- 1Department of Pediatric Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Pediatric Surgery, Guizhou Children Hospital, Zunyi, China
Splenic abscesses in children are very rare, and multiple splenic abscesses are rarer. These lesions are difficult to diagnose quickly because of their low incidence and the low specificity of the associated clinical and imaging findings. The treatment of splenic abscesses includes conservative treatment, percutaneous drainage, and splenectomy, but the selection criteria for treatment are still unclear. We present a case of a 13-year-old girl with multiple splenic abscesses. Her blood culture report was negative. We eventually confirmed the diagnosis by enhanced magnetic resonance imaging (MRI). The patient underwent a successful laparoscopic total splenectomy, and her symptoms were resolved thereafter.
Introduction
Splenic abscesses are rare but potentially fatal condition. This disease is more common in adults than in children (1) and is mostly unifocal. The most common causative organisms are Enterobacteriaceae, gram-positive cocci, and anaerobic bacteria (2–4). The predominant symptoms are fever, left upper quadrant abdominal pain, nausea and vomiting (5). Herein, we report a case of multiple splenic abscesses in a child and summarize the clinical characteristics, diagnosis and treatment of this disease.
Case
A 13-year-old girl presented with persistent dull pain in the left upper abdomen one week before admission; the pain worsened 1 day prior to admission, accompanied by vomiting. Fever was present during this period, but the specific temperature was unknown. No special treatment was given. The physical examination showed only tenderness in the left upper abdomen and no special conditions in other important organs and systems. Gastrointestinal ultrasonography indicated splenomegaly with multiple dark fluid-filled areas and pelvic effusion. Abdominal contrast-enhanced computed tomography (CT) indicated multiple low-density lesions of the spleen (Figure 1A). The enhanced scan showed annular enhancement, with the largest being 45 × 55 mm (Figure 1B). Vascular tumors or other lesions were considered. Splenomegaly and a small amount of pelvic effusion were also observed. Bone marrow aspiration on the second day after admission showed that the proportion of neutrophils increased with increasing NAP score and decreasing erythron series. Laboratory examination: white blood cell count (WBC) 14.45 × 109/L, neutrophil ratio (NE%) 82%, neutrophil (NE) 11.85 × 109/L, hypersensitive C-reactive protein (CRP) 165.086 mg/L, hemoglobin (HGB) 86 g/L. Female tumor-related antigen examination indicated carbohydrate antigen-125 112 and ferritin 477. After 3 days of cefminox treatment, WBC, NE%, NE and CRP decreased (Figure 2). Since the patient's HGB was low at 72 g/L, 1 unit of WBC-free erythrocyte suspension was given. MRI of the upper abdomen revealed splenomegaly with multiple abscesses and slight perisplenic exudation (Figures 3A,B). Because (i) MRI clearly indicated multiple splenic abscesses, (ii) the anti-inflammatory effect of cefminox was not obvious, and (iii) a fever spike occurred at night (39.1°C), we replaced the antibiotic with cefoperazone sodium and sulbactam sodium and planned to perform surgical treatment as soon as possible. The next day, 6 days after admission, the blood bacterial culture was negative. After HGB rose to 75 g/L, another unit of WBC-free erythrocyte suspension was given. On the 7th day, laboratory examination showed that WBC, NE% and NE had increased slightly, failing to reflect any obvious controlling effect of antibiotics. Based on the results of all examinations, laparoscopic total splenectomy was performed on the 8th day for radical treatment. Splenomegaly (15 × 12 × 8 cm) was observed during the operation. The surface of the spleen was uneven and partially white (Figure 4A), and the spleen was closely adhered to the greater omentum and liver, with edema. After the spleen was completely resected, the splenic tissue was placed into a specimen bag, cut up and removed, and bacterial culture was performed. No bacterial growth was observed. Postoperative pathological findings included chronic suppurative splenic inflammation with focal necrosis, abscess formation, local histiocytic hyperplasia, blood sinus dilatation and hemorrhage, and interstitial fibrous tissue hyperplasia (Figure 4C). The CRP decreased significantly on the first day after surgery. The platelet count (PLT) had been in the normal range before operation and increased rapidly after splenectomy. We believe that this is mainly due to diminished phagocytosis of aged cells after splenectomy. The patient's immunoglobulin M (IgM) level was 0.3 g/L before surgery and decreased to 0.26 g/L after surgery. The levels of the other immunoglobulins remained normal, as did the levels of complement C3 and C4. On the 13th day after admission, the bacterial culture of peritoneal drainage was negative. Hydrothorax ultrasound indicated bilateral pleural effusion. On the 14th day, inflammatory indicators began to recover. On the 18th day, WBC, NE%, NE and CRP completely returned to normal. Epstein‒Barr virus DNA and cytomegalovirus DNA were negative. The pleural effusion was well absorbed, although an ultrasound of pleural effusion in the chest and abdomen indicated some remaining pleural effusion on the left. On the 19th day, a reexamination of the upper abdominal enhanced CT revealed a small amount of fluid/blood in the operative area after splenectomy. The patient was discharged on day 21. We followed the patient up for three months after the operation. The patient was well, with a normal blood routine test and no recurrence of the abscesses.
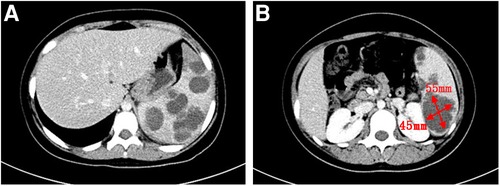
Figure 1. Contrast-enhanced abdominal CT scan. (A): Multiple low-density lesions of the spleen. (B): The largest lesion measures 45 × 55 mm.
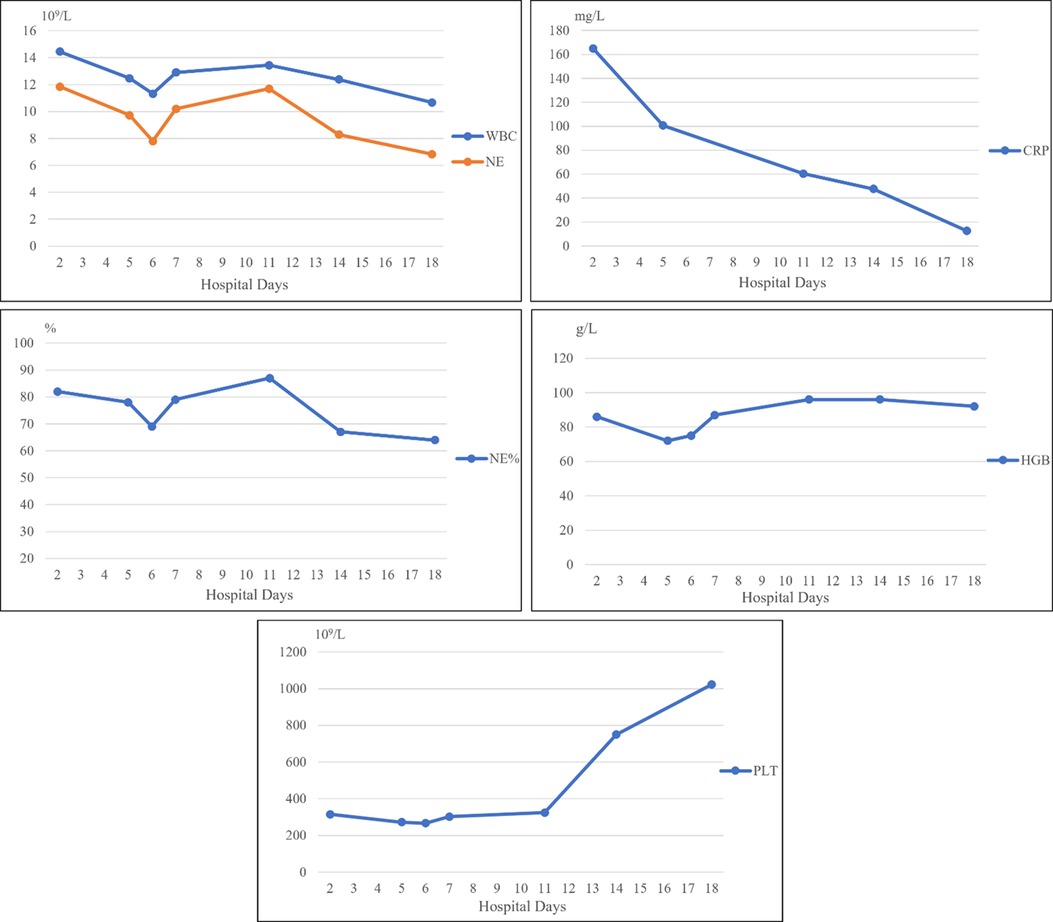
Figure 2. WBC, NE and NE% decreased after antibiotic treatment, increased slightly on the first postoperative day, and then continued to decline to the normal values. CRP continued to decrease after various interventions. These trends demonstrate that our treatment was effective. HGB continued to decline on admission, rebounded after we administered a blood transfusion, and was slightly low after surgery.
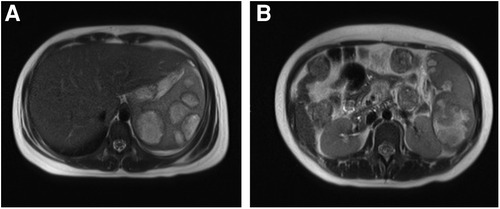
Figure 3. Contrast-enhanced MRI. (A): Splenomegaly with up to 7 abscesses in the same axial plane. (B): The largest abscesses.
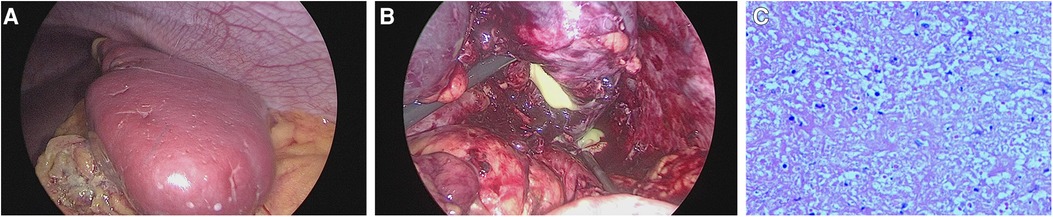
Figure 4. Intraoperative images and histopathology. (A): The spleen was enlarged (15 × 12 × 8 cm), and its surface was uneven and partially white. (B): Pus coming out of the spleen. (C): Chronic suppurative splenic inflammation with focal necrosis, abscess formation, local histiocytic hyperplasia, blood sinus dilatation and hemorrhage, and interstitial fibrous tissue hyperplasia.
Discussion
Splenic abscesses are very rare; a series of autopsy studies have found that their prevalence is 0.2%–0.7% (6, 7). We reviewed some cases of splenic abscesses in children. Clinical, laboratory, imaging, treatment and etiology variables in splenic abscesses in children are summarized (Table 1). Due to the physiological function of the spleen against infection, splenic abscesses are usually associated with other diseases or immunocompromised states, such as splenic trauma, malignant tumors, diabetes, or systemic infection (3). Immunodeficiency is also the main pathogenic factor of splenic abscesses (16). With the increasing incidence of malignant tumors, organ transplantation, immunosuppressive therapy, and AIDS, the risk of splenic abscesses is also gradually increasing (3, 17). However, as these underlying diseases are much less common than in adults, splenic abscesses are rare in children. The onset age of splenic abscesses in children is usually over 10 years old. The most common causes of splenic abscesses in children include secondary infections transmitted through the blood, hematological abnormalities, typhoid fever, and tuberculosis, among others. Infective endocarditis, otitis media and appendicitis are common causes of secondary infections. Among the causative hematological abnormalities, the most common is leukemia, followed by aplastic anemia and anemia of other etiologies (8, 11).
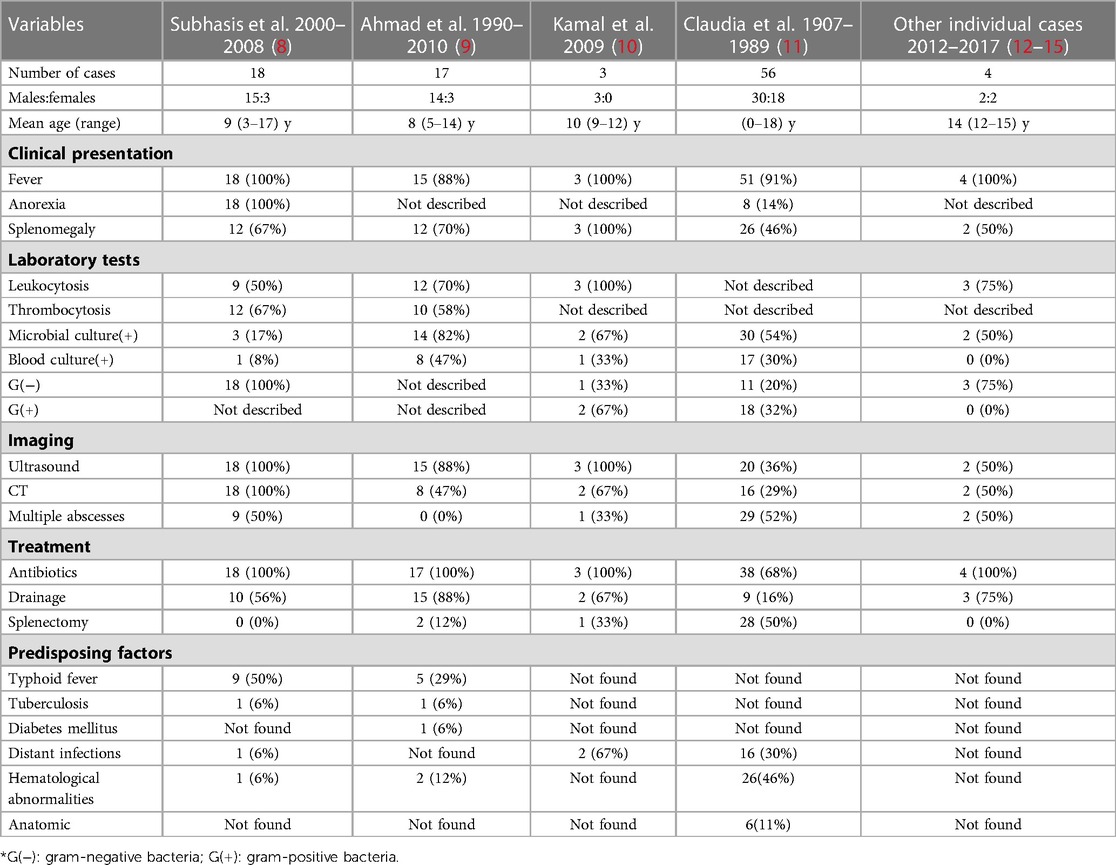
Table 1. Clinical, laboratory, imaging, treatment and etiology variables associated with splenic abscesses in children.
Splenic abscesses have no specific clinical manifestations. Most patients usually seek treatment due to fever and left upper abdominal pain, and their hematological examinations will generally find increased WBC (2, 5, 8, 9, 11). Blood culture is of great significance in clarifying the etiology and diagnosis. It has been reported that 24%–80% of splenic abscesses test positive on blood culture, and multiple abscesses are more likely to be the result of bloodborne transmission, while other routes of transmission mostly cause independent abscesses (2). Aseptic splenic abscesses also exist and are often the first manifestation of inflammatory bowel disease, especially Crohn's disease, with gastrointestinal symptoms (18). Our patient showed no signs of digestive tract inflammation, such as diarrhea, rectal tenesmus or changes in stool characteristics.
Due to the low sensitivity and specificity of clinical symptoms and laboratory markers, imaging plays a crucial role in diagnostic examination (19). Digital x-ray imaging can assist in diagnosis. In some patients, left diaphragmatic elevation, left pleural effusion, and abnormal soft tissue masses in the left upper quadrant can be observed (20), but these signs lack specificity. The sensitivity of abdominal ultrasound for splenic abscesses are estimated to be 75%–93% (4, 19). Most abscesses are oval or round lesions with low or almost no echo, irregular walls, and mild to moderate acoustic enhancement at the distal end, which can be considered somewhat specific (17). Splenic abscesses can occur as a complication of infective endocarditis, which can aggravate the disease and increase mortality (21, 22). Davido et al. proposed that any splenic abscesses found must be examined by cardiac ultrasound to exclude the possibility of endocarditis (2). Infective endocarditis is often characterized by fever, leukocytosis and splenomegaly, a symptom profile resembling that of splenic abscesses, but infective endocarditis develops more rapidly and poses a greater risk. Therefore, we also believe that when splenic abscesses are suspected, echocardiography should be performed to rule out infective endocarditis. It has been reported that the sensitivity of CT for detecting splenic abscesses can reach 100%, with the lesions typically presenting as low-density masses with peripheral enhancement (17). After injection of a contrast agent, uneven enhancement of splenic tissue is observed, and then its parenchyma gradually becomes evenly enhanced (19). Compared with CT, MRI has superior contrast, clarity and multidirectional imaging capabilities. MRI has higher resolution than CT for the surrounding soft tissue and can better judge the situation of lesions invading adjacent tissue. MRI is superior to CT in displaying splenic microabscesses (23). In our case, the initial CT examination did not definitively identify the lesions as abscesses. It was the clear indication on MRI that allowed us to diagnose splenic abscesses conclusively. Ultrasound and MRI are extremely sensitive in the early diagnosis of splenic infection and can be considered suitable early diagnosis methods (19, 24).
It has been suggested in the literature that the etiology of splenic abscesses is unknown in approximately one-third of cases (25); these cases can be considered primary splenic abscesses. However, Michale et al. proposed that primary splenic abscesses do not completely lack specific clinical characteristics (26). The patients they studied were all in good physical condition before onset, with rapid onset, large abscesses, and negative bacterial cultures. No other diseases were found in our case. The patient had no previous surgical history or history of infection, and her physical fitness was at the same level as that of normal children of the same age. A week after the onset of the disease, imaging examination found that the volume of the abscesses had occupied almost the entire spleen, and there were up to seven abscesses at the same level in the scan. After admission, blood culture and virus tests were negative. No other gastrointestinal symptoms were found before or after the course of the disease except vomiting for one day; therefore, this case did not meet the criteria for aseptic splenic abscesses. According to the findings from CT and MRI, we diagnosed the patients with splenic abscesses, a diagnosis that was confirmed during the operation. This case has no clear etiology, which is consistent with the clinical characteristics of primary multiple splenic abscesses as summarized in the literature.
There is no gold standard for the treatment of splenic abscesses, and individual treatment should be tailored to each patient's condition. The general treatment plan is antibiotics first. If there is no obvious improvement, we then choose either abscess drainage or splenectomy according to the patient's condition. To maintain the immune function of the spleen, many experts believe that the optimal treatment for pediatric splenic abscesses is broad-spectrum intravenous antibiotics and early percutaneous drainage, especially for isolated splenic abscesses, which respond well to treatment and do not require splenectomy (8, 9, 27). Although the success rate of splenectomy has been reported to be 86%–94%, it carries risks of surgical drainage and post-splenectomy sepsis and does not preserve splenic tissue which these experts consider very important; on this basis plus the high success rate and low cost of drainage, they believe that percutaneous drainage should be the preferred treatment for splenic abscesses (3, 17, 28). It has been suggested that preservation of the spleen is especially valuable for children with immunocompromised and incomplete immune function (29), and percutaneous drainage should be considered the preferred treatment at this time. However, some experts believe that splenectomy has a better therapeutic effect than percutaneous drainage or antibiotic therapy alone and that intravenous antibacterial therapy combined with splenectomy should be the first choice for the treatment of splenic abscesses (4, 16, 17). When percutaneous drainage fails due to multiple abscesses, tight adhesion around the spleen, or other reasons, a splenectomy or open drainage is needed. Follow-ups from medical centers showed that no patients had surgical complications or sequelae related to their spleen lesions (16). Although there was a slight decrease in our patient's inflammatory indicators after initial antibiotic treatment, the change was not significant, and there was no sign of reduced absorption in the abscesses. There were too many abscesses in our patient, occupying too large a volume in the spleen. Without timely intervention and treatment, there would have been a considerable risk of abscess rupture, leading to aggravation of disease and even death. According to the imaging findings, there was little normal splenic tissue left, and even if puncture drainage were performed, the effect would not be substantially different from that of splenectomy. There was also the risk of incomplete drainage of the abscesses, and the child was 13 years old, with mostly mature immune function; therefore, we directly performed a laparoscopic total splenectomy. After surgery, the child's inflammatory markers quickly returned to normal. No other symptoms appeared, and the patient made a good recovery. She was followed up for three months after the operation, and no physical discomfort was found. A routine blood test and ultrasound showed no obvious abnormalities.
Conclusion
Splenic abscesses in children are rare, and the associated clinical manifestations, laboratory test results and imaging findings are not highly specific. The possibility of splenic abscesses should be considered in the presence of unexplained fever, left upper abdominal pain, palpable abdominal mass, and changes in the imaging features of the spleen. CT may be preferred as a diagnostic aid, as it currently is at many centers, but in cases in which CT is inconclusive, our experience suggests that the addition of MRI is warranted. Blood culture should be carried out as early as possible, and sensitive antibiotics should be taken to block the development of the disease in time. Percutaneous drainage can be considered for a single large splenic abscess, and splenectomy can be considered for older children as well as children with multiple splenic abscesses.
WBC, NE and NE% decreased after antibiotic treatment, increased slightly on the first postoperative day, and then continued to decline to the normal values. CRP continued to decrease after various interventions. These trends demonstrate that our treatment was effective. HGB continued to decline on admission, rebounded after we administered a blood transfusion, and was slightly low after surgery.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-cY wrote the first draft of the manuscript. ZJ helped critically revise the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81760099) and by the Science and Technology Planning Project of Zunyi City, Guizhou Province, China [no. (2019)104].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
References
1. Alvi AR, Kulsoom S, Shamsi G. Splenic abscess: outcome and prognostic factors. J Coll Physicians Surg Pak. (2008) 18(12):740–3.19032885
2. Davido B, Dinh A, Rouveix E, Crenn P, Hanslik T, Salomon J. Splenic abscesses: from diagnosis to therapy. Rev Med Interne. (2017) 38(9):614–8. doi: 10.1016/j.revmed.2016.12.025
3. Liu YH, Liu CP, Lee CM. Splenic abscesses at a tertiary medical center in Northern Taiwan. J Microbiol Immunol Infect. (2014) 47(2):104–8. doi: 10.1016/j.jmii.2012.08.027
4. Chang KC, Chuah SK, Changchien CS, Tsai TL, Lu SN, Chiu YC, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol. (2006) 12(3):460–4. doi: 10.3748/wjg.v12.i3.460
5. Tung CC, Chen FC, Lo CJ. Splenic abscess: an easily overlooked disease? Am Surg. (2006) 72(4):322–5. doi: 10.1177/000313480607200409
6. Reid SE, Lang SJ. Abscess of the spleen. Am J Surg. (1954) 88(6):912–7. doi: 10.1016/0002-9610(54)90449-3
7. Chulay JD, Lankerani MR. Splenic abscess. Report of 10 cases and review of the literature. Am J Med. (1976) 61(4):513–22. doi: 10.1016/0002-9343(76)90331-4
8. Choudhury SR, Debnath PR, Jain P, Kushwaha AS, Puri A, Chadha R, et al. Conservative management of isolated splenic abscess in children. J Pediatr Surg. (2010) 45(2):372–5. doi: 10.1016/j.jpedsurg.2009.10.074
9. Faruque AV, Qazi SH, Arshad M, Anwar N. Isolated splenic abscess in children, role of splenic preservation. Pediatr Surg Int. (2013) 29(8):787–90. doi: 10.1007/s00383-013-3336-2
10. Rattan KN, Kadian YS, Saroha V, Jindal N. Splenic abscess in children: a report of three patients. Afr J Paediatr Surg. (2009) 6(2):106–9. doi: 10.4103/0189-6725.54774
11. Keidl CM, Chusid MJ. Splenic abscesses in childhood. Pediatr Infect Dis J. (1989) 8(6):368–73. doi: 10.1097/00006454-198906000-00009
12. Ahmed S, Oh HB, Kheng D, Krishnan P. Case report of successful partial splenectomy for a splenic abscess in a paediatric patient. Int J Surg Case Rep. (2017) 38:176–9. doi: 10.1016/j.ijscr.2017.07.050
13. Lee HW, Han SB. Large splenic abscess caused by non-typhoidal salmonella in a healthy child treated with percutaneous drainage. Children. (2020) 7(8):88. doi: 10.3390/children7080088.
14. Jordan AJ, Becker KP, Sertemir M, Neff KW, Adam R, Schroten H, et al. Multiple aseptic splenic abscesses in a 15 year old patient. BMC Gastroenterol. (2014) 14:20. doi: 10.1186/1471-230X-14-20
15. Yeom JS, Park JS, Seo JH, Park ES, Lim JY, Park CH, et al. Multiple large splenic abscesses managed with computed tomography-guided percutaneous catheter drainage in children. Pediatr Neonatol. (2013) 54(6):409–12. doi: 10.1016/j.pedneo.2013.01.008
16. Smith MD, Nio M, Camel JE, Sato JK, Atkinson JB. Management of splenic abscess in immunocompromised children. J Pediatr Surg. (1993) 28(6):823–6. doi: 10.1016/0022-3468(93)90336-J
17. Chiang IS, Lin TJ, Chiang IC, Tsai MS. Splenic abscesses: review of 29 cases. Kaohsiung J Med Sci. (2003) 19(10):510–5. doi: 10.1016/S1607-551X(09)70499-1
18. Favarel-Garrigues M, Cadiot G, Bermejo M, Boulagnon-Rombi C, Bani-Sadr F, Hentzien M. Aseptic splenic abscesses as the inaugural manifestation of Crohn's disease. Clin Res Hepatol Gastroenterol. (2022) 46(9):101989. doi: 10.1016/j.clinre.2022.101989
19. Nieciecki M, Kozuch M, Czarniecki M, Mlosek RK, Michno A, Olszewski W, et al. How to diagnose splenic abscesses? Acta Gastroenterol Belg. (2019) 82(3):421–6.31566331
20. Chun CH, Raff MJ, Contreras L, Varghese R, Waterman N, Daffner R, et al. Splenic abscess. Medicine. (1980) 59(1):50–65. doi: 10.1097/00005792-198001000-00003
21. Alnasser SA, Mindru C, Preventza O, Rosengart T, Cornwell L. Successful conservative management of a large splenic abscess secondary to infective endocarditis. Ann Thorac Surg. (2019) 107(4):e235–e7. doi: 10.1016/j.athoracsur.2018.08.065
22. Rasslan R, Alves V, Damous SHB, de Santis A, Tarasoutchi F, Menegozzo CAM, et al. Splenic abscesses in endocarditis: a rare disease with high mortality. The experience of a heart institute in Brazil. J Invest Surg. (2022) 35(11-12):1836–40. doi: 10.1080/08941939.2022.2130481
23. Alabousi A, Patlas MN, Scaglione M, Romano L, Soto JA. Cross-sectional imaging of nontraumatic emergencies of the spleen. Curr Probl Diagn Radiol. (2014) 43(5):254–67. doi: 10.1067/j.cpradiol.2014.04.002
24. Guo H, Wang Y, Yang Y, Liu W. Hepatosplenic brucella abscesses on computed tomography and magnetic resonance imaging: case series. Medicine. (2019) 98(24):e15881. doi: 10.1097/MD.0000000000015881
25. Singh AK, Karmani S, Samanta J, Gupta P, Gupta V, Yadav TD, et al. Splenic abscess in a tertiary care centre in India: clinical characteristics and prognostic factors. ANZ J Surg. (2021) 91(9):1819–25. doi: 10.1111/ans.16517
26. Gelfand M. Primary splenic abscess. Lancet. (1947) 2(6486):904. doi: 10.1016/S0140-6736(47)90913-6
27. Choudhury SR, Rajiv C, Pitamber S, Akshay S, Dharmendra S. Management of splenic abscess in children by percutaneous drainage. J Pediatr Surg. (2006) 41(1):e53–6. doi: 10.1016/j.jpedsurg.2005.10.085
28. Han SP, Galketiya K, Fisher D. Primary splenic abscess requiring splenectomy. ANZ J Surg. (2018) 88(7-8):E623–e4. doi: 10.1111/ans.13537
Keywords: splenic abscess, multiple splenic abscesses, children, laparoscopic total splenectomy, enhanced magnetic resonance imaging
Citation: Yang Y-c, Liu J, Zheng Z-b, Tang C-y, Zhu D-w, Xia X-r, Huang L, Du Q, Hao Y-x, Liu Y-m and Jin Z (2023) Case report: a case of multiple splenic abscesses in a child and literature review. Front. Pediatr. 11:1162527. doi: 10.3389/fped.2023.1162527
Received: 9 February 2023; Accepted: 17 April 2023;
Published: 5 May 2023.
Edited by:
Mario Lima, University of Bologna, ItalyReviewed by:
Katsuhiro Ogawa, Oita University, JapanLin Xiaokun, Wenzhou Medical University, China
Jad Ahmad Degheili, Children's Hospital of Eastern Ontario (CHEO), Canada
© 2023 Yang, Liu, Zheng, Tang, Zhu, Xia, Huang, Du, Hao, Liu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu Jin OTE1ODg0NzAwQHFxLmNvbQ==
 Yingchun Yang
Yingchun Yang Jian Liu1,2
Jian Liu1,2 Zebing Zheng
Zebing Zheng Chengyan Tang
Chengyan Tang Xingrong Xia
Xingrong Xia Lu Huang
Lu Huang Zhu Jin
Zhu Jin