
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 09 May 2023
Sec. General Pediatrics and Pediatric Emergency Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1163546
 Nader Shaikh*†
Nader Shaikh*† Marcia Kurs-Lasky
Marcia Kurs-Lasky Hui Liu
Hui Liu Vinod Rajakumar
Vinod Rajakumar Heba Qureini
Heba Qureini Isabella O. Conway
Isabella O. Conway Matthew C. Lee
Matthew C. Lee Sojin Lee
Sojin Lee
Background: The current reference standard for pediatric urinary tract infection (UTI) screening, the leukocyte esterase (LE) dipstick test, has suboptimal accuracy. The objective of this study was to compare the accuracy of novel urinary biomarkers to that of the LE test.
Methods: We prospectively enrolled febrile children who were evaluated for UTI based on their presenting symptoms. We compared the accuracy of urinary biomarkers to that of the test.
Results: We included 374 children (50 with UTI, 324 without UTI, ages 1–35 months) and examined 35 urinary biomarkers. The urinary biomarkers that best discriminated between febrile children with and without UTI were urinary neutrophil gelatinase–associated lipocalin (NGAL), IL-1β, CXCL1, and IL-8. Of all examined urinary biomarkers, the urinary NGAL had the highest accuracy with a sensitivity of 90% (CI: 82–98) and a specificity of 96% (CI: 93–98).
Conclusion: Because the sensitivity of the urinary NGAL test is slightly higher than that of the LE test, it can potentially reduce missed UTI cases. Limitations of using urinary NGAL over LE include increased cost and complexity. Further investigation is warranted to determine the cost-effectiveness of urinary NGAL as a screening test for UTI.
Diagnosis of urinary tract infection (UTI) relies on the urine culture, which typically requires 48–72 h. This forces clinicians to rely on bedside screening tests to determine which children need treatment with antimicrobials. However, neither the leukocyte esterase (LE) test nor the leukocyte count (WBC) obtained using conventional urine microscopy is sufficiently specific to serve as a screening test. LE and WBC were found to have a sensitivity and specificity of 79% and 87% and 74% and 86%, respectively (1). The low specificity of these tests results in the inappropriate use of antimicrobials (2); approximately 50% of children prescribed antimicrobials for UTI were eventually proven not to have a UTI (3). More specific markers for UTI would reduce unnecessary antimicrobial use. In addition, the low sensitivity of the available screening tests leads to missed UTIs, particularly UTIs caused by organisms other than Escherichia coli, where the sensitivity of the current screening tests approaches 50% (4).
Compared to urinary leukocytes, which can be elevated in various conditions, urinary proteins intricated in the host's response to a pathogen might be better suited to serve as screening tests for UTI. In a previous case–control study, urinary neutrophil gelatinase–associated lipocalin (NGAL) was more accurate than the available point-of-care tests currently used to screen children for UTI. However, because accuracy estimates are often inflated in case–control studies (5), we revisited the accuracy of urinary NGAL in our ongoing prospective study partly aimed at identifying better markers to screen for UTI in young febrile children.
Between June 2019 and May 2020, we enrolled consecutive children in a prospective study at the Children's Hospital of Pittsburgh Emergency Department. Children were included if they were between 1 month and 3 years of age, had a fever (temperature of ≥38°C) within 24 h of presentation, and had a urine sample collected via a catheter. We excluded children who had received systemic antimicrobials or corticosteroids within 72 h of enrolment, had other concurrent systemic bacterial infections, were immunodeficient, had a neurogenic bladder, or had major genitourinary abnormalities. Of note, routine practice at our institution is to perform bladder catheterization on all non–toilet-trained children with suspected UTI. The study was approved by the University of Pittsburgh Institutional Review Board, and no consent was required from the parents of participating children. We stored 0.2 ml aliquot of urine in a cryovial without preservatives at −80°C within 1 h of sample collection. However, if a delay was anticipated, samples were stored in a specimen refrigerator until processing.
We assessed 35 candidate markers. These markers were measured in urine using a commercially available 34-plex plate (ProcartaPlex, EPX340-12167-901, Thermo Fisher Scientific; see Table 2 for a listing of these markers) and Bioporto NGAL Human ELISA kit (KIT036RUO; Bioporto). On each plate, we included duplicates and control samples.
We first ran the 34-plex plate by following the manufacturer’s recommendations. We used 50 μl of the sample and processed samples in five batches. Plates were read on Bio-Plex MAGPIX instrument (Bio-Rad) after performing the recommended calibration and verification steps using MAGPIX Performance Verification Kit (Bio-Rad, MPX-PVER-K25) and MAGPIX Calibration Kit (Bio-Rad, MPX-CAL-K25). Data were processed using the Bio-Plex Manager Software (version 6.1, Bio-Rad). NGAL ELISA was performed per the manufacturer's instructions and using a 500-fold dilution; plates were read using BenchmarkPlus (Bio-Rad). We ran the NGAL ELISA's after the 34-plex plate because freeze-thaw cycles have little effect on NGAL values (6). We did not normalize biomarker values to urine creatinine because we previously found that this decreased accuracy (7–10). The laboratory technician was blinded to the target condition.
Urinary tract infection was the target condition examined. The reference standard for diagnosis of UTI was the urine culture. We defined UTI as the growth of at least one organism at ≥10,000 colony-forming units/milliliter (CFU/ml) from a catheterized specimen.
We used the t-test to compare levels of the respective urinary markers in those with and those without UTI. To account for multiple comparisons, we converted p-values to q-values using the Benjamini–Hochberg correction (11). For each marker, we calculated the area under the receiver operating characteristic (ROC) curve and the sensitivity and specificity values that would maximize the Youden index (J), where J is the sensitivity plus the specificity minus 1. Also, for each marker, we calculated the sensitivity and specificity values that would minimize the distance (D) from the ROC curve to the point (0,1), i.e., where 1 minus specificity equals 0 and sensitivity equals 1. We used linear regression to explore the effect of covariates (age, gender, race, and isolated organisms) on marker accuracy. We evaluated the accuracy of combinations of biomarkers in predicting UTI using a procedure that creates a decision tree model (SAS PROC HPSPLIT). Entropy criterion was specified for the GROW statement and cost complexity for the PRUNE statement.
Figure 1 shows the flow of patients in the study, 652 of whom were eligible for enrolment. Table 1 describes the demographic characteristics of the 374 children included in the analysis. The prevalence of UTI was 13.4% (50/374). As expected, children with UTI were mostly female (88.0%).
Biomarker levels, their respective AUC, and their respective sensitivity and specificity, which are determined using the cutoff that maximized the Youden index, are shown in Table 2. Urinary NGAL had the highest AUC (0.96; CI: 0.93–0.99), with a sensitivity of 90% (CI: 82–98) and specificity of 96% (CI: 93–98). Figure 2 shows NGAL levels in children with and without UTI. Three additional markers, IL-1β, CXCL1, and IL-8, also had AUCs greater than 0.85 and specificities greater than 76%. Supplement Table S3 shows the cutoff for each marker that maximized the Youden index with the corresponding sensitivity and specificity and the cutoff that minimized the distance to (0,1) on the ROC curve with the corresponding sensitivity and specificity.
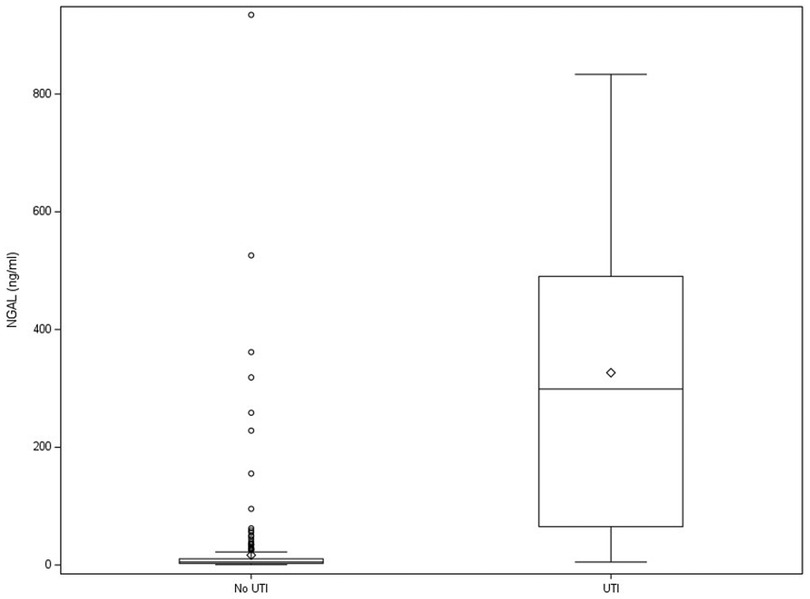
Figure 2. Box and whisker plot of urinary NGAL level in children with and without UTI. Dots represent outliers. Diamonds represent group means. NGAL, neutrophil gelatinase–associated lipocalin; UTI, urinary tract infection.
In children with UTI, E. coli was the most frequently isolated organism (92%) on conventional urine culture. Urinary NGAL levels did not differ significantly (p = 0.35) between children with non-E. coli UTIs and those with E. coli UTIs (75.0% and 91.3%, respectively, had urinary NGAL ≥39.9 ng/ml). In contrast, patients with non-E. coli UTIs had significantly lower LE values compared to those of patients with E. coli UTIs (LE ≥ trace in 25.0% vs. 93.5%, respectively, p = 0.004).
No association existed between any of the covariates and the levels of the top four biomarkers examined, i.e., NGAL, IL-1β, CXCL1, and IL-8. Combining markers did not improve accuracy; the best combination, which used four variables, had a sensitivity of 0.90 and a specificity of 0.98 (AUC = 0.94; CI: 0.90–0.99). Pearson correlation coefficients between duplicates were greater than 0.99 for urinary NGAL, IL-1β, CXCL1, and IL-8.
We found that urinary NGAL had a sensitivity and specificity of 90% (CI: 82–98) and 96% (CI: 93–98) in differentiating UTI from no UTI in febrile young children with suspected UTI. In comparison, leukocyte esterase in this study had a sensitivity of 88% (CI: 79–97) and specificity of 96% (CI: 94–98). Urinary NGAL is involved in sequestering iron required for bacterial growth within the urinary tract and is released from neutrophils and intercalated cells in the renal collecting duct in response to infection or injury (12). A number of studies (4, 13–16) and a recent meta-analysis (10) have found that urinary NGAL levels differ in children with and without UTI, which is consistent with our results.
In 1,000 children presenting with UTI, 100 of whom are assumed to have a UTI (based on the prevalence of UTI in more recent studies that use an algorithm to select children for testing), two fewer UTIs would have been missed. Given the higher costs of the urinary NGAL test, this marginal difference supports the continued use of the LE as the screening test for UTI. However, certain other factors need to be considered. First, our results differ from the results of a recent meta-analysis comparing urinary NGAL and the leukocyte esterase test (10). In that meta-analysis, urinary NGAL was found to be more accurate than in our current study. Conversely, LE was less accurate in the meta-analysis than in our current study. This could be due to the different spectrum of patients enrolled (e.g., febrile) or different urine collection methods. Second, although the number of children with non-E. coli UTI was small in the current study, our data suggest that urinary NGAL may be a better screening test for UTI due to pathogens other than E. coli. Third, in children with ongoing bladder inflammation, such as children with spina bifida, the tests may perform differently (17). For these reasons, further research on the potential of urinary NGAL is warranted.
We found that IL-8 was significantly elevated in children with UTI (9, 18). IL-8, which is secreted by the urothelium, plays a major role in the recruitment of neutrophils to the urinary tract (19). IL-8 levels have been shown to be elevated in adults and children with UTI (20–22). However, we found that the accuracy of IL-8 was lower than that of LE, which limits its clinical utility.
We also found that CXCL1 (also known as GRO-α) was elevated significantly in children with UTI. Like IL-8, CXCL1 is also involved in neutrophil recruitment (8). CXCL1 is produced by the urothelium, particularly in response to uropathogenic E. coli exposure (23). CXCL1 has been shown to be elevated in mouse urine with uropathogenic E. coli exposure (24), and some authors have found increased levels of CXCL1 in humans with UTI (25). As with IL-8, we found that the accuracy of CXCL-1 was lower than LE, which limits its clinical utility.
We found that IL-1β was elevated in children with UTI. IL-1β is a pro-inflammatory biomarker that is secreted by macrophages in response to infection and serves to recruit neutrophils and other leukocytes. IL-1β is released very early in response to infection (26, 27). IL-1β has been shown to be elevated in children with cystitis (28) and acute pyelonephritis (27, 29). However, IL-1β has not been studied as a screening biomarker for febrile UTIs in children. Our results indicate that IL-1β as a biomarker to detect UTI in febrile children deserves more study.
In a previous study (30), we reported that screening using the leukocyte esterase test could miss a significant proportion of infections caused by organisms other than E. coli. The current study, while small, seems to reinforce those findings; of the four children with infections caused by organisms other than E. coli, only 1 had a positive LE test, whereas three had high (≥39.9 ng/ml) urinary NGAL levels. Further research is needed to establish whether urinary NGAL could serve as an accurate marker for UTI secondary to organisms other than E. coli.
This study has several limitations. In order to allow us to compare the accuracy of various biomarkers to the LE test, we did not require a positive LE test in our definition of UTI. Thus, the misclassification of children with asymptomatic bacteriuria without pyuria as UTI is a potential limitation. Nevertheless, asymptomatic bacteriuria masquerading as UTI is highly unlikely because all children included were symptomatic (all were tested and treated for UTI) and because, based on the prevalence of asymptomatic bacteria without pyuria in this population (i.e., 0.21%) (31), no more than one child included in this sample likely has asymptomatic bacteriuria without pyuria. A second source of misclassification of children may have come from contaminated samples read as having UTI. We attempted to minimize this by only using catheterized samples.
Our findings further support the use of urinary NGAL as a screening test for UTI. More research regarding the utility of measuring urinary NGAL, especially in children with non-E. coli UTI and in children with spina bifida in whom the performance of the leukocyte esterase test is suboptimal, is needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the University of Pittsburgh Institutional Review Board. Written informed consent from the participants’ legal guardians/next of kin was not required to participate in this study in accordance with the national legislation and institutional requirements.
NS conceptualized and designed the study, collected data, interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript. MK-L performed the statistical analysis and reviewed and revised the manuscript. SL carried out the experiments and reviewed and revised the manuscript. VR interpreted the data and drafted the initial manuscript. HQ, IOC, and ML interpreted the data and reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK087870).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1163546/full#supplementary-material .
1. Williams GJ, Macaskill P, Chan SF, Turner RM, Hodson E, Craig JC. Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta-analysis. Lancet Infect Dis. (2010) 10:240–50. doi: 10.1016/S1473-3099(10)70031-1
2. Saha D, Patel J, Buckingham D, Thornton D, Barber T, Watson JR. Urine culture follow-up and antimicrobial stewardship in a pediatric urgent care network. Pediatrics. (2017) 139. doi: 10.1542/peds.2016-2103
3. Watson JR, Sanchez PJ, Spencer JD, Cohen DM, Hains DS. Urinary tract infection and antimicrobial stewardship in the emergency department. Pediatr Emerg Care. (2018) 34:93–5. doi: 10.1097/PEC.0000000000000688
4. Jung N, Byun HJ, Park JH, Kim JS, Kim HW, Ha JY. Diagnostic accuracy of urinary biomarkers in infants younger than 3 months with urinary tract infection. Korean J Pediatr. (2018) 61:24–9. doi: 10.3345/kjp.2018.61.1.24
5. Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. (1999) 282:1061–6. doi: 10.1001/jama.282.11.1061
6. Schuh MP, Nehus E, Ma Q, Haffner C, Bennett M, Krawczeski CD, et al. Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. (2016) 67:56–61. doi: 10.1053/j.ajkd.2015.04.040
7. Shaikh N, Martin JM, Hoberman A, Skae M, Milkovich L, Nowalk A, et al. Host and bacterial markers that differ in children with cystitis and pyelonephritis. J Pediatr. (2019) 209:146–53 e1. doi: 10.1016/j.jpeds.2019.01.012
8. Shaikh N, Martin JM, Hoberman A, Skae M, Milkovich L, McElheny C, et al. Biomarkers that differentiate false positive urinalyses from true urinary tract infection. Pediatr Nephrol. (2020) 35:321–9. doi: 10.1007/s00467-019-04403-7
9. Shaikh N, Liu H, Kurs-Lasky M, Forster CS. Biomarkers for febrile urinary tract infection in children. Pediatr Nephrol. (2022) 37:171–7. doi: 10.1007/s00467-021-05173-x
10. Shaikh K, Rajakumar V, Osio VA, Shaikh N. Neutrophil gelatinase-associated lipocalin for urinary tract infection and pyelonephritis: a systematic review. Pediatr Nephrol. (2021) 36(6):1481–7. doi: 10.1007/s00467-020-04854-3
11. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. (2001) 125:279–84. doi: 10.1016/S0166-4328(01)00297-2
12. Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, et al. alpha-intercalated cells defend the urinary system from bacterial infection. J Clin Invest. (2014) 124:2963–76. doi: 10.1172/JCI71630
13. Agarwal I, Iswarya J, Flemming J, Chaturvedi S, Mathew LG, Varkey SD, et al. Prospective study to determine the usefulness of urinary neutrophil gelatinase associated lipocalin (NAGL) as an early and sensitive marker of urinary tract infection (UTI) in children. Pediatr Nephrol. (2013) 28:1358. doi: 10.1007/s00467-013-2521-9
14. Lee HE, Kim DK, Kang HK, Park K. The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr Nephrol. (2015) 30:123–30. doi: 10.1007/s00467-014-2905-5
15. Lubell TR, Barasch JM, Xu K, Ieni M, Cabrera KI, Dayan PS. Urinary neutrophil gelatinase-associated lipocalin for the diagnosis of urinary tract infections. Pediatrics. (2017) 140. doi: 10.1542/peds.2017-1090
16. Valdimarsson S, Jodal U, Barregard L, Hansson S. Urine neutrophil gelatinase-associated lipocalin and other biomarkers in infants with urinary tract infection and in febrile controls. Pediatr Nephrol. (2017) 32:2079–87. doi: 10.1007/s00467-017-3709-1
17. Forster CS, Devarajan P. Neutrophil gelatinase-associated lipocalin: utility in urologic conditions. Pediatr Nephrol. (2017) 32:377–81. doi: 10.1007/s00467-016-3540-0
18. Bitsori M, Karatzi M, Dimitriou H, Christakou E, Savvidou A, Galanakis E. Urine IL-8 concentrations in infectious and non-infectious urinary tract conditions. Pediatr Nephrol. (2011) 26:2003–7. doi: 10.1007/s00467-011-1909-7
19. Agace WW, Hedges SR, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. (1993) 92:780–5. doi: 10.1172/JCI116650
20. Ko YC, Mukaida N, Ishiyama S, Tokue A, Kawai T, Matsushima K, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. (1993) 61:1307–14. doi: 10.1128/iai.61.4.1307-1314.1993
21. Krzemien G, Szmigielska A, Turczyn A, Panczyk-Tomaszewska M. Urine interleukin-6, interleukin-8 and transforming growth factor beta1 in infants with urinary tract infection and asymptomatic bacteriuria. Cent Eur J Immunol. (2016) 41:260–7. doi: 10.5114/ceji.2016.63125
22. Benson M, Jodal U, Agace W, Hellstrom M, Marild S, Rosberg S, et al. Interleukin (IL)-6 and IL-8 in children with febrile urinary tract infection and asymptomatic bacteriuria. J Infect Dis. (1996) 174:1080–4. doi: 10.1093/infdis/174.5.1080
23. Godaly G, Otto G, Burdick MD, Strieter RM, Svanborg C. Fimbrial lectins influence the chemokine repertoire in the urinary tract mucosa. Kidney Int. (2007) 71:778–86. doi: 10.1038/sj.ki.5002076
24. Ozer A, Altuntas CZ, Bicer F, Izgi K, Hultgren SJ, Liu G, et al. Impaired cytokine expression, neutrophil infiltration and bacterial clearance in response to urinary tract infection in diabetic mice. Pathog Dis. (2015) 73. doi: 10.1093/femspd/ftv002
25. Otto G, Burdick M, Strieter R, Godaly G. Chemokine response to febrile urinary tract infection. Kidney Int. (2005) 68:62–70. doi: 10.1111/j.1523-1755.2005.00381.x
26. Jung JH, Hong HJ, Gharderpour A, Cho JY, Baek BS, Hur Y, et al. Differential interleukin-1beta induction by uropathogenic Escherichia coli correlates with its phylotype and serum C-reactive protein levels in Korean infants. Sci Rep. (2019) 9:15654. doi: 10.1038/s41598-019-52070-3
27. Sheu JN, Chen MC, Cheng SL, Lee IC, Chen SM, Tsay GJ. Urine interleukin-1beta in children with acute pyelonephritis and renal scarring. Nephrology (Carlton). (2007) 12:487–93. doi: 10.1111/j.1440-1797.2007.00819.x
28. Martins SM, Darlin DJ, Lad PM, Zimmern PE. Interleukin-1B: a clinically relevant urinary marker. J Urol. (1994) 151:1198–201. doi: 10.1016/S0022-5347(17)35212-6
29. Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infection—a systematic review. Biomark Insights. (2009) 4:111–21. doi: 10.4137/BMI.S3155
30. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. (2016) 138. doi: 10.1542/peds.2016-0087
Keywords: urinary tract infection (UTI), diagnostic accuracy, biomarker, infectious disease, microbiome
Citation: Shaikh N, Kurs-Lasky M, Liu H, Rajakumar V, Qureini H, Conway IO, Lee MC and Lee S (2023) Biomarkers for febrile urinary tract infection in children. Front. Pediatr. 11:1163546. doi: 10.3389/fped.2023.1163546
Received: 10 February 2023; Accepted: 17 April 2023;
Published: 9 May 2023.
Edited by:
Ruud Gerard Nijman, Imperial College London, United KingdomReviewed by:
Yasaman Vali, Amsterdam University Medical Center, Netherlands© 2023 Shaikh, Kurs-Lasky, Liu, Rajakumar, Qureini, Conway, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nader Shaikh bmFkZXIuc2hhaWtoQGNocC5lZHU=
†ORCID Nader Shaikh orcid.org/0000-0002-1602-343X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.