- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, BC Children’s Hospital, Vancouver, BC, Canada
- 2Division of Pediatric Hematology-Oncology, CHU de Québec—Centre Mère-Enfant Soleil, Quebec City, QC, Canada
- 3Research Center of the CHU de Québec, Axe Reproduction, Santé de la Mère et de l’Enfant, Quebec City, Canada
- 4Department of Pediatrics, Schulich School of Medicine & Dentistry, Western University, London, ON, Canada
- 5Department of Pediatrics, Division of Hematology/Oncology, London Health Sciences Centre, London, ON, Canada
Thromboembolism is an infrequent complication in children with hemophilia that has been traditionally associated with the presence of a central venous access device. Novel rebalancing agents have shown promising results as prophylactic therapies to minimize the risk of bleeding but both thromboembolism and thrombotic microangiopathy have been reported as complications. The management of thrombosis in children with hemophilia is particularly challenging given the inherent risk of bleeding. In this paper, we present clinical vignettes to review the literature, highlight challenges, and describe our approach to managing thromboembolism in children with hemophilia.
1. Introduction
Hemophilia is an X-linked condition that results in the deficiency of factor VIII (FVIII) or factor IX (FIX) and can lead to life-threatening bleeding, hemarthrosis, and hemophilic arthropathy. The risk of bleeding is correlated with factor levels and is defined as mild (5%–40% factor level), moderate (1%–5% factor level) and severe (<1% factor level) (1). The use of prophylactic therapy to prevent bleeding complications is the cornerstone of hemophilia treatment in persons with severe hemophilia and those with moderate hemophilia with a bleeding phenotype. Frequent venipuncture can be painful and challenging in young children. Thus, a central venous access device (CVAD) is often needed for ease of administration and to institute optimal prophylaxis regimens at a young age. In the last few years, several non-factor replacement therapies have been developed to provide prophylaxis for persons with hemophilia. These novel agents are administered subcutaneously, making them substantially easier to administer in young children (2).
While it seems paradoxical that children with bleeding disorders would develop thrombosis, thrombotic complications are a rare but well-described complication in persons with hemophilia, especially with the use of CVAD. Clinical trials of non-replacement therapies have also reported thrombotic complications. However, there are no guidelines in the literature to guide the management of thrombotic events in children with hemophilia. In this article, we use clinical vignettes to present a review of the literature and our approach to the prevention and management of thrombotic complications in children with hemophilia. Of note, as gene therapy has not yet been studied in children, thrombosis related to gene therapy was outside the scope of this review.
2. Catheter-related thrombosis in children with hemophilia
Case 1: “A six-year-old male with severe hemophilia A presents with intermittent occlusion of his CVAD. He has a high-titre inhibitor and is currently receiving immune tolerance induction (ITI) with daily infusions of recombinant FVIII. His left-sided Port-A-Cath was put in place at the age of two to facilitate venous access in the setting of ITI. A compression ultrasound with Doppler is done to investigate the etiology of intermittent line occlusion and shows a non-occlusive thrombosis of the left internal jugular vein, at the tip of the CVAD. Is anticoagulation warranted for this child? Should the CVAD be removed?”
2.1. Use of CVAD in children with hemophilia
Persons with severe hemophilia require prophylactic administration of CFC to decrease the risk of bleeding and long-term consequences of arthropathy (3, 4). One of the challenging complications of CFC replacement therapy is the development of inhibitors or neutralizing antibodies (5). As inhibitors render CFC ineffective, they portend a very high risk of bleeding (6). Management of inhibitors in hemophilia generally involves intensive factor replacement therapy to induce tolerance (immune tolerance induction, ITI). ITI in children is particularly challenging because of the need of frequent, often daily, venous injections. The inability to ensure proper venous access may result in sub-optimal delivery of ITI and can have a tremendous negative effect on the quality of life of the child. CVADs have been shown to ensure timely, safe, and effective management of children with inhibitors (7). As such, the development of inhibitors in a young child typically results in the insertion of a CVAD. Even in young children without inhibitors, the use of a CVAD may facilitate effective delivery of prophylactic therapy. Blanchette et al. surveyed pediatric patients with hemophilia in North America and reported that approximately 80% of children on full dose prophylaxis therapy under the age of five required a CVAD (8). The installation of a CVAD comes with several risks such as perioperative bleeding, infection, thrombosis, and an increased need for inpatient admissions (9–11).
2.2. Epidemiology and risk factors of CVAD-associated thrombosis in children with hemophilia
Thromboembolism in persons with hemophilia is rarely reported in the literature probably due to the protective effect of hemophilia against blood clot formation. As such, data are scarce regarding their epidemiology and no established pediatric guidelines are available to guide treatment and follow-up.
One of the main causes of thrombus formation in children with a CVAD is endovascular injury (10). CVAD-specific characteristics such as the size, location, and type of catheter used may also contribute to the risk of thrombus formation (12). Persistently elevated endogenous levels of FVIII have been shown to increase the risk of TE in children and healthy individuals (13, 14). Thus, authors have hypothesized that frequent infusions of supratherapeutic doses of CFC may help contribute to the propagation of thrombosis once in place (15). Augustsson et al. described how the use of CFC can potentiate formation and propagation of thrombus through mechanisms that can be unrelated to tissue factor (16).
The incidence of thromboembolism in children with hemophilia who required a CVAD is highly variable in the literature, mainly due to variations in screening practices between institutions. In a systematic review and meta-analysis, Valentino et al. reviewed 2,973 cases of CVAD placement in 2,704 persons with hemophilia in different centers around the world. Fifty-five cases of thrombosis were identified, while 34.9% of the patients had CVAD placement for ITI. This review did not suggest a difference in rate of thrombosis in patients with and without factor inhibitors (17). Conversely, Van Dijk et al. noted that patients with hemophilia on ITI had higher rate of CVAD-related thrombosis (7.2 vs. 3.1/1,000 CVAD days), with 15% of all children with a CVAD in their cohort experiencing a thrombotic event (7). Journeycake et al. performed screening venography in 15 patients with hemophilia who had a CVAD for more than 12 months and reported eight (53%) patients with a DVT. All of the thromboses were found in patients with CVAD for more than 48 months (18). In the international immune tolerance study that surveyed 99 patients with 183 catheters, screening for asymptomatic thrombi was not performed and only one symptomatic DVT was identified (19). Carcao et al. hypothesized that continuation of frequent administration of factor replacement as part of ITI might cause a prothrombotic state, especially when the level of inhibitor is low, which might increase the risk of thrombus formation. The authors recommend monitoring of inhibitor titers and to stop frequent factor replacement when inhibitor titers drop below 2–3 Bethesda Unit (BU) (20).
2.3. Management of CVAD-related thrombosis in children with hemophilia
Management of CVAD-related thrombosis is challenging in patients with hemophilia. The treatment approach should be individualized based on the patient's thrombotic and bleeding risk as well as the ongoing need for a CVAD; most commonly used modalities include expectative treatment, CVAD removal, and anticoagulation at either a prophylactic or a therapeutic dosing (Figure 1). The decision to initiate anticoagulation should be carefully made in consultation with hematologists. One report suggested removal of the CVAD with no anticoagulation as an efficacious method to prevent further propagation of the clot (18). In such situations, close monitoring with compression ultrasound is needed to ensure stabilization or degradation of the clot.
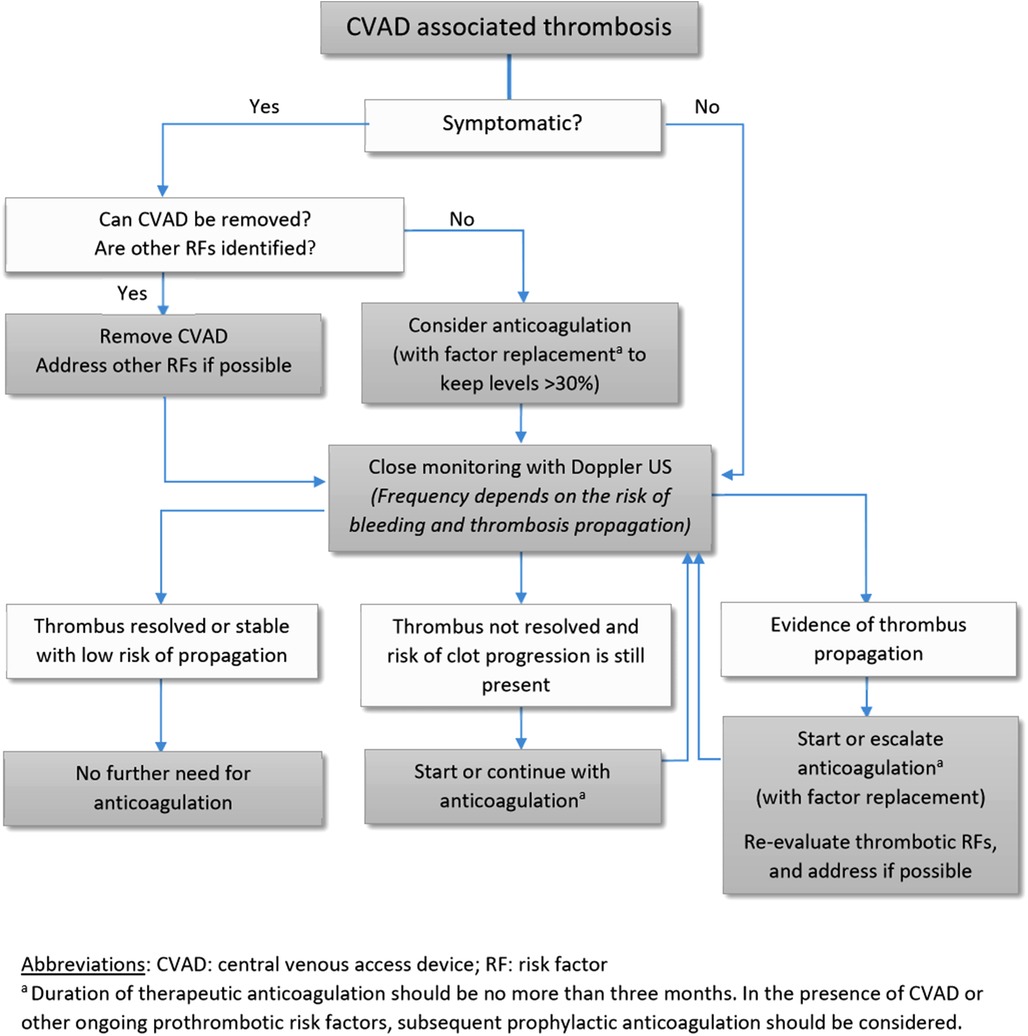
Figure 1. CVAD, central venous access device; RF, risk factor. aDuration of therapeutic anticoagulation should be no more than three months. In the presence of CVAD or other ongoing prothrombotic risk factors, subsequent prophylactic anticoagulation should be considered.
If anticoagulation is required, keeping the factor level above 30% might be required for safe anticoagulation (21) although this may be quite challenging or impossible in children with high-titer inhibitors. Dargaud et al. described the importance of balancing the use of anticoagulation and factor replacement, by aiming for therapeutic doses rather than reduced doses of anticoagulation in combination with CFC in such a way to have optimal anticoagulation activity around the factor plasma peak level (22). Alternatively, the use of Emicizumab (further described below) can be cautiously considered for prophylaxis while requiring anticoagulation. Weyand et al. reported a child with CVAD-related thrombosis in the context of bacteremia and ITI. The child was on prophylaxis with activated prothrombin complexes concentrates (aPCC) and developed significant enlargement of the thrombus, resulting in symptomatic obstruction of the right ventricular outflow tract. The child was transitioned to emicizumab and treated with low molecular weight heparin (LMWH) and received a total of six months of anticoagulation with removal of the CVAD at the three-month mark. A single hematoma at the emicizumab injection site was reported. Conversely, Barg et al. reported a child with a CVAD-related thrombosis treated with LMWH on emicizumab who experienced a fatal retroperitoneal bleed. As emicizumab results in thrombin generation of roughly 10%–15% FVIII-equivalent activity, emicizumab alone may be insufficient as prophylactic therapy while on therapeutic anticoagulation. Further data will be needed regarding the safety of anticoagulation while on emicizumab prophylaxis.
In persons with hemophilia who developed an inhibitor, given the high bleeding risk and the difficulty of achieving safe FVIII or FIX levels, one should be very careful before initiating anticoagulation. If the patient still necessitates a CVAD, anticoagulation with a lower therapeutic target, or using prophylactic dosing, might be considered to stabilize the clot and prevent propagation. Furthermore, existence of other risk factors for thrombosis propagation should be carefully evaluated for an informed decision.
When choosing anticoagulation, short-acting and reversible agents are recommended for patients with high risk of bleeding (21). In children, the most commonly used anticoagulants are unfractionated heparin and LMWH. While the use of direct oral anticoagulants (rivaroxaban and dabigatran) has recently been approved in children, there are no data on their use in persons with hemophilia and thus should be used with caution. The duration of anticoagulation should be tailored to the response to treatment and should be minimized to reduce risk of bleeding complication. We suggest performing a close follow-up ultrasound at six weeks, for possible discontinuation of anticoagulation should there be no thrombus propagation, based on the results of the KIDS-DOTT trial. Although not performed specifically in children with bleeding disorders, this randomized controlled trial has shown anticoagulation for 6 weeks to be non-inferior to a 3-month duration for thrombotic recurrence and clinically relevant bleeding for clearly provoked DVT when there is no residual occlusive thrombus at the 6-week radiological re-evaluation (23). Of note, this study excluded children with previous VTE, medical conditions putting the children at increased risk of recurrence, and high-risk thrombotic events (e.g., pulmonary embolism without DVT, more central PE, use or intent to use thrombolytic agents, etc.). If the risk factor for DVT is ongoing, anticoagulation therapy should continue in either therapeutic or prophylactic doses until the risk factor has resolved, based on the most recent pediatric guidelines for anticoagulation in children (24). Continuous assessment of the thrombotic and bleeding risk is crucial.
Thrombolysis may be indicated for life-threatening thromboembolism. For example, Carcao et al. reported a 10-year-old patient with severe hemophilia A presenting with superior vena cava syndrome necessitating mechanical thrombolysis. To minimize the risk of bleeding, his FVIII level was kept at >100% by frequent factor replacement alongside a continuous infusion of unfractionated heparin with a standard target heparin level between 0.3 and 0.7 IU/ml (20). The rationale of increasing the FVIII level to prevent major bleeding is supported by data published by analysis of 433 patients with moderate and mild hemophilia A. The study showed a decrease in bleeding episode by 18% for every 1% increase in factor VIII activity level (25).
In our case, we elected to remove the malfunctioning CVAD. As the patient was asymptomatic, and the thromboembolism was small and non-occlusive, no anticoagulation was used. Serial compression ultrasounds were performed to monitor for thromboembolism progression and showed thrombus stability. Peripheral intravenous injections were performed but ongoing difficulties with venous access prompted eventual placement of a second CVAD, with hemostatic coverage using bypassing agents. No secondary thromboprophylaxis was used.
3. Non-catheter related thrombosis in children with hemophilia
Thrombosis appears to happen very infrequently in children with hemophilia receiving CFC in the absence of a CVAD. Of note, a recent case series featured two children with mild hemophilia A (FVIII levels of 28% and 24%) who developed cerebral venous sinus thrombosis (CSVT) following head trauma. The first child had a concomitant subdural bleed and CSVT. She received intravenous FVIII replacement and, after ensuring stability of the intracranial bleed, LMWH for six weeks. Complete resolution of her subdural hematoma and cerebral venous sinus thrombosis was observed. The second case was initially treated with anticoagulation, which was discontinued due to an acute abdominal bleed. Regardless, she showed complete resolution of the CSVT. As shown by these examples, individualized management is required (26). In such cases, if possible, identification and elimination of any underlying modifiable risk factor for thrombosis is important.
4. Thrombosis associated with non-replacement therapies
Case 2: A three-year-old boy with severe hemophilia A with a high-titer inhibitor presents with a swollen right ankle after playing soccer with his siblings. He is on prophylaxis with subcutaneous emicizumab every 2 weeks. The emergency department physician calls for advice on hemostatic management. How should his bleed be treated?
4.1. Emicizumab
Non-factor replacement therapies are revolutionizing the prophylactic treatment of hemophilia. Emicizumab is a bi-specific monoclonal antibody which mimics the activity of factor VIII by bridging factor IXa and X. Hence, emicizumab accelerates FIXa induced factor X (FX) activation, which leads to thrombin generation (27, 28). Emicizumab provides a consistent, steady-state level of hemostasis, which has been approximated to a 10%–15% factor VIII activity level (29, 30). In an extensive clinical trial program, emicizumab has been demonstrated to significantly reduce annualized bleeding rates as compared to prophylaxis with bypassing agents in patients with inhibitors and FVIII prophylaxis in those without inhibitors (31–35). Emicizumab provides several potential benefits over traditional CFC replacement in children. It is administered subcutaneously every two to four weeks. Children, adolescents, and their caregivers have previously reported improvements in health-related quality of life outcomes (36). In children with inhibitors, there was a 99% reduction in annualized bleeding rates as compared to the use of bypassing agents, with 77% of children having zero bleeding events (32). As such, where available, emicizumab is quickly becoming the preferred prophylactic therapy for children with hemophilia A.
4.2. Management of bleeds for children with hemophilia receiving emicizumab
Despite the significant improvement in bleeding phenotype, children with hemophilia A on emicizumab can still experience breakthrough bleeding, especially in perioperative settings or after trauma. Management of bleeding in children on emicizumab requires personalised care based on the severity of bleed, bleeding phenotype of the patient, and the presence of an inhibitor.
In children without inhibitors, mild mucosal bleeding episodes may be treated with tranexamic acid alone. In moderate to severe bleeding, FVIII replacement should be given to target a FVIII level appropriate for the bleed severity. While FVIII has a binding affinity to FIXa and FX that is around 10 times higher than that of emicizumab (29). As such, FVIII preferentially binds to FIXa/FX when FVIII products are administered during a bleed. Several clinical trials have demonstrated the safety of concurrent FVIII therapy to treat bleeds or provide prophylaxis against surgery in children with hemophilia A, with no reported thrombotic events (33, 34, 37).
Special consideration should be given to the hemostatic management in children with hemophilia A with inhibitors. As with non-inhibitor patients, minor mucosal bleeding may be managed with tranexamic acid alone. FVIII replacement can be tried in patients with low-titers inhibitors (<5 BU), it is unlikely to be beneficial for patients with high-titer inhibitors. For moderate to severe bleeding bypassing therapy with aPCC or rFVIIa will be necessary (38–40). The Food and Drug Administration (FDA) has warranted a special warning for the use of aPCC products in patients receiving Emicizumab (41). As further explained below, emerging data pertaining to the synergistic prothrombotic effect of aPCC and emicizumab suggest that for the time being, rFVIIa should be prioritized to treat bleeds in these situations.
4.3. Thrombotic risk in persons with hemophilia receiving emicizumab
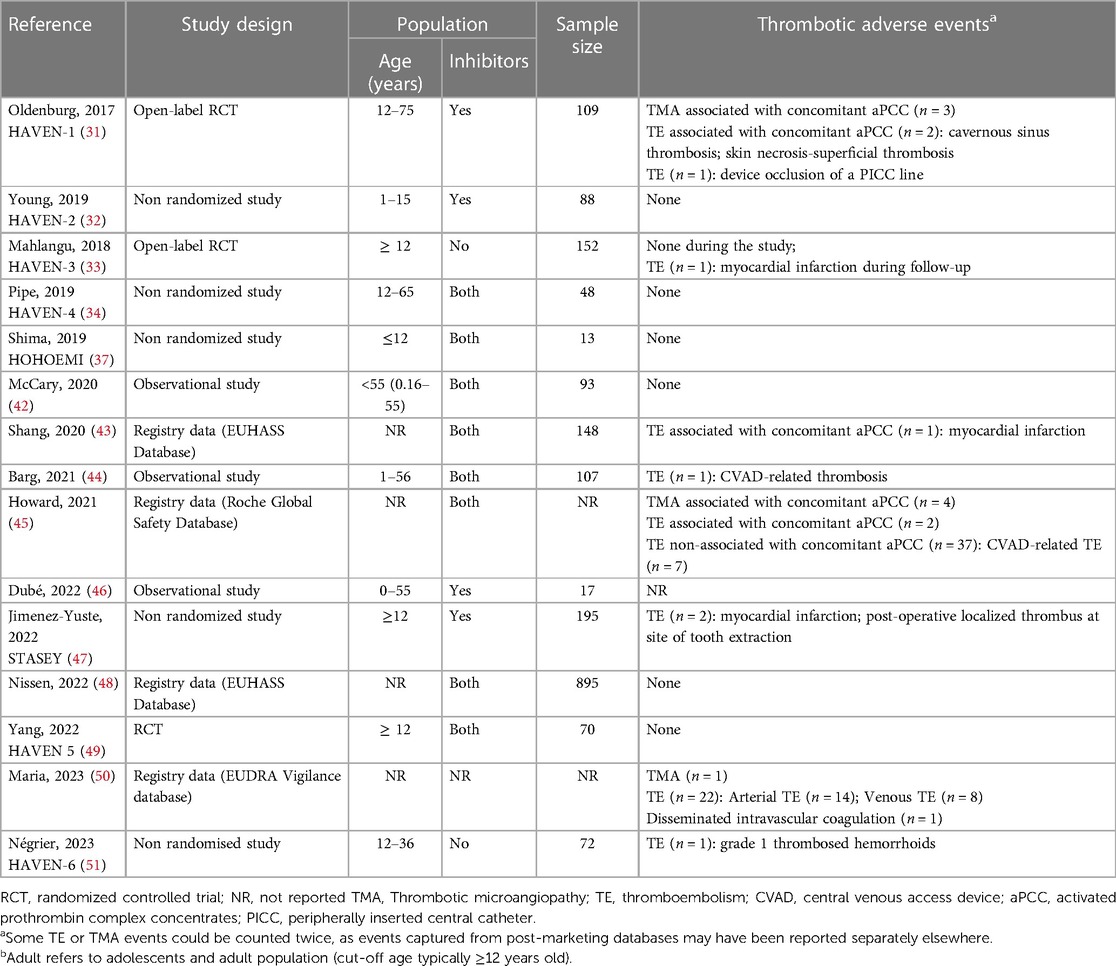
Multiple concerns of increased risk of thrombotic microangiopathy (TMA) and thromboembolism were reported in the literature with emicizumab (Table 1). In the HAVEN-1 clinical trial of emicizumab prophylaxis in adolescents and adults with hemophilia A with inhibitors, the development of thrombotic microangiopathy (TMA) was reported with the concurrent use of emicizumab and aPCC (31–34). Subsequently, out of 8 patients treated with aPCC (>100 U/Kg/24 h) for more than 24 h, 3 patients developed TMA. Two of the cases resolved while one individual experienced fatal rectal hemorrhage. An additional case of TMA was reported post FDA approval, that resolved with cessation of aPCC use (52). In addition, two cases of thromboembolism were also reported with the concurrent use of emicizumab and aPCC. These adverse events resulted in a FDA “black box” warning for TMA or thromboembolism when emicizumab and aPCC are used concomitantly. Nissen et al. reported 3 cases of TE and 0 cases of TMA in 985 patients on emicizumab. All 3 cases of thromboembolism occurred on emicizumab monotherapy, but patients had multiple other risk factors (48). To date, we are not aware of pediatric cases of TMA associated with emicizumab use, which may be explained by the “black box” warning being issued prior to pediatric approval rather than developmental differences in coagulation in children.
While emicizumab mimics the action of FVIII by binding the activated FIXa (enzyme) to the FX (substrate), several notable differences exist in its mechanism of action that likely contribute to its thrombotic potential. Compared to FVIII, emicizumab has a low affinity for the enzyme and its substrate, and does not distinguish between the zymogen and the enzyme (e.g., FIX vs. FXIa and FX vs. FXa) (29, 53). In addition to bridging FIXa and FX, FVIII promotes phospholipid binding and stabilizes the FIXa active site. As emicizumab cannot complete these additional actions, its action is not limited to the platelet cell surface and excessive thrombin generation may occur anywhere in the vasculature, including in the endothelium. Finally, the action of FVIII is highly regulated, which is not the case with emicizumab, which leads to a persistent, low-grade activation of the coagulation system (53). When aPCC is administered to a patient receiving emicizumab, this leads to a prolonged activation of procoagulant pathways by providing large amounts of FIX (and of FIXa, to a lesser extent) (29, 54). In a seminal study, Hartmann and colleagues have shown that the in vitro combination of a sequence-identical analog of emicizumab and aPCC leads to a 17-fold, synergistic increase of thrombin generation (54).
Recently, Kizilocak et al. reported the in vitro and in vivo effect of escalating doses of aPCC in nine persons with hemophilia with inhibitors, aged 5–27 years old, on emicizumab. While excessive thrombin generation was seen in vitro with standard dosing of aPCC, most patients had normal thrombin generation in vivo with doses of aPCC up to 75 units/kg, suggesting that low doses of aPCC may be considered in specific circumstances (55). Importantly, suboptimal in vivo endogenous thrombin generation was noted with subtherapeutic doses of aPCC.
In addition to TMA, there have been several reported episodes of thromboembolism associated with the use of Emicizumab (Table 1). As noted above, during the HAVEN-1 clinical trial two cases of thromboembolism occurred with concurrent aPCC use, which included one case each of cavernous sinus thrombosis and superficial thrombophlebitis. Both cases were managed with discontinuation of aPCC without the need for anticoagulation. Emicizumab was restarted in one patient without any further thromboembolism recorded (31). Importantly, no thrombotic events were reported in children in the HAVEN clinical trial program, nor the HOHOEMI single arm study of prophylaxis in young children (37). However, a real-world study of emicizumab reported an infant with a CVAD-related thrombosis without concurrent use of bypassing agent therapy. Unfortunately, this child had a fatal bleed while on concurrent anticoagulation therapy with LMWH (44).
Levy et al. analysed data from HAVEN 1–4 trials and reported no cases of TMA or TE with the concomitant use of emicizumab and rFVIIa (56). A recent publication reported an adult with hemophilia A and with inhibitor on emicizumab who received rFVIIa to treat an ankle bleed and experienced a mycocardial infarction and pulmonary embolism. However, the patient had multiple comorbidities that may have contributed the thrombotic event (57).
While not related to acute bleed management, an important consideration in assessing thrombotic risk is the use of frequent, high-dose FVIII replacement as part of ITI while on emicizumab prophylaxis, often times using a CVAD. Batsuli and colleagues reported the “Atlanta” protocol with the concurrent use of emicizumab and ITI with no thrombotic events noted, while the results were promising for inhibitor eradication (58). The ongoing MOTIVATE trial (ClinicalTrials.gov Identifier: NCT04023019) is investigating the safety of this approach prospectively.
Based on these observations, rFVIIa (eptacog alfa) is the treatment of choice in children with hemophilia A with inhibitors who experience breakthrough bleeding or require surgery while on emicizumab. The National Hemophilia Foundation recommends the use of standard initial dosing of rFVIIa at 90–120 mcg/kg at no more than a q2 h interval. Alternatively, in adolescents older than 12 years of age and adults, an alternate rFVIIa product, ectacog beta, may be used. The recommended dosing is 75 mcg/kg at no more than q3 h interval. While duration of therapy is individualized, most bleeding events are expected to resolve with 2–3 doses of rFVIIa (59). In situations where rFVIIa is not available or if the bleed is non-responsive to rFVIIa, low dose aPCC can be used, using doses up to 50 units/kg with a maximal daily dose of 100 units/kg (60). Frequent blood work to monitor for TMA should be performed, including twice-daily complete blood count, reticulocyte count, blood smear looking for schistocytes, bilirubin, haptoglobin, creatinine, lactate dehydrogenase, and d-dimers (61). Should TMA develop, the recommendation is to stop aPCC and monitor the patient closely. Most of the published cases reported resolution of TMA with discontinuation of aPCC and supportive care, and occasionally plasmapheresis (31).
For our case, we opted to treat the ankle hemarthrosis with rFVIIa (eptacog alfa) with initial dose of 90 mcg/kg. The child required a second dose to achieve satisfactory hemostasis and was admitted for observation. Given the availability and response to rFVIIa, the use of low dose aPCC was not considered. He was evaluated by physiotherapy and given recommendations around weight-bearing and stretching. The benefits of inhibitor eradication, namely the ability to use FVIII to treat bleeds in tolerized children was re-discussed with the family and initiation of ITI was planned.
5. Rebalancing agents
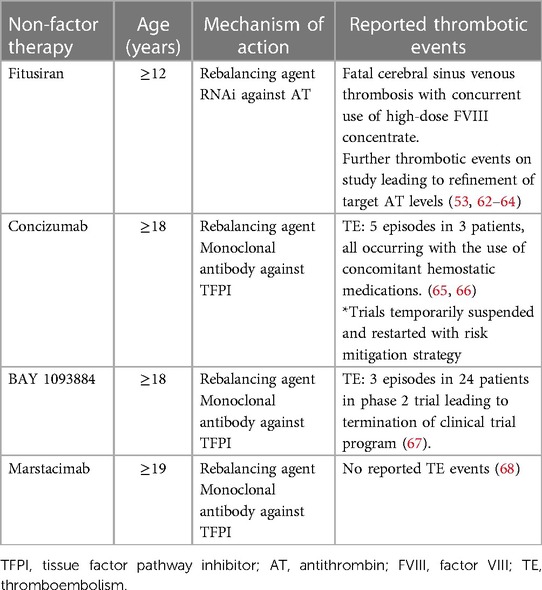
Other promising therapeutic agents for patients with severe factor deficiencies include rebalancing agents, targeting natural anticoagulants. This concept stems from “rebalanced coagulation” observed with liver disease, in which levels of procoagulants and anticoagulants are similarly reduced, such that overall thrombin generation remains near normal (53). Additionally, it was recognized that co-inheritance of a thrombophilic trait along with hemophilia led to a milder bleeding phenotype. Several products are currently in development, and none are currently approved and marketed. Fitusiran is a small interfering RNA (siRNA) that silences post-transcriptional hepatic expression of the SERPINC1 gene, thereby reducing antithrombin levels in a dose-dependent manner. Concizumab and Marstacimab are humanized monoclonal antibodies targeting the Tissue Factor Pathway Inhibitor (TFPI) binding site for FXa. Table 2 summarizes thrombotic complications encountered in clinical trials with rebalancing agents. Considering the little combined experience using these agents and the scarcity of data, caution and monitoring when providing procoagulant therapy for surgery or breakthrough bleeds will be required when using these products. This is especially true for young children, given the well-known concept of developmental hemostasis (69) and its implication of the fine balance between bleeding and thrombosis.
6. Conclusion
Despite being at high risk of bleeding, children with hemophilia can still develop complications related to thrombosis. The two presented cases reflect the most frequent (CVAD-related) as well as novel (TE risk with rebalancing agents) causes of TE encountered in children with hemophilia A. We advocate for an individualized approach to management with balancing the risks of bleeding and clotting. When opting not to treat TE in children with hemophilia, close radiological surveillance for thrombus progression is suggested. The development of several novel rebalancing agents as prophylactic therapy for persons with severe hemophilia A bring the promise significant improvements in bleeding and, most importantly for children, the possibility of subcutaneous administration. Consequently, the uptake of such therapies may result in a marked decrease in the use of CVADs, the biggest risk factor for TE in children. At this time, however, there remains an incomplete understanding of the thrombotic risks of rebalancing agents, and how to effectively use concomitant hemostatic therapy when there is breakthrough bleeding. In the future, it will be vital to have real-world studies assessing the long-term risks of not only bleeding and arthropathy, but also TE in children with hemophilia.
Author contributions
JE wrote the first draft of the manuscript. ST and MP wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Ali Amid's research is supported by Naiman-Vickars Endowment Fund.
Conflict of interest
Dr. Tole has done consultancy work with Roche, Novo Nordisk, Sanofi, and Octapharma. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aPCC, activated prothrombin complex concentrate; AT, antithrombin; BU, Bethesda units; CFC, clotting factor concentrates; CSVT, cerebral sinus venous thrombosis; CVAD, central venous access device; DOAC, direct oral anticoagulant; FDA, Food and Drug Administration; FVIII, factor VIII; FIX, factor IX; ITI, immune tolerance induction; LMWH, low molecular weight heparin; NR, not reported; RCT, randomized controlled trial; rFVIIa, recombinant factor VIIa; TE, thromboembolism; TMA, thrombotic microangiopathy.
References
1. Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. (2014) 12(11):1935–9. doi: 10.1111/jth.12672
2. Pelland-Marcotte MC, Carcao MD. Hemophilia in a changing treatment landscape. Hematol Oncol Clin North Am. (2019) 33(3):409–23. doi: 10.1016/j.hoc.2019.01.007
3. Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. (1992) 232(1):25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x
4. Blanchette VS, Manco-Johnson M, Santagostino E, Ljung R. Optimizing factor prophylaxis for the haemophilia population: where do we stand? Haemophilia. (2004) 10(Suppl 4):97–104. doi: 10.1111/j.1365-2516.2004.00998.x
5. Young G. How I treat children with haemophilia and inhibitors. Br J Haematol. (2019) 186(3):400–8. doi: 10.1111/bjh.15942
6. van den Berg HM, Fischer K, Carcao M, Chambost H, Kenet G, Kurnik K, et al. Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood. (2019) 134(3):317–20. doi: 10.1182/blood.2019000658
7. Van Dijk K, Van Der Bom JG, Bax KN, Van Der Zee DC, Van Den Berg MH. Use of implantable venous access devices in children with severe hemophilia: benefits and burden. Haematologica. (2004) 89(2):189–94. doi: 10.3324/x
8. Blanchette VS, McCready M, Achonu C, Abdolell M, Rivard G, Manco-Johnson MJ. A survey of factor prophylaxis in boys with haemophilia followed in North American haemophilia treatment centres. Haemophilia. (2003) 9(Suppl 1):19–26; discussion 26. doi: 10.1046/j.1365-2516.9.s1.12.x
9. Langley AR, Stain AM, Chan A, McLimont M, Chait S, Wu J, et al. Experience with central venous access devices (CVADs) in the Canadian hemophilia primary prophylaxis study (CHPS). Haemophilia. (2015) 21(4):469–76. doi: 10.1111/hae.12713
10. Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. (2009) 23(5):225–9. doi: 10.1016/j.blre.2009.07.002
11. Buckley B, Dreyfus J, Prasad M, Gayle J, Kendter J, Hall E. Burden of illness and costs among paediatric haemophilia patients with and without central venous access devices treated in US hospitals. Haemophilia. (2018) 24(3):e93–e102. doi: 10.1111/hae.13404
12. Lasagni D, Nosadini M, Molinari AC, Saracco P, Pelizza MF, Piersigilli F, et al. Systemic catheter-related venous thromboembolism in children: data from the Italian registry of pediatric thrombosis. Front Pediatr. (2022) 10:843643. doi: 10.3389/fped.2022.843643
13. Kraaijenhagen RA, in’t Anker PS, Koopman MM, Reitsma PH, Prins MH, van den Ende A, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. (2000) 83(1):5–9. doi: 10.1055/s-0037-1613747
14. Kreuz W, Stoll M, Junker R, Heinecke A, Schobess R, Kurnik K, et al. Familial elevated factor VIII in children with symptomatic venous thrombosis and post-thrombotic syndrome: results of a multicenter study. Arterioscler Thromb Vasc Biol. (2006) 26(8):1901–6. doi: 10.1161/01.ATV.0000227510.36653.ed
15. Weyand AC, Dorfman AL, Shavit JA, Pipe SW. Emicizumab prophylaxis to facilitate anticoagulant therapy for management of intra-atrial thrombosis in severe haemophilia with an inhibitor. Haemophilia. (2019) 25(3):e203–5. doi: 10.1111/hae.13721
16. Augustsson C, Persson E. In vitro evidence of a tissue factor-independent mode of action of recombinant factor VIIa in hemophilia. Blood. (2014) 124(20):3172–4. doi: 10.1182/blood-2014-05-576892
17. Valentino LA, Ewenstein B, Navickis RJ, Wilkes MM. Central venous access devices in haemophilia. Haemophilia. (2004) 10(2):134–46. doi: 10.1046/j.1365-2516.2003.00840.x
18. Journeycake JM, Quinn CT, Miller KL, Zajac JL, Buchanan GR. Catheter-related deep venous thrombosis in children with hemophilia. Blood. (2001) 98(6):1727–31. doi: 10.1182/blood.V98.6.1727
19. Hay CR, DiMichele DM, I.I.T. Study. The principal results of the international immune tolerance study: a randomized dose comparison. Blood. (2012) 119(6):1335–44. doi: 10.1182/blood-2011-08-369132
20. Carcao MD, Connolly BL, Chait P, Stain AM, Acebes M, Massicotte P, et al. Central venous catheter-related thrombosis presenting as superior vena cava syndrome in a haemophilic patient with inhibitors. Haemophilia. (2003) 9(5):578–83. doi: 10.1046/j.1365-2516.2003.00791.x
21. Martin K, Key NS. How I treat patients with inherited bleeding disorders who need anticoagulant therapy. Blood. (2016) 128(2):178–84. doi: 10.1182/blood-2015-12-635094
22. Dargaud Y, Meunier S, Negrier C. Haemophilia and thrombophilia: an unexpected association!. Haemophilia. (2004) 10(4):319–26. doi: 10.1111/j.1365-2516.2004.00906.x
23. Goldenberg NA, Kittelson JM, Abshire TC, Bonaca M, Casella JF, Dale RA, et al. Effect of anticoagulant therapy for 6 weeks vs 3 months on recurrence and bleeding events in patients younger than 21 years of age with provoked venous thromboembolism: the kids-DOTT randomized clinical trial. JAMA. (2022) 327(2):129–37. doi: 10.1001/jama.2021.23182
24. Monagle P, Cuello CA, Augustine C, Bonduel M, Brandao LR, Capman T, et al. American society of hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. (2018) 2(22):3292–316. doi: 10.1182/bloodadvances.2018024786
25. den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. (2011) 17(1):41–4. doi: 10.1111/j.1365-2516.2010.02383.x
26. Sheikh IN, Srivaths L, Li E, Steinberg-Shemer O, Mandel-Shorer N, Kenet G, et al. Cerebral sinus venous thrombosis in children with inherited bleeding disorders: a case series. Pediatr Blood Cancer. (2022) 69(10):e29902. doi: 10.1002/pbc.29902
27. Kitazawa T, Esaki K, Tachibana T, Ishii S, Soeda T, Muto A, et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. (2017) 117(7):1348–57. doi: 10.1160/TH17-01-0030
28. Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. (2016) 127(13):1633–41. doi: 10.1182/blood-2015-06-650226
29. Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. (2017) 130(23):2463–8. doi: 10.1182/blood-2017-08-801662
30. Donners A, Rademaker CMA, Bevers LAH, Huitema ADR, Schutgens REG, Egberts TCG, et al. Pharmacokinetics and associated efficacy of emicizumab in humans: a systematic review. Clin Pharmacokinet. (2021) 60(11):1395–406. doi: 10.1007/s40262-021-01042-w
31. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. (2017) 377(9):809–18. doi: 10.1056/NEJMoa1703068
32. Young G, Liesner R, Chang T, Sidonio R, Oldenburg J, Jimenez-Yuste V, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. (2019) 134(24):2127–38. doi: 10.1182/blood.2019001869
33. Mahlangu J, Oldenburg J, Paz-Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. (2018) 379(9):811–22. doi: 10.1056/NEJMoa1803550
34. Pipe SW, Shima M, Lehle M, Shapiro A, Chebon S, Fukutake K, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. (2019) 6(6):e295–e305. doi: 10.1016/S2352-3026(19)30054-7
35. Callaghan MU, Negrier C, Paz-Priel I, Chang T, Chebon S, Lehle M, et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1–4 studies. Blood. (2021) 137(16):2231–42. doi: 10.1182/blood.2020009217
36. Mancuso ME, Mahlangu J, Sidonio R Jr., Trask P, Uguen M, Chang T, et al. Health-related quality of life and caregiver burden of emicizumab in children with haemophilia A and factor VIII inhibitors-results from the HAVEN 2 study. Haemophilia. (2020) 26(6):1009–18. doi: 10.1111/hae.14183
37. Shima M, Nogami K, Nagami S, Yoshida S, Yoneyama K, Ishiguro A, et al. A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. (2019) 25(6):979–87. doi: 10.1111/hae.13848
38. Shapiro AD, Mitchell IS, Nasr S. The future of bypassing agents for hemophilia with inhibitors in the era of novel agents. J Thromb Haemost. (2018) 16(12):2362–74. doi: 10.1111/jth.14296
39. Makris M, Kasper C. The world federation of hemophilia guideline on management of haemophilia. Haemophilia. (2013) 19(1):1. doi: 10.1111/hae.12074
40. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. (2014) 124(23):3365–72. doi: 10.1182/blood-2014-05-577643
41. Tiede A. Thromboembolic risks of non-factor replacement therapies in hemophilia. Hamostaseologie. (2017) 37(4):307–10. doi: 10.5482/20170004
42. McCary I, Guelcher C, Kuhn J, Butler R, Massey G, Guerrera MF, et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. (2020) 26(4):631–6. doi: 10.1111/hae.14005
43. Shang A, Selak Bienz N, Gadiraju R, Chang T, Kuebler P. Real-world safety of emicizumab: the first interim analysis of the European haemophilia safety surveillance (EUHASS) database. Blood. (2020) 136(Suppl 1):29–30. doi: 10.1182/blood-2020-134905
44. Barg AA, Budnik I, Avishai E, Brutman-Barazani T, Bashari D, Misgav M, et al. Emicizumab prophylaxis: prospective longitudinal real-world follow-up and monitoring. Haemophilia. (2021) 27(3):383–91. doi: 10.1111/hae.14318
45. Howard M, McKinley D, Sanabria F, Ko RH, Nissen F. Evaluation of the safety of emicizumab prophylaxis in persons with hemophilia A: an updated summary of thrombotic events and thrombotic microangiopathies. Blood. (2021) 138(Suppl 1):3186. doi: 10.1182/blood-2021-146147
46. Dube E, Merlen C, Bonnefoy A, Pilon J, Zourikian N, Gauthier J, et al. Switching to emicizumab: a prospective surveillance study in haemophilia A subjects with inhibitors. Haemophilia. (2023) 29(1):348–51. doi: 10.1111/hae.14685
47. Jimenez-Yuste V, Peyvandi F, Klamroth R, Castaman G, Shanmukhaiah C, Rangarajan S, et al. Safety and efficacy of long-term emicizumab prophylaxis in hemophilia A with factor VIII inhibitors: a phase 3b, multicenter, single-arm study (STASEY). Res Pract Thromb Haemost. (2022) 6(8):e12837. doi: 10.1002/rth2.12837
48. Nissen F, Jiang Y, Hiew HJ, Aizenas M, Tobaruela G, Jew L. Real-world safety of emicizumab: interim analysis of the European haemophilia safety surveillance (EUHASS) database. Blood. (2022) 140(Suppl 1):469–70. doi: 10.1182/blood-2022-157528
49. Yang R, Wang S, Wang X, Sun J, Chuansumrit A, Zhou J, et al. Prophylactic emicizumab for hemophilia A in the Asia-pacific region: a randomized study (HAVEN 5). Res Pract Thromb Haemost. (2022) 6(2):e12670. doi: 10.1002/rth2.12670
50. Maria A, Alessandro C, Declan N, Peyvandi F. Hemorrhagic and thrombotic adverse events associated with emicizumab and extended half-life factor VIII replacement drugs: eudraVigilance data of 2021. J Thromb Haemost. (2023) 546–52. doi: 10.1016/j.jtha.2023.01.010
51. Negrier C, Mahlangu J, Lehle M, Chowdary P, Catalani O, Bernardi RJ, et al. Emicizumab in people with moderate or mild haemophilia A (HAVEN 6): a multicentre, open-label, single-arm, phase 3 study. Lancet Haematol. (2023). e168–77. doi: 10.1016/s2352-3026(22)00377-5
52. Genentech I. HEMLIBRA (emicizumab-kxwh) injection, for subcutaneous use, prescribing information. (2023). Available at: https://www.gene.com/download/pdf/hemlibra_prescribing.pdf.
53. Hoffman M. Thrombosis and novel hemophilia therapies: the fine line between clotting and bleeding. Blood Adv. (2021) 5(18):3736. doi: 10.1182/bloodadvances.2021004802C
54. Hartmann R, Feenstra T, Valentino L, Dockal M, Scheiflinger F. In vitro studies show synergistic effects of a procoagulant bispecific antibody and bypassing agents. J Thromb Haemostasis. (2018) 16(8):1580–91. doi: 10.1111/jth.14203
55. Kizilocak H, Marquez-Casas E, Malvar J, Young G. Safety of FEIBA and emicizumab (SAFE): dose escalation study evaluating the safety of in vivo administration of activated prothrombin complex concentrate in haemophilia A patients on emicizumab. Haemophilia. (2023) 29(1):100–5. doi: 10.1111/hae.14684
56. Levy GG, Asikanius E, Kuebler P, Benchikh El Fegoun S, Esbjerg S, Seremetis S. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: experience from the HAVEN clinical program. J Thromb Haemost. (2019) 17(9):1470–7. doi: 10.1111/jth.14491
57. Gundabolu K, Goldsweig A, Bhatt VR, Koepsell SA, Harper JL. ST-segment elevation myocardial infarction (STEMI) and pulmonary embolism in a hemophilia A patient receiving emicizumab and recombinant activated factor VII. Haemophilia. (2020) 26(1):e5–e8. doi: 10.1111/hae.13871
58. Batsuli G, Zimowski KL, Tickle K, Meeks SL, Sidonio RF Jr. Immune tolerance induction in paediatric patients with haemophilia A and inhibitors receiving emicizumab prophylaxis. Haemophilia. (2019) 25(5):789–96. doi: 10.1111/hae.13819
59. Foundation, N.H. Recommendation on the Use and Management of Emicizumab-kxwh (Hemlibra®) for Hemophilia A with and without Inhibitors (2023). Available at: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-268-recommendation-on-the-use-and-management-of-emicizumab-kxwh-hemlibrar-for-hemophilia-a-with-and-without-inhibitors [Cited February 13, 2023].
60. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. (2020) 26(Suppl 6):1–158. doi: 10.1111/hae.14046
61. Wang CP, Young G, Thornburg CD. Safety evaluation of emicizumab prophylaxis in individuals with haemophilia A. Expert Opin Drug Saf. (2021) 20(4):387–96. doi: 10.1080/14740338.2021.1893303
62. Young G, Srivastava A, Kavakli K, Ross C, Sathar J, Tran H, et al. Efficacy and safety of fitusiran prophylaxis, an siRNA therapeutic, in a multicenter phase 3 study (ATLAS-INH) in people with hemophilia A or B, with inhibitors (PwHI). Blood. (2021) 138(Suppl 1):4. doi: 10.1182/blood-2021-150273
63. Kenet G NB, Zulfikar B, Antmen B, Kampmann P, Matsushita T, You C, et al. A Phase 3 study (ATLAS-PPX) to evaluate efficacy and safety of fitusiran, an siRNA therapeutic, in people with haemophilia A or B who have switched from prior factor or bypassing agent prophylaxis [abstract].
64. WFH. Alnylam Suspends Fitusiran Dosing due to Thrombotic Event in Phase 2 open-label extension study (2017). Available at: https://news.wfh.org/alnylam-suspends-fitusiran-dosing-due-thrombotic-event-phase-2-open-label-extension-study/
65. Shapiro AD, Angchaisuksiri P, Astermark J, Benson G, Castaman G, Eichler H, et al. Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors. Blood Adv. (2022) 6(11):3422–32. doi: 10.1182/bloodadvances.2021006403
66. Seremetis SV, Cepo K, Skovgaard Rasmussen J, Høyer Rose T, Tamer S, Porstmann T, et al. Risk mitigation strategy for concizumab clinical trials after pause due to non-fatal thrombotic events. Blood. (2020) 136(Suppl 1):40. doi: 10.1182/blood-2020-139563
67. Mancuso ME, Ingham SJM, Kunze M. Befovacimab, an anti-tissue factor pathway inhibitor antibody: early termination of the multiple-dose, dose-escalating phase 2 study due to thrombosis. Haemophilia. (2022) 28(5):702–12. doi: 10.1111/hae.14595
68. Mahlangu JN, Lamas JL, Morales JC, Malan DR, Salek SZ, Wang M, et al. A phase 1b/2 clinical study of marstacimab, targeting human tissue factor pathway inhibitor, in haemophilia. Br J Haematol. (2023) 200(2):229–39. doi: 10.1111/bjh.18420
Keywords: hemophilia, thrombosis, children, emicizumab, CVAD
Citation: El Maamari J, Amid A, Pelland-Marcotte M-C and Tole S (2023) Between Scylla and Charybdis: thrombosis in children with hemophilia. Front. Pediatr. 11:1173549. doi: 10.3389/fped.2023.1173549
Received: 24 February 2023; Accepted: 27 April 2023;
Published: 23 May 2023.
Edited by:
Tomasz Szczepanski, Medical University of Silesia, PolandReviewed by:
Giovanni Del Borrello, IRCSS Istituto Giannina Gaslini, ItalyLeonardo Rodrigues Campos, Hospital Universitário Antônio Pedro, Brazil
© 2023 El Maamari, Amid, Pelland-Marcotte and Tole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Claude Pelland-Marcotte bWFyaWUtY2xhdWRlLnBlbGxhbmQtbWFyY290dGUuMUB1bGF2YWwuY2E=
†These authors share senior authorship
 Jad El Maamari
Jad El Maamari Ali Amid
Ali Amid Marie-Claude Pelland-Marcotte
Marie-Claude Pelland-Marcotte Soumitra Tole4,5,†
Soumitra Tole4,5,†