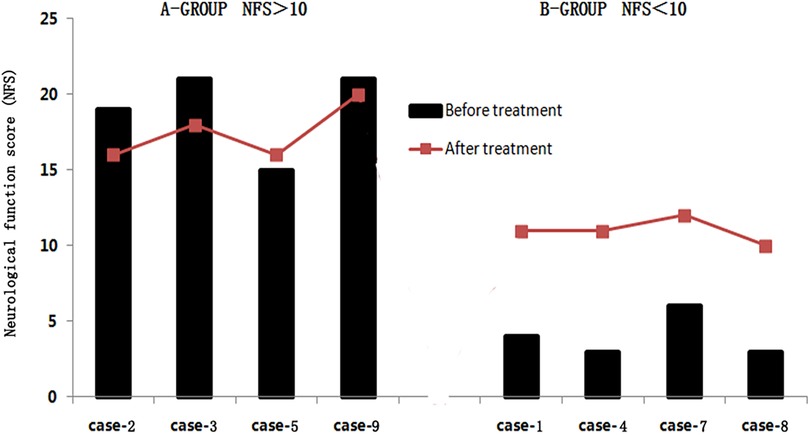
Figure 2. Changes of neurological function score (NFS) by sirolimus-treated patients. A-Group: NFS > 10. B-Group: NFS < 10.
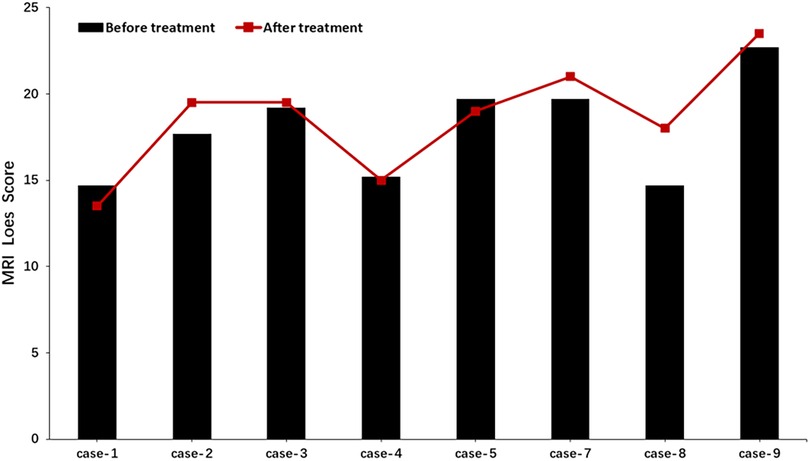
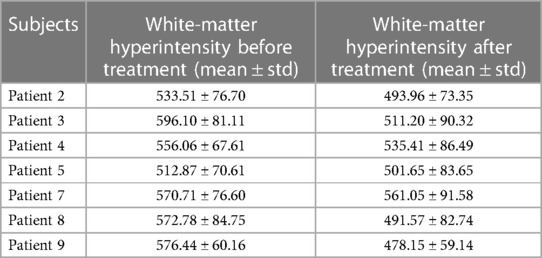
After three months of treatment, as measured with the Loes score, two of eight patients decreased by 0.5–1 point, and one patient remained unchanged, and the other five increased by 0.5–3.5 points (Figure 3). Considering that the Loes score cannot accurately reflect the changes in the volume of WMH, we processed the MRI images to better evaluate the change of WMH in the brain. Because patient 1 could not obtain the consistent scanning parameters before and after treatment, seven patients participated in the comparison of WMH in the brain. The results of processed image are shown in Figure 4. The statistical results of WMH are shown in Table 3. The difference in the volume of WMH before and after treatment in seven patients was statistically significant (p = 0.0156) (Figure 5).
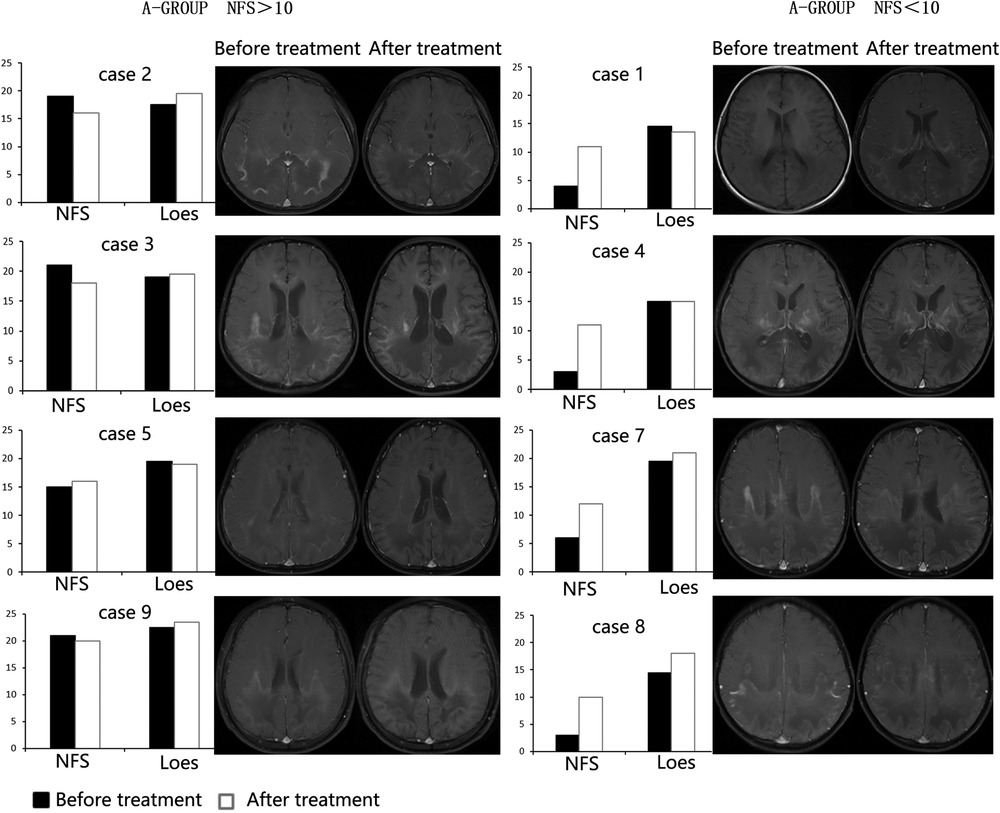
Figure 4. The MRI images after processed (left: before treatment, right: after treatment; case 5 could not be compared due to inconsistent scanning parameters before and after treatment and was excluded.).
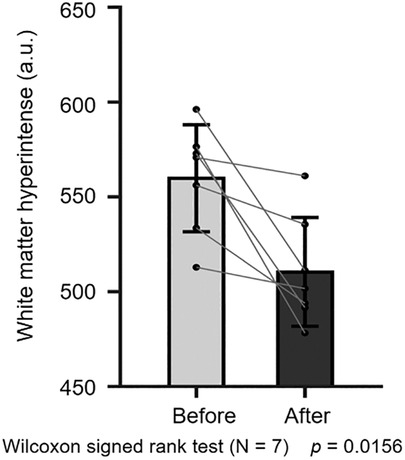
Figure 5. Statistical comparison of white-matter hyperintensity before and after treatment (n = 7, p = 0.0156).
Discussion
In this study, sirolimus was used to treat patients in the advanced stage of CCALD based on emergency humanitarianism. Our results showed that the autophagy inducer sirolimus could be safe for patients with CCALD, and the efficacy of sirolimus in improving the clinical symptoms of advanced ALD requires further studies with larger sample size and long-term follow-up.
CCALD can lead to death in young childhood. HSCT and hematopoietic stem cell gene therapy are only effective at an early stage, and there are no other effective treatments for CCALD. Moreover, there are risks of transplant failure and complication of the conditioning regimen (21, 22). In addition, the disease of CCALD progresses rapidly, and patients often have a rapid spread of demyelination in the brain, with death in a few years (23). Given the rapid progression of the disease and the absence of a treatment option to stop progression, and based on the possible efficacy of sirolimus in X-ALD proposed by Launay et al. in 2014, we tried this treatment regimen clinically. All enrolled patients were in the advanced stage and did not meet the criteria for HSCT and hematopoietic stem cell gene therapy, and these patients had no other treatment options.
No serious life-threatening adverse events were found, and the biochemical indicators remained stable, indicating that sirolimus is relatively safe for treating CCALD. Fever was considered to be a common side-effect of sirolimus. Three patients did develop the aggravation of hypertonia after sirolimus treatment, and the mechanism is not entirely clear. We cannot definitively assume that it was a direct sirolimus-related adverse event, because these patients also had oral ulcers caused by sirolimus. Pain associated with oral ulcers may also cause exacerbation of hypertonia. No other SAE occurred, which to some extent, indicated the safety of sirolimus in CCALD patients.
Sirolimus, as an autophagy inducer, can inhibit the mTOR pathway, reduce axonal degeneration, and arrest disease progression in Abcd1−/Abcd2−/− mice (12). Among patients with NFS score < 10, there was progress in scoring, which was thought to be related to the shorter follow-up period. Among patients with NFS score >10, we found an improvement of 1–3 points. However, the follow-up time was shorter, so we cannot determine the impact on quality of life. At present, the reported effective treatments of CCALD are HSCT and hematopoietic stem-cell gene therapy. However, they are only effective in patients in the early stage of CCALD (21, 22). Based on these studies, we considered whether sirolimus, like HSCT and hematopoietic stem cell gene therapy, is only effective against CCALD in the early stage. However, the CCALD patients enrolled in our study were all in the advanced stage. Therefore, whether sirolimus is effective for ALD patients in the early stage of the disease requires further long-term follow-up studies with large sample size.
Brain MRI is an important method of diagnosing ALD. Neuroimaging studies have shown that the MRI pattern of demyelination in ALD is related to disease progression and outcome (24), and the progress of Loes score is also closely related to survival rate (24, 25). Our results showed that the more severe the clinical symptoms, the higher the Loes score; that is, the severity of brain MRI is related to the severity of clinical symptoms. This is consistent with previous research. In addition, Loes scores declined or stabilized in three of the eight patients, which also provides some hope for patients in the advanced stage without effective regimens. However, the sample size of this study is small, which needs to be confirmed by further studies. The traditional Loes score is evaluated according to the affected area, but it cannot accurately reflect the changes in the volume of WMH. So, we quantitatively analyzed the WMH, and finally found that the volume of WMH decreased after sirolimus treatment, and the difference was statistically significant. This suggests that the relationship between sirolimus and demyelination in the brain deserves further attention. In addition, it has been reported that longitudinal changes in WMH volume were progressive in most patients (26, 27). Some mild patients may have multiple possibilities for the changes in WMH volume: progression, regression, or stability, and most of the regression is related to the intervention (28). In this study, the patients were in advanced stage of disease, so it is unlikely that a reduction in WMH would have occurred without intervention. After sirolimus treatment, WMH volume decreased and the difference was statistically significant, suggesting the role of sirolimus to some extent. However, the analysis of WMH has certain limitations. There seem progressive atrophic changes in the brain including the white matter, as a result, comparison areas of WMH may represent atrophic changes. Therefore, the effect of brain atrophy on WMH volume change cannot be ruled out.
For the pathogenic mechanism, it has been reported that inflammatory demyelination of cerebral ALD seems to be related to autoimmunity, such as increased serum antibody responses against myelin oligodendrocyte glycoprotein (29, 30). The mTOR inhibitor sirolimus has shown remarkable clinical efficacy in autoimmune diseases such as systemic lupus erythematosus and multiple sclerosis (31, 32). Therefore, it would be of interest to monitor the immunological status, such as routine detection of antinuclear and myelin basic protein and myelin oligodendrocyte auto-antibodies to demonstrate efficacy of sirolimus in patients with ALD. However, the limitation of this study is that patients' immune status was not monitored during treatment. We expect to complete the detection of autoantibody status in future studies.
Glucocorticoids have been reported to inhibit inflammation (33). In this study, hydrocortisone treatment was performed in 2 of the 8 patients who completed the study, however, it is worth noting that, the 2 patients had been taking hydrocortisone (0.22–0.49 mg/kg/day) for a long time before enrollment. In addition, the original dose of the drug did not change after enrollment, which reduced the influence of hydrocortisone to some extent.
Although it is strictly designed, it still has some limitations in this study. First, there was no control group in this study. Since all of the patients enrolled in this study are CCALD in the advanced stage with rapid disease progression, and death can occur within several years. Moreover, their parents were willing to take various treatments for their children instead of waiting, so this study based on emergency humanitarianism treated CCALD patients in the advanced stage of the disease with sirolimus. In addition, for patients who received HSCT previously, they were all at an early stage, so no suitable historical control has been found. Based on the above reasons, this study was set as a self-controlled study of sirolimus add-on therapy, and all patients maintained their original oral drug dose after enrollment. Second, it is based on the principle of maximizing the benefits of patients, so during the treatment, all patients did not change their original treatment drugs, which led to bias of research results, and the interference of drug interactions could not be ruled out. The last, since sirolimus was used for the first time in ALD patients, we designed this research as a pilot study in consideration of the safety, so the sample size is small and the follow-up time of three months is relatively short, and it was not clear whether sirolimus could benefit ALD patients in the long term. Therefore, it is necessary to further study the efficacy, optimal dose, treatment duration, and indications of sirolimus in larger randomized controlled trials. Despite this, our study suggests that sirolimus could be a safe choice for CCALD patients.
Conclusion
The autophagy inducer sirolimus may be safe to treat CCALD patients. However, larger sample sizes and longer follow-up studies are needed to clarify the efficacy of sirolimus in patients with advanced CCALD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Beijing Jingdu Children's Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
X-ML, L-YL, Q-HW, designed the study and finished investigation, data acquisition, drafting of the manuscript. L-PZ and S-JL provided formulation or evolution of overarching research goals and finished the design of methodology, study supervision, critical revision. Y-YW finished data acquisition, data analysis. JW finished data interpretation. X-YY taken part in data acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the “The Capital Health Research and Development of Special (grant number: 2022-1-5081)”.
Acknowledgments
The authors thank the parents or guardians of all participants for their cooperation and persistence to this Study. The author thanks the clinicians and radiologists who helped in this study. The author thanks the China Adrenoleukodystrophy Care Center for its assistance in the patient recruitment phase.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1187078/full#supplementary-material.
References
1. Son J, Shim J, Kim K, Ha J, Han J. Neuronal autophagy and neurodegenerative diseases. Exp Mol Med. (2012) 44(2):89–98. doi: 10.3858/emm.2012.44.2.031
2. Ghavami S, Shojaei S, Yeganeh B, Ande S, Jangamreddy J, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. (2014) 112:24–49. doi: 10.1016/j.pneurobio.2013.10.004
3. Zhang J, Lachance V, Schaffner A, Li X, Fedick A, Kaye L, et al. A founder mutation in VPS11 causes an autosomal recessive leukoencephalopathy linked to autophagic defects. PLoS Genet. (2016) 12(4):e1005848. doi: 10.1371/journal.pgen.1005848
4. Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, et al. Cargo recognition failure is responsible for inefficient autophagy in huntington's disease. Nat Neurosci. (2010) 13(5):567–76. doi: 10.1038/nn.2528
5. Senkevich K, Gan-Or Z. Autophagy lysosomal pathway dysfunction in Parkinson's disease; evidence from human genetics. Parkinsonism Relat Disord. (2020) 73:60–71. doi: 10.1016/j.parkreldis.2019.11.015
6. Boland B, Kumar A, Lee S, Platt F, Wegiel J, Yu W, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. (2008) 28(27):6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008
7. Wiesinger C, Eichler F, Berger J. The genetic landscape of X-linked adrenoleukodystrophy: inheritance, mutations, modifier genes, and diagnosis. Appl Clin Genet. (2015) 8:109–21. doi: 10.2147/TACG.S49590
8. Mallack E, Gao K, Engelen M, Kemp S. ABCD1Structure And function of the variant database: 20 years, 940 pathogenic variants, and 3400 cases of adrenoleukodystrophy. Cells. (2022) 11(2):283. doi: 10.3390/cells11020283
9. Fourcade S, Ferrer I, Pujol A. Oxidative stress, mitochondrial and proteostasis malfunction in adrenoleukodystrophy: a paradigm for axonal degeneration. Free Radical Biol Med. (2015) 88:18–29. doi: 10.1016/j.freeradbiomed.2015.05.041
10. Raymond G, Aubourg P, Paker A, Escolar M, Fischer A, Blanche S, et al. Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25(3):538–48. doi: 10.1016/j.bbmt.2018.09.036
11. Moser H, Mahmood A, Raymond G. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. (2007) 3(3):140–51. doi: 10.1038/ncpneuro0421
12. Launay N, Aguado C, Fourcade S, Ruiz M, Grau L, Riera J, et al. Autophagy induction halts axonal degeneration in a mouse model of X-adrenoleukodystrophy. Acta Neuropathol. (2015) 129(3):399–415. doi: 10.1007/s00401-014-1378-8
13. Singh A, Kashyap M, Tripathi V, Singh S, Garg G, Rizvi S. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol Neurobiol. (2017) 54(8):5815–28. doi: 10.1007/s12035-016-0129-3
14. Dai C, Ciccotosto G, Cappai R, Wang Y, Tang S, Hoyer D, et al. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction, and apoptosis through the activation of autophagy and mTOR/akt/CREB signaling pathways. ACS Chem Neurosci. (2018) 9(4):824–37. doi: 10.1021/acschemneuro.7b00323
15. He W, Chen J, Wang Y, Zhang M, Qian LU, Wang Q, et al. Sirolimus improves seizure control in pediatric patients with tuberous sclerosis: a prospective cohort study. Seizure. (2020) 79:20–6. doi: 10.1016/j.seizure.2020.03.018
16. Chen X, Wang Y, Zhang M, Lu Q, Pang L, Liu L, et al. Sirolimus can increase the disappearance rate of cardiac rhabdomyomas associated with tuberous sclerosis: a prospective cohort and self-controlled case series study. J Pediatr. (2021) 233:150–5.e4. doi: 10.1016/j.jpeds.2021.02.040
17. Shen Y, Wang Y, Zhang M, Xu Y, Lu Q, He W, et al. Sirolimus treatment for tuberous sclerosis complex prior to epilepsy: evidence from a registry-based real-world study. Seizure. (2022) 97:23–31. doi: 10.1016/j.seizure.2022.03.003
18. Loes D, Hite S, Moser H, Stillman A, Shapiro E, Lockman L, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol. (1994) 15(9):1761–6.7847225
19. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. (2001) 5(2):143–56. doi: 10.1016/S1361-8415(01)00036-6
20. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. (2002) 17(2):825–41. doi: 10.1006/nimg.2002.1132
21. Eichler F, Duncan C, Musolino P, Orchard P, De Oliveira S, Thrasher A, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. (2017) 377(17):1630–8. doi: 10.1056/NEJMoa1700554
22. Chiesa R, Boelens J, Duncan C, Kühl J, Sevin C, Kapoor N, et al. Variables affecting outcomes after allogeneic hematopoietic stem cell transplant for cerebral adrenoleukodystrophy. Blood Adv. (2022) 6(5):1512–24. doi: 10.1182/bloodadvances.2021005294
23. Engelen M, Kemp S, de Visser M, van Geel B, Wanders R, Aubourg P, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. (2012) 7:51. doi: 10.1186/1750-1172-7-51
24. Loes D, Fatemi A, Melhem E, Gupte N, Bezman L, Moser H, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. (2003) 61(3):369–74. doi: 10.1212/01.WNL.0000079050.91337.83
25. Moser H, Loes D, Melhem E, Raymond G, Bezman L, Cox C, et al. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. (2000) 31(5):227–39. doi: 10.1055/s-2000-9236
26. Xu X, Gao Y, Liu R, Qian L, Chen Y, Wang X, et al. Progression of white matter hyperintensities contributes to lacunar infarction. Aging Dis. (2018) 9(3):444–52. doi: 10.14336/AD.2017.0808
27. Scharf E, Graff-Radford J, Przybelski S, Lesnick T, Mielke M, Knopman D, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: the mayo clinic study of aging. Stroke. (2019) 50(11):3037–44. doi: 10.1161/STROKEAHA.119.025822
28. Jiang J, Yao K, Huang X, Zhang Y, Shen F, Weng S. Longitudinal white matter hyperintensity changes and cognitive decline in patients with minor stroke. Aging Clin Exp Res. (2022) 34(5):1047–54. doi: 10.1007/s40520-021-02024-5
29. Bernheimer H, Budka H, Müller P. Brain tissue immunoglobulins in adrenoleukodystrophy: a comparison with multiple sclerosis and systemic lupus erythematosus. Acta Neuropathol. (1983) 59(2):95–102. doi: 10.1007/BF00691593
30. Schmidt S, Marrosu G, Kölsch H, Haase C, Ferenczik S, Sokolowski P, et al. Genetic variations and humoral immune responses to myelin oligodendroglia glycoprotein in adult phenotypes of X-linked adrenoleukodystrophy. J Neuroimmunol. (2003) 135:148–53. doi: 10.1016/S0165-5728(02)00445-9
31. Lai Z, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet (London, England). (2018) 391(10126):1186–96. doi: 10.1016/S0140-6736(18)30485-9
32. Vakrakou A, Alexaki A, Brinia M, Anagnostouli M, Stefanis L, Stathopoulos P. The mTOR signaling pathway in multiple sclerosis; from animal models to human data. Int J Mol Sci. (2022) 23(15):8077. doi: 10.3390/ijms23158077
Keywords: childhood cerebral adrenoleukodystrophy, autophagy, sirolimus, white matter hyperintensities, demyelination
Citation: Luo X-M, Liu L-Y, Wang Q-H, Wang Y-Y, Wang J, Yang X-Y, Li S-J and Zou L-P (2023) Exploratory study of autophagy inducer sirolimus for childhood cerebral adrenoleukodystrophy. Front. Pediatr. 11:1187078. doi: 10.3389/fped.2023.1187078
Received: 15 March 2023; Accepted: 5 May 2023;
Published: 6 June 2023.
Edited by:
Hong Ni, Children's Hospital of Soochow University, ChinaReviewed by:
Mushfiquddin Khan, Medical University of South Carolina, United StatesDan Yu, Sichuan University, China
© 2023 Luo, Liu, Wang, Wang, Wang, Yang, Li and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Jun Li bGlzajMwMUBxcS5jb20= Li-Ping Zou em91bGlwaW5nMjFAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiao-Mei Luo1,2,†
Xiao-Mei Luo1,2,† Yang-Yang Wang
Yang-Yang Wang Jing Wang
Jing Wang Shi-Jun Li
Shi-Jun Li Li-Ping Zou
Li-Ping Zou