- 1Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: This study aims to evaluate the value of the proportion of large platelets (PLCR) and platelet crit (PCT) in predicting necrotizing enterocolitis (NEC) in low birth weight (LBW) neonates.
Methods: A total of 155 LBW (<2,500 g) neonates with NEC, who were admitted to the neonatal intensive care unit (NICU) of the hospital from January 1, 2017, to November 30, 2019, were included in the case group. According to the 1:3 case–control study design, a total of 465 LBW neonates without NEC (three for each LBW neonate with NEC), who were admitted to the NICU and born ≤24 h before or after the birth of the subjects, were included in the control group.
Results: During the study period, a total of 6,946 LBW neonates were born, of which 155 had NEC, including 92 who also had sepsis. Neonatal sepsis was the most important risk factor and confounding factor for NEC in LBW neonates. Further stratified analysis showed that in LBW neonates without sepsis, anemia [P = 0.001, odds ratio (OR) = 4.367, 95% confidence interval (CI): 1.853–10.291], high PLCR (P < 0.001, OR = 2.222, 95% CI: 1.633–3.023), and high PCT (P = 0.024, OR = 1.368, 95% CI: 1.042–1.795) increased the risk of NEC and the receiver operating characteristic curve area of PLCR, sensitivity, specificity, and cutoff value were 0.739, 0.770, 0.610, and 33.55, respectively.
Conclusions: The results showed that 2/100 LBW neonates were at risk for NEC, and the stratified analysis of the confounding factors of sepsis identified the risk factors of NEC in LBW neonates. This study first reported the significance of PLCR in the early prediction of NEC occurrence in LBW neonates without sepsis.
Introduction
Neonatal necrotizing enterocolitis (NEC) is a neonate-specific inflammatory necrotizing disease that involves the ileum and/or colon and is common among premature infants, severely threatening the life of neonates (1–3). The mortality rate has declined due to continuous advances in the treatment of premature infants in recent years, but the incidence of NEC has not decreased significantly.
Statistically, the incidence of NEC in premature infants weighing <1,500 g was 5%–10%, the mortality rate was 20%–30%, and >30%–50% of NEC neonates required surgical treatment (4, 5). Despite decades of research, the current understanding of the diagnosis and treatment of neonatal NEC is limited, the rate of neonatal NEC mortality remains high, and neonatal surgical advances have not significantly improved the prognosis in NEC survivors (6, 7). Therefore, NEC intervention, especially in low birth weight (LBW) neonates with NEC, should be under intensive focus with respect to identifying the causes and related factors for early diagnosis and treatment.
Hitherto, although NEC pathogenesis remains unclear, several studies have shown that it is caused by a combination of factors. Some studies have reported that preterm birth (6, 7), low birth weight (6–9), and race (8) were critical risk factors. Recent studies have shown that maternal infection (10), congenital pneumonia (11), asphyxia (12), blood transfusion (12), anemia (13, 14), and neonatal sepsis are also potential contributing factors. Furthermore, it has been suggested that NEC pathogenesis is multifactorial, involving a combination of abnormal bacterial colonization, a cascade amplification of inflammation, gut immaturity, and ischemia–reperfusion (I/R) injury. The differences in the immune response to mucosal damage and the microbiota may also be responsible for the increased inflammatory response in NEC (15). NEC induced by gut immaturity and I/R injury is not significantly associated with sepsis. Based on the epidemiological and clinical theories, sepsis can confound the diagnosis of clinical complications and the use of inflammatory proteins as the NEC marker. Both sepsis and NEC require careful differential diagnosis, as both may be lethal if not properly diagnosed and treated.
Regarding gut immaturity in NEC infants, the degree and duration of thrombocytopenia are associated with the severity of bowel injury and adverse clinical outcomes. NEC infants develop thrombocytopenia with a platelet (PLT) count of <100 × 109/L. This low PLT count is yet an unresolved clinical dilemma (16, 17). A robust NEC biomarker different from that of sepsis could improve bedside management, reduce morbidity and mortality rates, and allow patients to select potential treatments in the clinic. Animal studies (18) have shown that PLT activation during NEC-like intestinal injury is an early, thrombin-mediated process that antedates both mucosal damage and the rise in bacterial products in plasma. Hence, it is a crucial pathophysiological event during neonatal intestinal injury. In clinical practice, the PLT count in NEC patients is monitored periodically, but PLT activation indicators, such as the mean platelet volume (MPV), platelet crit (PCT), platelet distribution width (PDW), and platelet large cell ratio (PLCR), are usually neglected (19, 20). These PLT indicators are valuable in the clinical diagnosis and prognostic prediction of cardiovascular and metabolic diseases (21, 22); however, their clinical significance, reference value, and utilization value are still being investigated.
Intriguingly, the high mortality rate of NEC patients could be ascribed to the difficulty in diagnosing and treating the condition promptly. Radiographic evidence, such as pneumatosis intestinalis, is used to diagnose severe or advanced disease but has a sensitivity of only 44% with limited specificity and a lack of interpretation concordance (2, 23). Several studies have adopted 1:1 or 1:2 case–control cohorts with low statistical efficiency (24, 25). Herein, we hypothesized that sepsis, anemia, and PLT activation index are vital NEC predictors in LBW neonates. Furthermore, a 1:3 case–control study with sufficient statistical efficiency was conducted to verify the predictive value of anemia, sepsis, and PLT activation indexes in the incidence of LBW NEC, to achieve earlier diagnosis and treatment.
Methods
Participants
A total of 155 LBW (<2,500 g) neonates with NEC, who are born in the Women's Hospital, Zhejiang University School of Medicine, Hangzhou, China, and admitted to the neonatal intensive care unit (NICU) of the hospital from January 1, 2017, to November 30, 2019, were included in the case group. According to the 1:3 case–control study design, a total of 465 LBW neonates without NEC (three for each LBW neonate with NEC), who are admitted to the NICU and born ≤24 h before or after the birth of the subjects, were included in the control group.
The exclusion criteria for the case and control groups were as follows: neonates who were unlikely to survive or had significant gastrointestinal anomalies or those who were discharged at their own will in 3 days. Among the neonates excluded in our study, none with severe abdominal distension was suspected of having fulminant NEC.
All patient information was obtained from the hospital medical record database.
Neonates were diagnosed with NEC (stage II or above) if they met the diagnostic standard in the Practical Neonatology and Bell staging (26, 27) and had clinical symptoms such as abdominal distension, vomiting, and bloody stool triad and if the abdominal plain x-ray scan revealed abdominal intestinal aeration, intestinal obstruction, intestinal pneumatosis, or intrahepatic portal venous gas.
Sepsis in infants is defined by the presence of signs and symptoms of infection with positive blood culture. It is classified as early-onset sepsis (EOS) if symptoms start before 72 h of life and late-onset sepsis (LOS) if symptoms start after 72 h of life. NEC with sepsis is diagnosed in infants with signs and symptoms of infection and positive blood cultures before NEC.
The hospital routinely monitors the complete blood count (CBC) of high-risk infants with stable condition weekly, and the frequency of examination will be increased accordingly for infants with unstable condition. Neonatal anemia is defined as Hb levels less than the fifth percentile, with Hb levels varying with gestational age (28).
Identification of factors
The main influencing factors of the included subjects were listed, and clinical information of the subjects, including maternal factors [age, nationality, number of fetuses, hypertensive disorders complicating pregnancy (HDCP), gestational diabetes mellitus (GDM), placenta previa (PP), placenta abruption, and premature rupture of membrane (PROM)] and neonatal factors [date of birth, birth weight, NEC diagnosis age (the timespan from the date of birth to NEC diagnosis), neonatal sepsis, patent ductus arteriosus (PDA), anemia, hypoglycemia, birth asphyxia (29), blood transfusion, mycoplasma infection (30), and hyperglycemia], were obtained from the hospital medical record database every 3 months. The complications in the subjects occurred before NEC diagnosis. PLT indicators (such as PLT count, PDW, MPV, PCT, and PLCR) of the case group were recorded as the latest data 2 days before the date of NEC diagnosis, whereas those of the control group were recorded 1 day before and after the date of NEC diagnosis of the subjects.
Assignment of main study variables
Dependent variables
NEC neonates were assigned 1, and the controls were assigned 0.
Independent variables
All independent variables (quantitative or qualitative) were converted to qualitative variables by assignment. Since there were no definitive clinical reference values for PLT indicators for neonates, especially for neonates with LBW or low gestational age, the independent variables were stratified and assigned values using the quartile (Q) method, namely, quartile 1 (Q1), quartile 2 (Q2), and quartile 3 (Q3) (Table 1).
Statistical analysis
The general characteristics were compared between the case and control groups. The birth weight of the NEC neonates was determined to be normally distributed by the Shapiro–Wilk test, and Student’s t-test was used to compare the birth weight of the NEC neonates with and without sepsis. Spearman's method was used for the analysis of gestational age, birth weight, and NEC diagnostic age. In the 1:3 case–control analysis, the occurrence of NEC was evaluated by analyzing the variables using univariate non-conditional logistic regression. A factor with P < 0.5 was analyzed using stepwise multivariate conditional logistic regression (Cox regression). The factors, after being stratified by sepsis, were analyzed using univariate and multivariate non-conditional logistic regression. The receiver operating characteristic (ROC) curve was used to evaluate the PLCR and PCT in NEC diagnosis. SPSS 22.0 software was used for statistical analyses and image drawing. Data conforming to the normal distribution were expressed as mean ± standard deviations, while those not conforming to the normal distribution were expressed as median (minimum, maximum). The odds ratio (OR) and the 95% confidence interval (CI) were calculated. An OR with 95% CI not containing the value of 1 was of statistical significance. All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Results of the general information
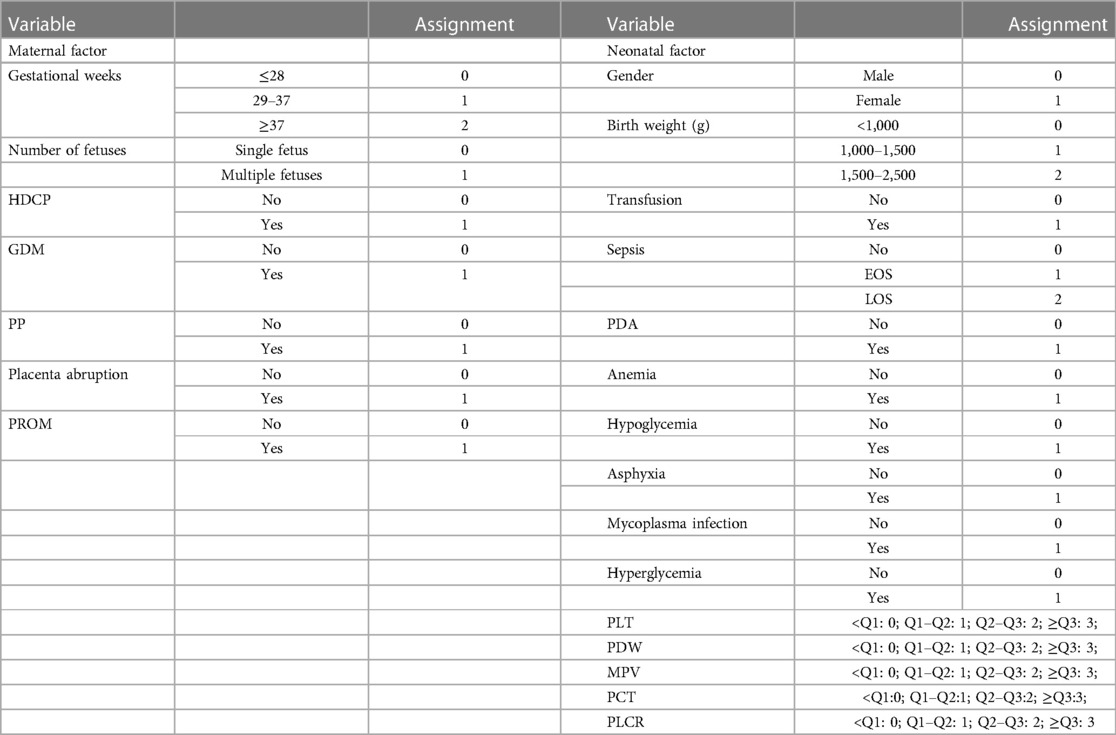
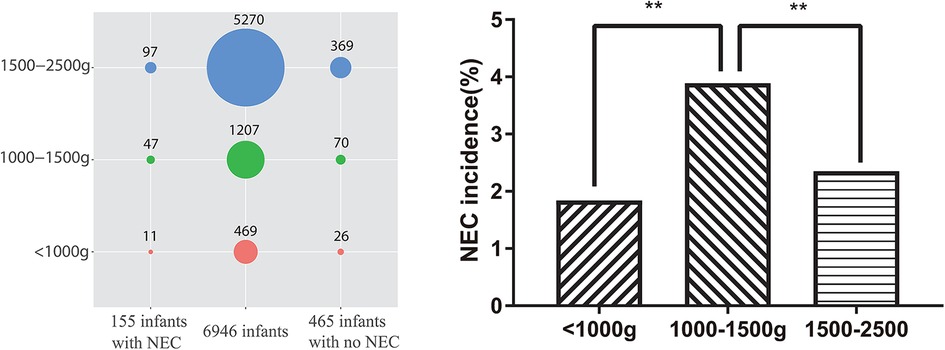
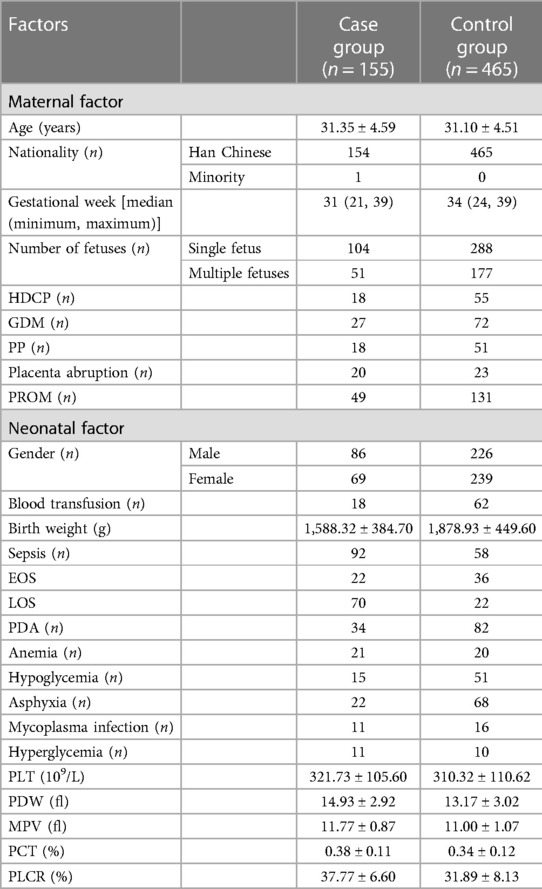
During the study period, a total of 58,507 mothers gave birth to a total of 60,182 neonates in the hospital. Among the neonates, 6,946 (11.54%) had LBW, and 155 (2.23%) had LBW and NEC [including 92 (59.35%) who also had sepsis]. Among the 465 neonate controls with LBW in this study, 58 (12.47%) also had sepsis. Among the 6,946 LBW neonates, 5,270 (75.87%) had a birth weight between 1,500 and 2,500 g [including 97 (1.84%) with NEC], 1,207 (17.38%) had a birth weight between 1,000 and 1,500 g [including 47 (3.89%) with NEC], and 469 (6.75%) had a birth weight of <1,000 g [including 11 (2.35%) with NEC], which are evaluated using the chi-square test, as shown in Figure 1. Moreover, one of the NEC neonates had a mother with Lahu nationality, and the others had mothers with Han nationality. There was no statistically significant difference between the case and control groups in gestational age, P > 0.05. The general information on LBW neonates and their mothers is shown in Table 2.
Univariate analysis between NEC occurrence and baseline characteristics and clinical information of mothers
The cause of NEC is complex and might be affected by multiple maternal factors (such as general characteristics and complications). The univariate analysis of the main complications revealed that the gestational week and placenta abruption were statistically correlated (Table 3). Placenta abruption (OR = 2.847, 95% CI: 1.517–5.343, P = 0.001) was identified as a risk factor for NEC occurrence, whereas gestational week (OR = 0.321, 95% CI: 0.201–0.512, P < 0.001) was the independent protective factor.
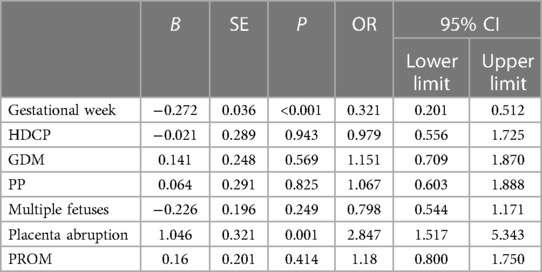
Table 3. Univariate analysis between NEC occurrence and baseline characteristics and clinical information of mothers.
Univariate analysis between NEC occurrence and baseline characteristics and clinical information of LBW neonates
The literature showed that a correlation has been in the spotlight between NEC occurrence and baseline characteristics and complications in neonates. The univariate analysis revealed that in neonates, sepsis, PDA, anemia, hyperglycemia, and birth weight were statistically correlated. Sepsis (P < 0.001, OR = 10.247, 95% CI: 06.717–15.633), PDA (P = 0.018, OR = 1.680, 95% CI: 1.094–2.580), anemia (P < 0.001, OR = 3.482, 95% CI: 1.835–6.627), and hyperglycemia (P = 0.005, OR = 3.476, 95% CI: 1.447–8.351) were identified as risk factors for NEC occurrence, whereas gestational week (OR = 0.606, 95% CI: 0.453–0.812, P = 0.001) was the independent protective factor. Conversely, hypoglycemia (OR = 0.870, 95% CI: 0.474–1.596, P = 0.651), asphyxia (OR = 0.966, 95% CI: 0.575–1.623, P = 0.895), wet lung (31) (OR = 1.483, 95% CI: 0.986–2.231, P = 0.058), mycoplasma infection (OR = 2.144, 95% CI: 0.973–4.725, P = 0.059), and blood transfusion (OR = 0.854, 95% CI: 0.488–2.231, P = 0.580) were not statistically correlated with NEC occurrence in LBW neonates. In addition, the analysis of the correlation between NEC occurrence and PLT indicators in LBW neonates showed that the PLT count (OR = 1.494, 95% CI: 0.953–1.322, P = 0.167) was not statistically correlated, whereas PDW (OR = 1.920, 95% CI: 1.597–2.307, P < 0.001), MPV (OR = 2.093, 95% CI: 1.735–2.525, P < 0.001), PCT (OR = 1.441, 95% CI: 1.217–1.705, P < 0.001), and PLCR (OR = 2.156, 95% CI: 1.777–2.017, P < 0.001) were statistically correlated with NEC occurrence in LBW neonates (Table 4).
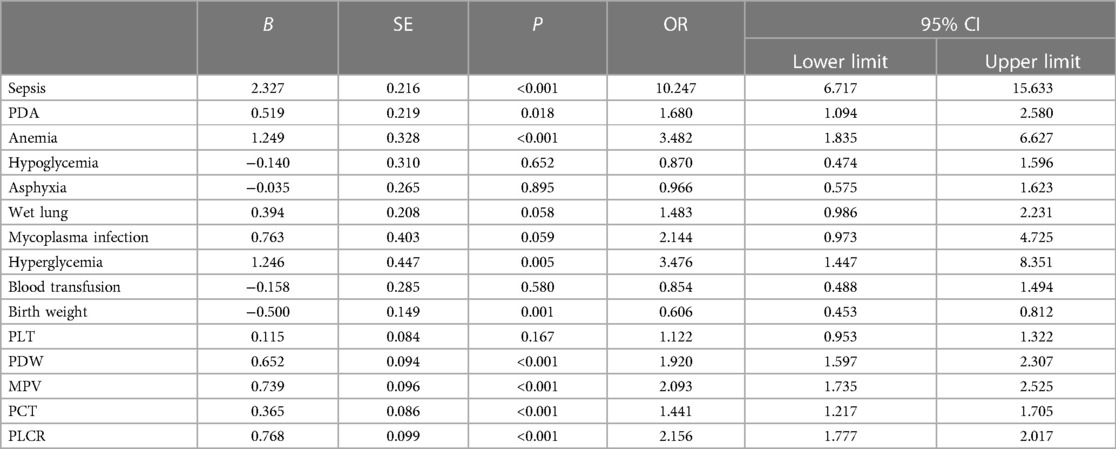
Table 4. Univariate analysis between NEC occurrence and baseline characteristics and clinical information of LBW neonates.
Analysis of the effect of multiple factors and their correlation on NEC occurrence
To avoid missing the critical clinical factors, we considered 16 variables with P < 0.05 in the univariate regression analysis result as independent variables for the 1:3 case–control analysis using the stepwise multivariate conditional logistic regression (the survival function was assessed by Cox regression). The results showed that sepsis, PLCR, and PCT were the final factors in the regression model. EOS (OR = 2.424, 95% CI: 1.461–4.021, P = 0.001) and LOS (OR = 4.291, 95% CI: 3.001–6.138, P < 0.001) increased the risk of NEC in LBW neonates. Increased PLCR (OR = 1.451, 95% CI: 1.220–1.724, P < 0.001) and increased PCT (OR = 1.225, 95% CI: 1.056–1.422, P = 0.007) could be the risk factors for NEC occurrence, as shown in Table 5.
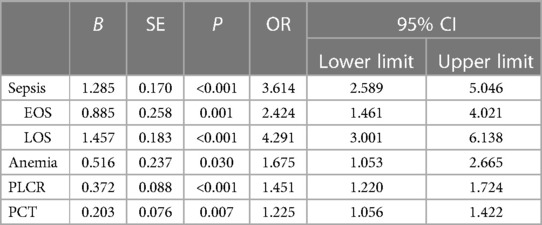
Table 5. Multivariate logistic regression (survival function Cox regression) analysis of NEC occurrence in LBW neonates.
Analysis of the effect of multiple factors and their correlation on NEC occurrence in neonates without sepsis
The effect of sepsis weighted the most among the above factors of statistical significance. Sepsis could lead to systemic inflammatory response, involving multiple organ system damages and alteration of the evaluation indicators, such as PLT count. Whereupon, the information of NEC patients without sepsis was analyzed using univariate regression, and 17 variables with P < 0.05 were considered as independent variables for stepwise multivariate non-conditional logistic regression analysis. The results showed that anemia, PLCR, and PCT were the final significant indicators in the model. Anemia (OR = 4.367, 95% CI: 1.853–10.291, P = 0.001) increased the risk of NEC in LBW neonates without sepsis, and increased PLCR (OR = 2.222, 95% CI: 1.633–3.023, P < 0.001) and PCT (OR = 1.368, 95% CI: 1.042–1.795, P = 0.024) could be the indicators in predicting the risk of NEC in LBW neonates without sepsis, as shown in Table 6.

Table 6. Multivariate logistic regression analysis of NEC occurrence in LBW neonates without sepsis.
Analysis of the effect of multiple factors and their correlation on NEC occurrence in neonates with sepsis
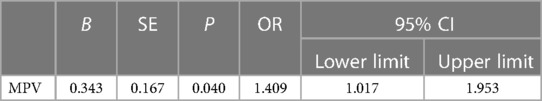
The information on NEC patients with sepsis was analyzed using univariate regression, and 10 variables with P < 0.05 were considered as independent variables for stepwise multivariate non-conditional logistic regression analysis. The results showed that only MPV was the final significant indicator entered into the model. Increased MPV (OR = 1.409, 95% CI: 1.017–1.953, P = 0.040) was the indicator to predict the risk of NEC in LBW neonates with sepsis (Table 7).
PLCR and PCT in predicting NEC occurrence
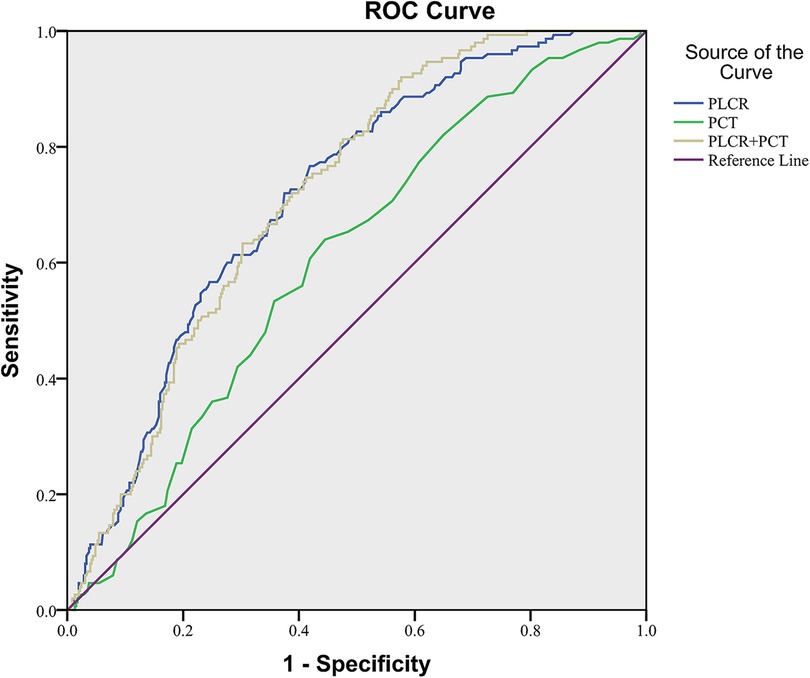
The ROC curve fitting analysis was used to evaluate the PLCR, PCT, and the combination of the two in NEC diagnosis. In all NEC patients in this study, the ROC curve area of PLCR diagnosis was 0.717 (P < 0.001), the sensitivity was 0.767, the specificity was 0.581, and the cutoff value was 33.55. The ROC curve area of PCT diagnosis was 0.606 (P < 0.001), the sensitivity was 0.640, the specificity was 0.560, and the cutoff value was 0.3350. The ROC curve area of PLCR–PCT diagnosis was 0.719 (P < 0.001), the sensitivity was 0.920, the specificity was 0.423, and the cutoff value was 0.1566 (Figure 2).
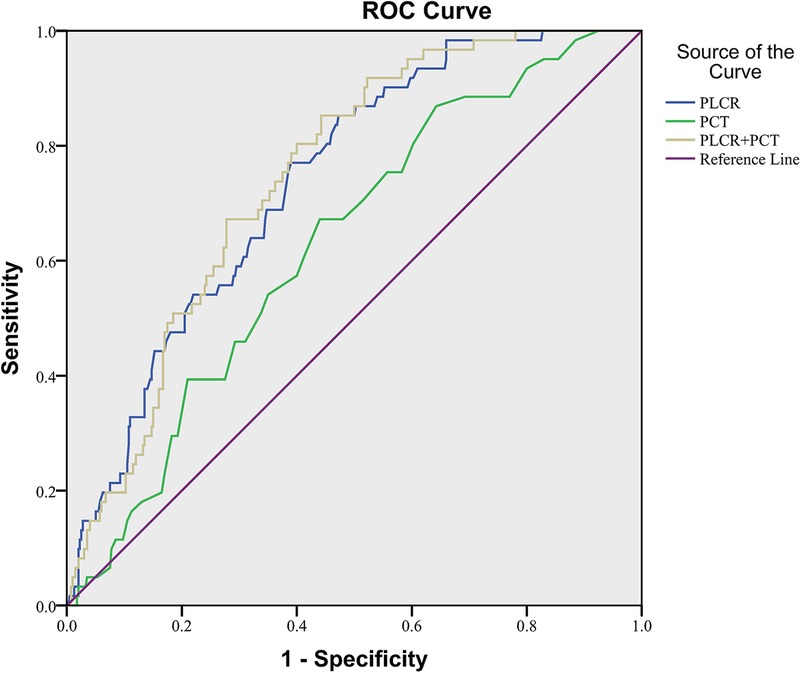
In NEC patients without sepsis, the ROC curve area of PLCR diagnosis was 0.739 (P < 0.001), the sensitivity was 0.770, the specificity was 0.610, and the cutoff value was 33.55. The ROC curve area of PCT diagnosis was 0.629 (P = 0.001), the sensitivity was 0.672, the specificity was 0.560, and the cutoff value was 0.3350. The ROC curve area of PLCR–PCT diagnosis was 0.748 (P < 0.001), the sensitivity was 0.852, the specificity was 0.557, and the cutoff value was 0.1074. In conclusion, the value of the combination of PLCR and PCT and PCT alone in NEC diagnosis was not significantly higher than PLCR alone (Figure 3).
The values of these ROCs were acceptable.
Discussion
The present study aimed to provide a pooled estimation of NEC in LBW neonates in China. The results showed that 2/100 LBW neonates developed NEC and that sepsis and anemia were the risk factors for NEC occurrence in LBW neonates. This might be the first study to show the superior value of PLT activation (especially PLCR), rather than PLT count, in predicting NEC occurrence in LBW neonates. Most studies postulate that neonatal sepsis is a major risk factor for NEC (10, 12, 27, 32). Currently, the correlation between anemia and NEC occurrence is under intensive focus, but the findings of such studies are yet controversial. The association between PDA and the occurrence of NEC due to hemodynamic changes is consistent with the findings of most studies (26). In this study, our results are suggestive that there is a correlation between anemia and NEC occurrence. The present study identified the risk factor for NEC occurrence in LBW neonates in NICUs in China and emphasized the value of PLT activation in NEC diagnosis of LBW neonates, thereby providing a new approach for future studies on NEC pathogenesis.
Correlation between neonatal sepsis and NEC occurrence
Stratified analysis of sepsis identified the risk factors for NEC in LBW neonates. This study found that every 6/10 NEC patients presented sepsis and both EOS and LOS were risk factors for NEC, suggesting that the control of intrauterine infection and hospital infection is crucial in preventing NEC. In the event of sepsis infection, pathogen-produced toxins may directly damage the intestinal mucosa or activate immune cells to produce cytokines, thereby altering vascular permeability and tissue damage (3). These phenomena result in the accumulation of PLTs and white blood cells in capillaries, which in turn causes intestinal damage and eventually NEC by blocking blood flow, aggravating the intestinal mucosa, and initiating excessive multiplication of intestinal bacteria. Another study showed that sepsis increases the risk of NEC by threefold (27), which is similar to the current finding. Typically, neonatal sepsis induced by different types of microbes manifests as a variety of pathophysiology and can result in several complications and outcomes (32, 33). Therefore, neonatal sepsis is deemed a vital confounding factor in the etiological analysis of NEC. Importantly, we also found that the risk factors for NEC differed between neonates with and without sepsis. These patients were further grouped into NEC subjects with and without sepsis for analysis. The results showed that NEC occurrence was correlated with anemia, PLCR, and PCT in NEC subjects without sepsis under the interplay of multiple factors. However, only MPV was weakly correlated with NEC occurrence in NEC subjects with sepsis.
Correlation between PLT activation indicators and NEC occurrence
PLT activation, rather than PLT count, was the earlier predictor for NEC occurrence. Pups with 2,4,6-trinitro-benzene sulfonic acid (TNBS)-mediated acute necrotizing ileocolitis had increased immature PLT fractions, high MPV, and increased megakaryocyte number/ploidy in the bone marrow, which are consistent with the clinical observations in human NEC (34, 35). These manifestations favored peripheral PLT consumption, but not decreased production, as the kinetic basis for thrombocytopenia (36). However, the literature showed that the PLT count decreased significantly in NEC patients compared to that in non-NEC patients and was correlated with the severity of NEC (37). Our results were different from the previous literature. The main reasons might be that the PLT in this study was counted 2 days before NEC diagnosis when NEC is in the early stage and the bone marrow is producing compensatory blood vigorously to maintain normal PLT count or increase PLT count. Additionally, PLCR and PCT were sensitive and increased at the early stage. Apparently, PLCR and PCT, not PLT count, could be used as indicators to predict the early risk in NEC patients without sepsis, indicating that PLT activation was the early predictor for NEC occurrence. Various types of sepsis affect the PLT through different pathways. PLT activation and depletion during NEC disrupt the mucosal wall established and lead to bacterial translocation across the damaged mucosa (18). PLTs repair bacterial damage to the vascular endothelium, resulting in increased platelet consumption, activated immune system, and enhanced PLT apoptosis (38). Other common viral infections, such as Torch or fungal infection, directly destroy the megakaryocytes or PLTs, inhibit bone marrow hematopoiesis, induce the production of autoantibodies to accelerate PLT destruction, and affect their levels in peripheral blood. Severe bacterial infections [such as GBS (group B streptococcus) infection] disrupt bone marrow hematopoiesis, leading to thrombocytopenia. Therefore, it could be speculated that the value of PLT indicators in predicting the occurrence of NEC in neonates with sepsis was not significant.
Correlation between anemia and NEC occurrence
Anemia was a risk factor for NEC occurrence in patients without sepsis but not in those with sepsis. Previous studies on anemia promoting NEC occurrence yielded varied results. Patel et al. (13) and Singh et al. (26) speculated that anemia, rather than blood transfusion, was correlated with a high risk of NEC occurrence; however, other retrospective studies did not find such a significant effect (27, 39). Nonetheless, no stratified analysis of sepsis was performed in these studies. Herein, we proposed that anemia, rather than blood transfusion, increased the risk of NEC occurrence in patients without sepsis because anemia affects splanchnic perfusion and causes hypoxia, anaerobic metabolism, and accumulation of anaerobic metabolism products, such as lactic acids. These by-products disrupt the intestinal vascular regulation resulting in ischemic injury, thereby increasing the risk of NEC occurrence (40). Various microbes causing sepsis give rise to anemia, hypoxia, and intestinal tissue damage in the body and, hence, are deemed confounding factors that complicate the analysis of the results. In the NEC and sepsis subgroup, the lack of association between anemia and NEC development may be due to a series of severe lesions caused by sepsis that interfere with this factor; hence, more follow-up research is needed.
Nevertheless, the present study had some limitations. First, all the results were based on the largest women's hospital in Zhejiang Province (one of the largest women's hospitals in China), but the information recorded did not reflect NEC occurrence and the associated risk factors nationwide. Therefore, multicenter clinical studies are essential to further investigate the correlation between anemia, increased PLCR, increased PCT, and NEC occurrence in LBW neonates. Second, this case–control study had a retrospective design and, thus, was subject to information bias. Hence, a cohort study, as well as a randomized controlled clinical experiment, should be carried out in the future to substantiate the current findings.
Conclusion
PLCR has a significant value in the early prediction of NEC incidence in LBW neonates without sepsis.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Women’s Hospital, Zhejiang University School of Medicine (IRB-20210068-R). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study was a retrospective case–control study.
Author contributions
ZJ designed the study and was the principal author of the manuscript. GY supervised data collection, did the analysis, and reviewed the manuscript. SZ supervised and contributed to the design, supervised the data analysis, and co-wrote the manuscript. LZ obtained epidemiology data and did the analysis. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Junnan Huang for her substantial assistance with the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NEC, necrotizing enterocolitis; LBW, low birth weight; PLCR, the value of the proportion of large platelets; PCT, platelet crit; MPV, mean platelet volume; PDW, platelet distribution width; HDCP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus; PP, placenta previa; PROM, premature rupture of membrane; PDA, patent ductus arteriosus.
References
1. Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr. (2017) 171:83–8. doi: 10.1001/jamapediatrics.2016.2708
2. Heath M, Buckley R, Gerber Z, Davis P, Linneman L, Gong Q, et al. Association of intestinal alkaline phosphatase with necrotizing enterocolitis among premature infants. JAMA Netw Open. (2019) 2:e1914996. doi: 10.1001/jamanetworkopen.2019.14996
3. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. (2017) 5:31. doi: 10.1186/s40168-017-0248-8
4. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364:255–64. doi: 10.1056/NEJMra1005408
5. Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. II. Outcome assessment. Am J Dis Child. (1981) 135:608–11. doi: 10.1001/archpedi.1981.02130310014006
6. Thyoka M, de Coppi P, Eaton S, Khoo K, Hall NJ, Curry J, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg. (2012) 22:8–12. doi: 10.1055/s-0032-1306263
7. Gaynes RP, Palmer S, Martone WJ, Holt CL, Buchter DS, Frawley LW, et al. The role of host factors in an outbreak of necrotizing enterocolitis. Am J Dis Child. (1984) 138:1118–20. doi: 10.1001/archpedi.1984.02140500024007
8. Wilson R, Kanto WP Jr., McCarthy BJ, Burton T, Lewin P, Terry J, et al. Epidemiologic characteristics of necrotizing enterocolitis: a population-based study. Am J Epidemiol. (1981) 114:880–7. doi: 10.1093/oxfordjournals.aje.a113258
9. Ahle M, Drott P, Andersson RE. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987–2009. Pediatrics. (2013) 132:e443–51. doi: 10.1542/peds.2012-3847
10. Wojkowska-Mach J, Rozanska A, Borszewska-Kornacka M, Domanska J, Gadzinowski J, Gulczynska E, et al. Necrotising enterocolitis in preterm infants: epidemiology and antibiotic consumption in the Polish neonatology network neonatal intensive care units in 2009. PLoS One. (2014) 9:e92865. doi: 10.1371/journal.pone.0092865
11. Boo NY, Cheah IG. Risk factors associated with necrotising enterocolitis in very low birth weight infants in Malaysian neonatal intensive care units. Singapore Med J. (2012) 53:826–31.23268157
12. Lu Q, Cheng S, Zhou M, Yu J. Risk factors for necrotizing enterocolitis in neonates: a retrospective case-control study. Pediatr Neonatol. (2017) 58:165–70. doi: 10.1016/j.pedneo.2016.04.002
13. Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. (2016) 315:889–97. doi: 10.1001/jama.2016.1204
14. MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. (2019) 10:3494. doi: 10.1038/s41467-019-11199-5
15. McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. Neoreviews. (2011) 12:e517–26. doi: 10.1542/neo.12-9-e517
16. Maheshwari A. Immunologic and hematological abnormalities in necrotizing enterocolitis. Clin Perinatol. (2015) 42:567–85. doi: 10.1016/j.clp.2015.04.014
17. Mlynarczyk M, Chauhan SP, Baydoun HA, Wilkes CM, Earhart KR, Zhao Y, et al. The clinical significance of an estimated fetal weight below the 10th percentile: a comparison of outcomes of <5th vs. 5th–9th percentile. Am J Obstet Gynecol. (2017) 217:198.e1–11. doi: 10.1016/j.ajog.2017.04.020
18. Namachivayam K, MohanKumar K, Shores DR, Jain SK, Fundora J, Everett AD, et al. Targeted inhibition of thrombin attenuates murine neonatal necrotizing enterocolitis. Proc Natl Acad Sci U S A. (2020) 117:10958–69. doi: 10.1073/pnas.1912357117
19. Arik OZ, Ozkan B, Kutlu R, Karal H, Sahin DY, Kaypakli O, et al. Relationship between platelet indices and international normalized ratio in patients with non-valvular atrial fibrillation. Platelets. (2014) 25:311–6. doi: 10.3109/09537104.2013.821603
20. Peng F, Li Z, Yi C, Guo Q, Yang R, Long H, et al. Platelet index levels and cardiovascular mortality in incident peritoneal dialysis patients: a cohort study. Platelets. (2017) 28:576–84. doi: 10.1080/09537104.2016.1246716
21. Faber J, Hvas AM, Kristensen SD, Grove EL, Adelborg K. Immature platelets and risk of cardiovascular events among patients with ischemic heart disease: a systematic review. Thromb Haemost. (2021) 121:659–75. doi: 10.1055/s-0040-1721386
22. Nardin M, Verdoia M, Barbieri L, De Luca G, Novara Atherosclerosis Study Group (NAS). Impact of metabolic syndrome on mean platelet volume and its relationship with coronary artery disease. Platelets. (2019) 30:615–23. doi: 10.1080/09537104.2018.1499885
23. Tam AL, Camberos A, Applebaum H. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: predictive value of radiologic findings. J Pediatr Surg. (2002) 37:1688–91. doi: 10.1053/jpsu.2002.36696
24. Chatziioannou AC, Wolters JC, Sarafidis K, Thomaidou A, Agakidis C, Govorukhina N, et al. Targeted LC-MS/MS for the evaluation of proteomics biomarkers in the blood of neonates with necrotizing enterocolitis and late-onset sepsis. Anal Bioanal Chem. (2018) 410:7163–75. doi: 10.1007/s00216-018-1320-3
25. Angura P, Velaphi S. Risk factors for necrotising enterocolitis in an HIV-endemic region. Paediatr Int Child Health. (2014) 34:208–15. doi: 10.1179/2046905514Y.0000000126
26. Singh R, Visintainer PF, Frantz ID 3rd, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. (2011) 31:176–82. doi: 10.1038/jp.2010.145
27. Wang ZL, An Y, He Y, Hu XY, Guo L, Li QY, et al. Risk factors of necrotizing enterocolitis in neonates with sepsis: a retrospective case–control study. Int J Immunopathol Pharmacol. (2020) 34:2058738420963818. doi: 10.1136/archdischild-2018-315387
28. Henry E, Christensen RD. Reference intervals in neonatal hematology. ClinPerinatol. (2015) 42:483–97. doi: 10.1111/j.1471-0528.2006.01086.x
29. Lu CY, Liu KF, Qiao GX, Luo Y, Cheng HQ, Du SZ. Risk factors for necrotizing enterocolitis in preterm infants: a meta analysis. Zhongguo Dang Dai Er Ke Za Zhi. (2022) 24(8):908–16. doi: 10.1542/peds.2009-0582
30. Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed. (2014) 99(1):F87–92. doi: 10.1136/archdischild-2012-303351
31. Sdona E, Papamichail D, Panagiotopoulos T, Lagiou P, Malamitsi-Puchner AJ. Cluster of late preterm and term neonates with necrotizing enterocolitis symptomatology: descriptive and case-control study. Matern Fetal Neonatal Med. (2016) 29(20):3329–34. doi: 10.1038/pr.2017.7
32. Wang J, Kortsalioudaki C, Heath PT, Buttery J, Clarke P, Gkentzi D, et al. Epidemiology and healthcare factors associated with neonatal enterococcal infections. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F480–5. doi: 10.1136/archdischild-2018-315387
33. Hakansson S, Kallen K. Impact and risk factors for early-onset group B streptococcal morbidity: analysis of a national, population-based cohort in Sweden 1997–2001. BJOG. (2006) 113:1452–8. doi: 10.1111/j.1471-0528.2006.01086.x
34. Baer VL, Lambert DK, Henry E, Christensen RD. Severe thrombocytopenia in the NICU. Pediatrics. (2009) 124:e1095–100. doi: 10.1542/peds.2009-0582
35. Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. (2006) 26:348–53. doi: 10.1038/sj.jp.7211509
36. Namachivayam K, MohanKumar K, Garg L, Torres BA, Maheshwari A. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr Res. (2017) 81:817–24. doi: 10.1038/pr.2017.7
37. Panesso-Gomez S, Shimamura M, Conces M, Talavera MM, Moallem M, Sanchez PJ, et al. Detection of cytomegalovirus in intestinal tissue of infants with necrotizing enterocolitis or spontaneous intestinal perforation. J Pediatr. (2019) 214:34–40. doi: 10.1016/j.jpeds.2019.07.038
38. Saber AM, Aziz SP, Almasry AZE, Mahmoud RA. Risk factors for severity of thrombocytopenia in full term infants: a single center study. Ital J Pediatr. (2021) 47:7. doi: 10.1186/s13052-021-00965-1
39. Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. (2011) 127:635–41. doi: 10.1542/peds.2010-3178
Keywords: NEC, anemia, sepsis, the value of the proportion of large platelets, platelet crit
Citation: Jiang Z, Ye G, Zhang S and Zhang L (2023) Association of anemia and platelet activation with necrotizing enterocolitis with or without sepsis among low birth weight neonates: a case–control study. Front. Pediatr. 11:1172042. doi: 10.3389/fped.2023.1172042
Received: 23 February 2023; Accepted: 15 August 2023;
Published: 31 August 2023.
Edited by:
Rozeta Sokou, Nikaia General Hospital “Aghios Panteleimon”, GreeceReviewed by:
Andreas G. Tsantes, National and Kapodistrian University of Athens, GreeceVasiliki Mougiou, National and Kapodistrian University of Athens, Greece
© 2023 Jiang, Ye, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songying Zhang emhhbmdzb25neWluZ0B6anUuZWR1LmNu Long Zhang anl6aGFuZ2xvbmdAemp1LmVkdS5jbg==
 Zhou Jiang
Zhou Jiang Guangyong Ye2
Guangyong Ye2 Songying Zhang
Songying Zhang Long Zhang
Long Zhang