- 1Department of Rheumatology, National Medical Research Center of Children's Health, Moscow, Russia
- 2Russian Federation Department of Pediatrics, Sechenov First Moscow State Medical University, Moscow, Russia
- 3Association of Pediatric Rheumatologists, Moscow, Russia
- 4Pediatric Pulmonology, Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
- 5Pediatric Oncology, Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
- 6Hospital Pediatry, Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
Non-bacterial osteomyelitis (NBO) is a rare chronic inflammatory bone disease related to immune system dysregulation. This disease belongs to a family of autoinflammatory diseases. It often coexists with other TNF-α-mediated immune-mediated diseases such as juvenile idiopathic arthritis (JIA) and inflammatory bowel diseases. Previously, interleukin-1-driven inflammation was described predominantly in monogenic cases of NBO, such as DIRA syndrome or Majeed syndrome. However, the association between NBO and JIA with systemic onset (soJIA) has not been described yet.
Herein, we describe the cases of two patients with soJIA with inflammatory bone lesions wherein canakinumab (anti-interleukin-1β antibodies) caused remission.
Case descriptions: Patient 1–A 6-month-old boy with typical soJIA suffered a destruction of the 7th to 9th ribs and the left pubic bone. Antibiotics, IVIG, and cyclosporine proved ineffective. Corticosteroids were effective, but due to the factor of corticosteroid dependence, which has some disadvantages, canakinumab with a dosage of 4 mg/kg was initiated every 4 weeks, which completely controlled the disease and allowed to taper corticosteroids.
Patient 2—A 2-year-old girl developed chronic non-bacterial osteomyelitis of the 5th rib 2 months after taking corticosteroids prescribed for typical soJIA. She underwent surgical debridement removal, and several courses of antibiotics proved ineffective. She developed macrophage activation syndrome, following which anakinra was prescribed, which resulted in only temporary improvement. Therefore, this drug was switched to canakinumab, which caused corticosteroid-free remission.
Conclusion: This is the first description of a rare association of soJIA with inflammatory bone lesions with the proven efficacy of IL-1 blockade. The association of two autoinflammatory conditions should indicate IL-1-driven mechanisms and a possible genetic basis. Follow-up genetic and functional studies are required to better understand the pathogenesis of such overlapping diseases.
Introduction
Non-bacterial osteomyelitis (NBO) is a rare chronic inflammatory bone destructive disease with unknown etiology (1). NBO belongs to a family of autoinflammatory diseases (2). Innate immune system dysregulation is the basis of its pathogenesis (3, 4). Several previous studies demonstrated the hyperproduction of IL-1β, TNF-α, IL-18, and IL-17a, with a lack of production of anti-inflammatory cytokines, such as IL-10, IL19, and IL-4 [(5–8]). NBO is often associated with TNF-α-mediated diseases such as juvenile spondyloarthritis, psoriatic arthritis, and inflammatory bowel diseases (9). According to the ARC/CARRA treatment plan, TNF-α inhibitors should be used in cases where non-biologic DMSRDs fail (10). There are several monogenic forms of NBO such as DIRA syndrome and Majeed syndrome, with a hyperproduction of IL-1 and successful treatment with IL-1 inhibitors, but the number of such cases is few (11, 12). In real clinical practice, IL-1 inhibitors are used rarely compared with TNF-α inhibitors (9).
Juvenile idiopathic arthritis with systemic onset (soJIA) is a typical autoinflammatory disease with a hyperproduction of several cytokines such as IL-1, IL-6, IL-18, TNF-α, IL-17A, interferon γ, and others (13, 14). Despite the broad spectrum of cytokines operating in soJIA pathogenesis, only the inhibition of IL-1 and IL-6 has shown the best results (15–17). The inhibition of TNF-a has partial efficacy and works better in patients in whom systemic features have disappeared and the disease has taken a chronic articular course (18).
Compared with NBO, soJIA is a rare autoinflammatory disease associated with other rheumatic diseases. Sometimes in the literature, the soJIA phenotype was described in autoinflammatory diseases (AID), e.g., TRAPS syndrome or CAPS syndrome (19, 20). However, the association between soJIA and NBO has not been described yet.
Herein, we describe the cases of two patients with soJIA overlapping with NBO and who were successfully treated with the IL-1β inhibitor, canakinumab.
Case description
Patient 1 was a boy who contracted the disease at 6 months of age with spiking fever, ankle arthritis, and a typical maculopapular rash associated with fever spikes (Figures 1A–C). He had anemia (Hb 77 g/L; n.v. 110–135 g/L), neutrophilic leukocytosis (WBC 28 × 109/L; n.v. 6–15 × 109/L), thrombocytosis—773 × 109/L (n.v.150–480 × 109/L)), ESR 74 mm/h (2–20 mm/h), increased CRP—136 mg/L (n.v. < 5 mg/L), increased ALT—92 U/L (n.v. < 40 U/L), AST—120 U/L (n.v. < 40 U/L), LDH—441 U/L (n.v. 91–295 U/L), ferritin—1,306 ng/ml (n.v. 12–327 U/L), and triglycerides 5 mmol/L (n.v. 0.34–1.6 mmol/L).
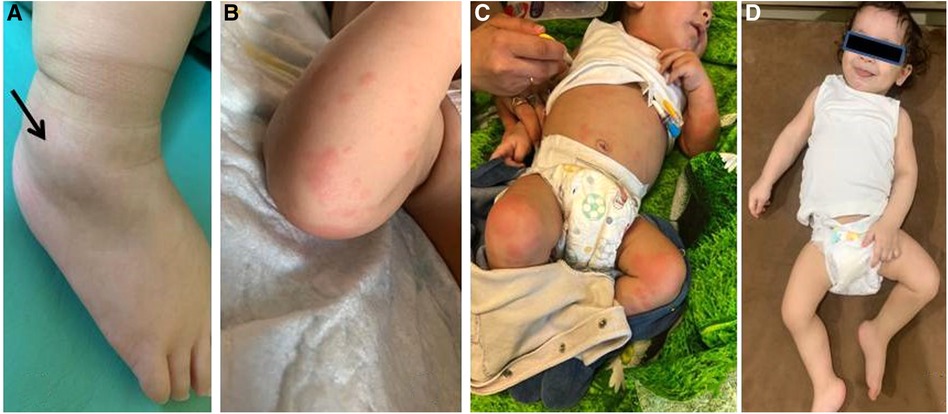
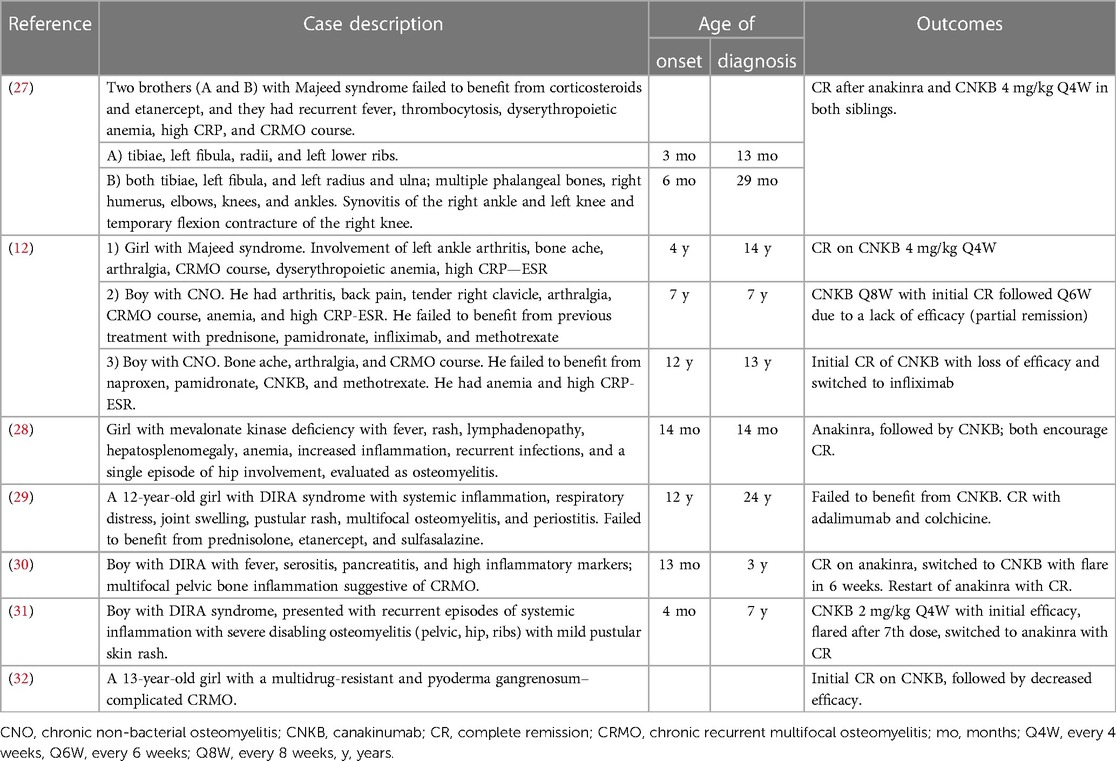
Figure 1. Patient 1: (A) right ankle swelling (arrow); (B) typical maculopapular rash; (C) the patient’s appearance before (rash and severe general condition of the patient) and (D) 3 months after (no rash, improvement of general condition) canakinumab treatment.
An examination revealed lymphadenopathy (supraclavicular, axillar, mesenteric, and retroperitoneal), arthritis of both hips and soft tissue swelling surrounding both ankles. Viral and bacterial infections and malignancy were ruled out, and empiric antibiotic therapy proved ineffective. This led to a diagnosis of soJIA with MAS, and the patient was started on systemic corticosteroids, cyclosporine A, and IVIG. All features of the disease disappeared and laboratory tests showed normal results. The patient was discharged from the hospital with a recommendation of corticosteroid tapering and continued cyclosporine A.
One month later, the patient developed a flare: spiking fever, typical rash, and knee, elbow, and hip arthritis with similar laboratory findings as at onset.
A chest CT taken after a prolonged fever revealed 7th right rib destruction with a swelling of the surrounding tissues and a focal destruction of the left pubic bone close to symphysis (Figure 2A). These findings were absent in CT and MRI done 1 month previously. Several courses of IV antibiotics and antimycotics proved ineffective. Pubic bone biopsy revealed signs of chronic osteomyelitis (infiltration with lymphocytes, plasma cells and monocytes, and bone sclerosis), which was confirmed by a second opinion sought from an alternative center. Further investigations in the following year (whole-body MRI) revealed bone marrow edema of the 8th and 9th right ribs and the left pubic bone, soft tissue swelling on the medial part of the left hip, and arthritis of the 7th costovertebral joint, elbows, hips, shoulders (Figures 2B,C).
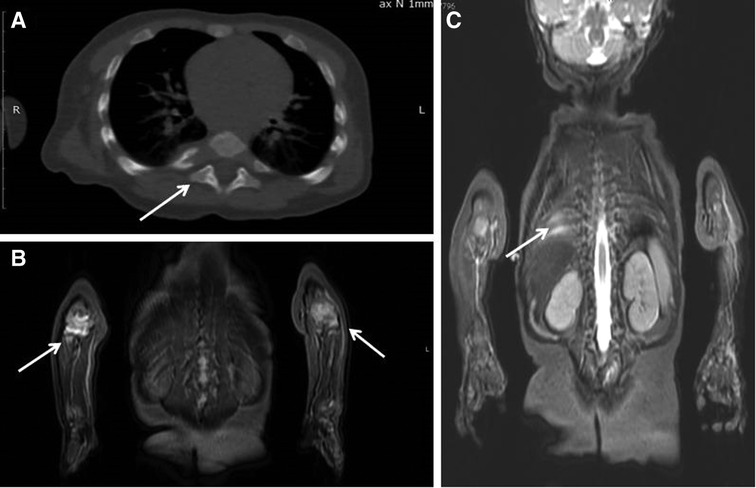
Figure 2. Patient 1: (A) chest CT: the destruction of the head of the 7th right rib (arrow). (B,C) Whole-body MRI (COR STIR sequence) Bone marrow edema of the 8th and 9th right ribs (C), synovitis of both elbows (B).
Whole-exome sequencing was done, which revealed a heterozygous c.2282G > A (R761H) MEFV variant. This variant is characterized as “likely pathogenic”. The results of basic immunology tests (immunoglobulin levels, T-/B-/NK-cell counts, and oxidative burst test) were unremarkable.
The presence of sJIA with MAS, MEFV variant, and failure of previous non-biologic DMARD led to the initiation of canakinumab at a dosage of 4 mg/kg every 4 weeks. When the canakinumab course was under way, the features of the disease in the patient disappeared (Figure 1D), which was proved by normal laboratory test results, following which the dosage of corticosteroids was reduced to 0.125 mg/kg.
Patient 2. A 2-year-old girl developed high spiking fever, rash (flat, non-itchy salmon-pink macules and papules coinciding with fever), arthritis, leukocytosis with neutrophilia, thrombocytosis, increased CRP, ESR, anemia, hyperferritinemia, and increased LDH with a positive effect of systemic corticosteroids. Two months later when the corticosteroids were tapered, she developed a mass lesion of the right anterior chest wall with axillar lymphadenopathy. A repeated chest CT scan revealed a destruction of the 5th right rib with periosteal reaction and an extraosseous mass lesion 25 × 48 × 56 mm under the musculus pectoralis major with foci of contrast uptake (Figure 3). Two surgeries were performed. Specific infections (tuberculosis and mycosis) and malignancy were ruled out. A morphological examination revealed chronic bone inflammation (infiltration with lymphocytes, plasma cells with a small count of neutrophils, and bone sclerosis). A CT revealed rib osteosclerosis. The fever and rash persisted, indicating the poor efficacy of antibiotics. Episodical corticosteroid injections for rash reduced the fever and rash. After 5 months the girl was admitted to our department of immunology and allergology, where she again developed fever and rash, leukocytosis, neutrophilia, and thrombocytosis-increased CRP. Due to possible immunodeficiency, antibiotics were started again, but her condition worsened. She developed a highly persistent non-spiking fever and rash and also a full-blown macrophage activation syndrome—WBC 4.6 × 109/L; platelets 135 × 109/L; Hb 66 g/L, CRP 59 mg/L, AST 105 U/L, LDH 1,263 U/L, ferritin 260 ng/ml, D-dimer 2,486 ng/ml (n.v. < 500), fibrinogen 1.4 g/L). The known immune deficiency was ruled out, and WES did not reveal any relevant findings. Antibiotics were discontinued, and whole-body MRI, chest, and abdominal CT revealed axillar, abdominal, mesenteric, inguinal lymphadenopathy, hepatosplenomegaly, joint effusion, and 5th rib osteosclerosis. There were no lung infiltrates. Due to the inefficacy of antibiotics and the positive effect of corticosteroids administered earlier, anakinra with a dosage of 5 mg/kg/day was initiated, which had good efficacy. In a span of 2 weeks, the patient developed a flare, and in the following 2 weeks, anakinra was switched to canakinumab at a dosage of 4 mg/kg every 4 weeks, which resulted in a complete resolution of the disease. Now she is in remission upon canakinumab treatment.
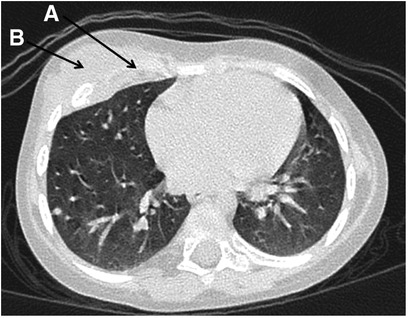
Figure 3. Patient 2: the destruction of the 5th right rib (A) and a mass lesion of the right anterior chest wall (B).
Discussion
Herein, we describe two cases of patients with soJIA with inflammatory bone lesions. To our knowledge, this is the first description of such a combination of two conditions with autoinflammatory mechanisms. Both cases show similarities in terms of young onset age, initial involvement of the plane bones, initial features of sJIA, and efficacy of the IL-1β inhibitor canakinumab. Typical NBO starts in preschool-age children with low systemic inflammation (low-grade fever, low CRP, no leucocytosis, and no thrombocytosis) (2). An extremely young onset age (before 1 year) is usually a hallmark of the monogenic origin of the disease (e.g., DIRA syndrome) or primary immunodeficiency syndrome (chronic granulomatosis disease). The pattern of bone involvement in our patients bore a close resemblance with bone involvement in monogenic autoinflammatory diseases, such as DIRA-syndrome or Majeed syndrome, because of very young onset age and association with systemic inflammation. We feel that “soJIA with inflammatory bone lesions” is the more appropriate term for the diagnosis of our patients, because their bone inflammation does not correspond to classical NBO. Both patients are fit candidates for performing whole-genome sequencing to find the possible candidate genes, due to their young onset age and features, which are similar to those of monogenic autoinflammatory diseases.
The efficacy of IL-1 inhibition serves as an indirect confirmation of clear autoinflammatory mechanisms in the pathogenesis of the diseases (21). The known monogenic forms of NBO - the DIRA syndrome or osteomyelitis, sterile multifocal, with periostitis and pustulosis; OMPP (OMIM: 612852) is a rare inherrited disorder caused by homozygous mutation in the IL!RN (Interleukin 1 receptor antagonist) gene (147679). The loss of IL1RN function results in the activation of Interleukin 1 receptor signaling, and its phenotype resembles chronic non-bacterial osteomyelitis (11). The inhibition of IL-1β signaling with canakinumab limits the progression of sterile osteomyelitis.
Classical NBO usually required TNF-α inhibitors, and the outcomes of anti-IL-1 treatment are limited. After a PubMed search, 27 manuscripts on interleukin-1 antagonists were found, predominantly anakinra (n = 19) and rare canakinumab (n = 7), and one manuscript was discovered on rilonacept in six DIRA patients (22).
The closest to soJIA is adult-onset Still’s disease. It has a similar IL-1 upregulation pathogenesis, which was confirmed by an identical transcriptional profile (23). There is only one published case on the overlapping of adult-onset Still’s disease with chronic recurrent multifocal osteomyelitis with a complete remission of anakinra (24).
The outcomes of anti-IL-1 treatment are predominantly limited to monogenic autoinflammatory diseases such as DIRA or Majeed syndrome (25).
In the biggest case series of anakinra in chronic non-bacterial osteomyelitis, unresponsiveness to NSAIDs and bisphosphonate improvement were achieved (decreasing VAS, ESR, CRP, and number of lesions), but new lesions occurred and some of them were symptomatic (26) In this study, there were no patients who were unresponsive to TNF-α inhibitors before anakinra administration. The data on canakinumab are scarce and limited to seven manuscripts on 10 patients. The cumulative data are presented in Table 1.
Canakinumab showed complete remission in 5/10 (50%) of patients, partial remission in 1/10 (10%), and ineffective remission in 4/10 (40%), with initial short-term efficacy in three children.
Conclusion
This study is the first description of a rare association of soJIA with inflammatory bone lesions with the proven efficacy of IL-1 blockade. The association of two autoinflammatory conditions should indicate IL-1-driven mechanisms and a possible genetic basis. Follow-up genetic and functional studies are required to better understand the pathogenesis of such overlapping diseases.
Data availability statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the manuscript revision, and all of them read and approved the submitted version.
Conflict of interest
EA received grant/research support from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Sanofi, Amgen, and Novartis and participated in Speakers’ bureau sessions for Roche, AbbVie, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer. TD received grant/research support from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Amgen, and Novartis and participated in Speakers’ bureau sessions for Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. King SM, Laxer RM, Manson D, Gold R. Chronic recurrent multifocal osteomyelitis: a noninfectious inflammatory process. Pediatr Infect Dis J. (1987) 6:907–11. doi: 10.1097/00006454-198710000-00009
2. Hofmann SR, Kapplusch F, Girschick HJ, Morbach H, Pablik J, Ferguson PJ, et al. Chronic recurrent multifocal osteomyelitis (CRMO): presentation, pathogenesis, and treatment.. Curr Osteoporos Rep Curr Osteoporos Rep. (2017) 15:542–54. doi: 10.1007/s11914-017-0405-9
3. Brandt D, Sohr E, Pablik J, Schnabel A, Kapplusch F, Mäbert K, et al. CD14 + monocytes Contribute to inflammation in chronic nonbacterial osteomyelitis (CNO) through increased NLRP3 inflammasome expression. Clin Immunol. (2018) 196:77–84. doi: 10.1016/j.clim.2018.04.011
4. Hedrich CM, Hofmann SR, Pablik J, Morbach H, Girschick HJ. Autoinflammatory bone disorders with special focus on chronic recurrent multifocal osteomyelitis (CRMO). Pediatr Rheumatol Online J. (2013) 11(1):47. doi: 10.1186/1546-0096-11-47
5. Hofmann SR, Kubasch AS, Ioannidis C, Rosen-Wolff A, Girschick HJ, Morbach H, et al. Altered expression of IL-10 family cytokines in monocytes from CRMO patients result in enhanced IL-1beta expression and release. Clin Immunol. (2015) 161:300–7. doi: 10.1016/j.clim.2015.09.013
6. Scianaro R, Insalaco A, Bracci Laudiero L, De Vito R, Pezzullo M, Teti A, et al. Deregulation of the IL-1β axis in chronic recurrent multifocal osteomyelitis. Pediatr Rheumatol Online J. (2014) 12:30. doi: 10.1186/1546-0096-12-30
7. Hofmann SR, Kubasch AS, Range U, Laass MW, Morbach H, Girschick HJ, et al. Serum biomarkers for the diagnosis and monitoring of chronic recurrent multifocal osteomyelitis (CRMO). Rheumatol Int. (2016) 36(6):769–79. doi: 10.1007/s00296-016-3466-7
8. Kostik MM, Makhova MA, Maletin AS, Magomedova SM, Sorokina LS, Tsukasaki M, et al. Cytokine profile in patients with chronic non-bacterial osteomyelitis, juvenile idiopathic arthritis, and insulin-dependent diabetes mellitus. Cytokine. (2021) 143:155521. doi: 10.1016/j.cyto.2021.155521
9. Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: a series of 486 cases from the eurofever international registry. Rheumatology. (2018) 57:1504. doi: 10.1093/rheumatology/key143
10. Zhao Y, Wu EY, Oliver MS, Cooper AM, Basiaga ML, Vora SS, et al. Chronic nonbacterial osteomyelitis/chronic recurrent multifocal osteomyelitis study group and the childhood arthritis and rheumatology research alliance scleroderma, vasculitis, autoinflammatory and rare diseases subcommittee (2018) consensus treatment plans for chronic nonbacterial osteomyelitis refractory to nonsteroidal antiinflammatory drugs and/or with active spinal lesions. Arthritis Care Res (Hoboken). (2018) 70(8):1228–37. doi: 10.1002/acr.23462
11. Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. (2009) 360(23):2426–37. doi: 10.1056/NEJMoa0807865
12. Moussa T, Bhat V, Kini V, Fathalla BM. Clinical and genetic association, radiological findings and response to biological therapy in seven children from Qatar with non-bacterial osteomyelitis. Int J Rheum Dis. (2017) 20(9):1286–96. doi: 10.1111/1756-185X.12940
13. Hinze T, Kessel C, Hinze CH, Seibert J, Gram H, Foell D. A dysregulated interleukin-18-interferon-γ-CXCL9 axis impacts treatment response to canakinumab in systemic juvenile idiopathic arthritis. Rheumatology (Oxford). (2021) 60(11):5165–74. doi: 10.1093/rheumatology/keab113
14. Yasin S, Schulert GS. Systemic juvenile idiopathic arthritis and macrophage activation syndrome: update on pathogenesis and treatment. Curr Opin Rheumatol. (2018) 30(5):514–20. doi: 10.1097/BOR.0000000000000526
15. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. (2012) 367(25):2396–406. doi: 10.1056/NEJMoa1205099
16. Yokota S, Itoh Y, Morio T, Origasa H, Sumitomo N, Tomobe M, et al. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann Rheum Dis. (2016) 75(9):1654–60. doi: 10.1136/annrheumdis-2015-207818
17. Giancane G, Papa R, Vastert S, Bagnasco F, Swart JF, Quartier P, et al. Anakinra in patients with systemic juvenile idiopathic arthritis: long-term safety from the pharmachild registry. J Rheumatol. (2022) 49(4):398–407. doi: 10.3899/jrheum.210563
18. Horneff G, Schulz AC, Klotsche J, Hospach A, Minden K, Foeldvari I, et al. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res Ther. (2017) 19(1):256. doi: 10.1186/s13075-017-1462-2
19. Nirmala N, Grom A, Gram H. Biomarkers in systemic juvenile idiopathic arthritis: a comparison with biomarkers in cryopyrin-associated periodic syndromes. Curr Opin Rheumatol. (2014) 26(5):543–52. doi: 10.1097/BOR.0000000000000098
20. Manki A, Nishikomori R, Nakata-Hizume M, Kunitomi T, Takei S, Urakami T, et al. Tumor necrosis factor receptor-associated periodic syndrome mimicking systemic juvenile idiopathic arthritis. Allergol Int. (2006) 55(3):337–41. doi: 10.2332/allergolint.55.337
21. Broderick L, Hoffman HM. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat Rev Rheumatol. (2022) 18(8):448–63. doi: 10.1038/s41584-022-00797-1
22. Garg M, de Jesus AA, Chapelle D, Dancey P, Herzog R, Rivas-Chacon R, et al. Rilonacept maintains long-term inflammatory remission in patients with deficiency of the IL-1 receptor antagonist. JCI Insight. (2017) 2(16):e94838. doi: 10.1172/jci.insight.94838
23. Feist E, Quartier P, Fautrel B, Schneider R, Sfriso P, Efthimiou P, et al. Efficacy and safety of canakinumab in patients with still's disease: exposure-response analysis of pooled systemic juvenile idiopathic arthritis data by age groups. Clin Exp Rheumatol. (2018) 36(4):668–675 .
24. Rech J, Manger B, Lang B, Schett G, Wilhelm M, Birkmann J. Adult-onset still's disease and chronic recurrent multifocal osteomyelitis: a hitherto undescribed manifestation of autoinflammation. Rheumatol Int. (2012) 32(6):1827–9. doi: 10.1007/s00296-011-2020-x
25. Stern SM, Ferguson PJ. Autoinflammatory bone diseases. Rheum Dis Clin North Am. (2013) 39(4):735–49. doi: 10.1016/j.rdc.2013.05.002
26. Pardeo M, Pires Marafon D, Messia V, Garganese MC, De Benedetti F, Insalaco A. Anakinra in a cohort of children with chronic nonbacterial osteomyelitis. J Rheumatol. (2017) 44(8):1231–8. doi: 10.3899/jrheum.160690
27. Herlin T, Fiirgaard B, Bjerre M, Kerndrup G, Hasle H, Bing X, et al. Efficacy of anti-IL-1 treatment in majeed syndrome. Ann Rheum Dis. (2013) 72(3):410–3. doi: 10.1136/annrheumdis-2012-201818
28. Coppola T, Becken B, Van Mater H, McDonald MT, Panayotti GM. A case report of mevalonate kinase deficiency in a 14-month-old female with fevers and lower extremity weakness. BMC Pediatr. (2019) 19(1):245. doi: 10.1186/s12887-019-1617-1
29. Kutukculer N, Puel A, Eren Akarcan S, Moriya K, Edeer Karaca N, Migaud M, et al. Deficiency of interleukin-1 receptor antagonist: a case with late onset severe inflammatory arthritis, nail psoriasis with onychomycosis and well responsive to Adalimumab therapy. Case Reports Immunol. (2019) 2019:1902817. doi: 10.1155/2019/1902817
30. Kuemmerle-Deschner JB, Welzel T, Hoertnagel K, Tsiflikas I, Hospach A, Liu X, et al. New variant in the IL1RN-gene (DIRA) associated with late-onset, CRMO-like presentation. Rheumatology (Oxford). (2020) 59(11):3259–63. doi: 10.1093/rheumatology/keaa119
31. Mendonça LO, Grossi A, Caroli F, de Oliveira RA, Kalil J, Castro FFM, et al. A case report of a novel compound heterozygous mutation in a Brazilian patient with deficiency of interleukin-1 receptor antagonist (DIRA). Pediatr Rheumatol Online J. (2020) 18(1):67. doi: 10.1186/s12969-020-00454-5
Keywords: chronic non-bacterial osteomyelitis 1, juvenile idiopathic arthritis with systemic onset 2, canakinumab 3, autoinflammation 4, chronic recurrent multifocal osteomyelitis 5, interleukine-1 6
Citation: Alexeeva EI, Dvoryakovskaya TM, Tsulukiya IT, Kondrateva NM, Solomatina NM, Kondratiev GV, Peshekhonova LV and Kostik MM (2023) Juvenile idiopathic arthritis with systemic onset with inflammatory bone lesions: two case reports of patients successfully treated with canakinumab and experience gained from literature. Front. Pediatr. 11:1163483. doi: 10.3389/fped.2023.1163483
Received: 10 February 2023; Accepted: 2 May 2023;
Published: 31 May 2023.
Edited by:
Klaus Tenbrock, RWTH Aachen University, GermanyReviewed by:
Henner Morbach, University Hospital Würzburg, GermanyKayoko Tao, National Cancer Center Hospital, Japan
© 2023 Alexeeva, Dvoryakovskaya, Tsulukiya, Kondrateva, Solomatina, Kondratiev, Peshekhonova and Kostik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail M. Kostik bWlraGFpbC5rb3N0aWtAZ21haWwuY29t; a29zdC1taWtoYWlsQHlhbmRleC5ydQ==
 Ekaterina I. Alexeeva1,2,3
Ekaterina I. Alexeeva1,2,3 Irina T. Tsulukiya
Irina T. Tsulukiya Natalia M. Kondrateva
Natalia M. Kondrateva Mikhail M. Kostik
Mikhail M. Kostik