
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 12 April 2023
Sec. Pediatric Orthopedics
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1161421
This article is part of the Research TopicNovel Insights into Syndromic Micrognathia: from Pathogenesis to Clinical ManagementView all 8 articles
 Zhengqiang Cang1,†
Zhengqiang Cang1,† Jiangbo Cui1,†
Jiangbo Cui1,† Jiaomiao Pei1,†
Jiaomiao Pei1,† Zheng Wang2
Zheng Wang2 Yichen Du1
Yichen Du1 Siqi Mu1
Siqi Mu1 Wenjie Dou1
Wenjie Dou1 Xing Fan1*
Xing Fan1* Xi Zhang1*
Xi Zhang1* Yang Li1*
Yang Li1*
Purpose: This systematic review aimed to analyze the characteristics of different diagnostic techniques for micrognathia, summarize the consistent diagnostic criteria of each technique, and provide a simple and convenient prenatal diagnosis strategy for micrognathia.
Methods: In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the search was undertaken in three international databases (PubMed, Scopus, and Web of Science). The three reviewers assessed all papers and extracted the following variables: author's name and year of publication, country, study design, number of participants, gestational age, equipment for prenatal examination, biometric parameters related to micrognathia, main results.
Results: A total of 25 articles included in the analysis. Nineteen articles described cross-sectional studies (76 percent), 4 (16 percent) were case-control studies, and 2 (8 percent) were cohort studies. Fifteen studies (60 percent) had a prospective design, 9 (36 percent) had a retrospective design, and one (4 percent) had both prospective and retrospective design. Thirty-two percent of the studies (n = 8) were performed in USA, and the remaining studies were performed in China (n = 4), Israel (n = 3), Netherlands (n = 3), UK (n = 1), France (n = 1), Italy (n = 1), Belgium(n = 1), Germany (n = 1), Spain (n = 1), and Austria (n = 1). The prenatal diagnosis of micrognathia can be performed as early as possible in the first trimester, while the second and third trimester of pregnancy were the main prenatal diagnosis period. The articles that were included in the qualitative synthesis describe 30 biometric parameters related to the mandible.
Conclusion: Of the 30 biometric parameters related to the mandible, 15 can obtain the simple and convenient diagnostic criteria or warning value for micrognathia. Based on these diagnostic criteria or warning value, clinicians can quickly make a preliminary judgment on facial deformities, to carry out cytologic examination to further clarify the diagnosis of micrognathia.
Micrognathia is a facial malformation characterized by small mandibular size, giving the fetus the appearance of a small jaw and overbite on profile facial views (1–3). As one of the most common craniofacial deformities, micrognathia is usually accompanied by retrognathia, glossoptosis and obstruction of the upper airways, which may greatly damage infants' appearance, complicate infant feeding, and even bring great risk for infant survival (4–12). Feeding problems is the main cause of poor infant growth and development because infants with upper airway obstruction lack the pulmonary reserve necessary to support the additional respiratory effort required for oral feeding, and have higher caloric consumption caused by repeated attempts to clear their upper airway (9, 12). Respiratory problem may cause perinatal emergency because severe upper airway obstruction may lead to hypoxemia in newborns with micrognathia and glossoptosis (7, 8). Prenatal diagnosis of micrognathia is vital to minimize these risks and avoid unprepared emergencies involving invasive interventions (e.g., tracheostomy) as it facilitates triage for delivery at a tertiary care center equipped with experienced management teams for micrognathia, helps clinicians increase their preparedness and take decisions regarding the management plan in advance, and improves family education.
Prenatal diagnosis of micrognathia was initially mainly determined by observing midsagittal ultrasound imaging of the fetal facial profile without using definitive metrics (13, 14). Prenatal ultrasound screening of the facial profile, length, and sagittal position of the mandible most often depends on subjective standards, usually yielding a low and unsatisfying sensitivity (14). For higher sensitivity and accuracy, various objective techniques have been introduced to help better define micrognathia in utero, including the inferior facial angle (IFA), fronto-naso-mental angle (FNMA), mandibular length, jaw index, etc (15–20). However, controversies about simple and efficient methods to evaluate micrognathia still exist, and the diagnostic criterion of specific method for micrognathia lacks consistency in the data. We performed a review of all published studies on prenatal imaging diagnosis of micrognathia to analyze the characteristics of different diagnostic techniques, try to summarize the consistent diagnostic criteria of each technique for micrognathia, and provide a simple and convenient prenatal diagnosis strategy for micrognathia. To the best of our knowledge, this is the first systematic review of prenatal diagnosis of micrognathia.
The literature search was undertaken in three international databases: the PubMed, Scopus, and Web of Science until 2022. The Medical Subject Heading (MeSH) terms “micrognathism”, “pierre pobin syndrom”, and “prenatal diagnosis” and all their entry terms were used. A more detailed search strategy for PubMed is available in Supplementary File S1. Search terms and strategy were translated for use with alternative databases.
Three reviewers (ZQC, JBC and JMP) screened title, abstract, and finally full-text articles against inclusion and exclusion criteria, as shown in Table 1.
After performing a search in the databases, the necessary data were transferred to the Endnote v9 (Clarivate Analytics, Philadelphia, Pa.) for publishing and managing bibliographies. The process of data extraction was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The articles were downloaded from the databases, after which duplicates identified by title and author, were removed. Three reviewers (ZQC, JBC and JMP) screened titles and abstracts of the remaining records. Then, the full-text of relevant articles were read and examined according to the inclusion criteria, to decide whether to include them in the systematic review. Where the three reviewers disagreed, consensus was achieved by discussion with the fourth reviewer (YL). The following information was extracted from each study: author's name and year of publication, country, study design, number of participants, gestational age (GA), equipment for prenatal examination, biometric parameters related to micrognathia, main results. Meta-analysis was not conducted, as the studies were heterogeneous.
Figure 1 shows the flow chart of records selection. A total of 1,150 studies were retrieved through the search queries in all three databases. Following electronic removal of duplicates (n = 418), 732 abstracts were screened independently by the three reviewers (ZQC, JBC and JMP) against the eligibility criteria. After excluding 678 abstracts that did not meet the inclusion criteria, 54 full-text articles were further screened by the same two reviewers. After full-text screening, 29 studies were excluded, leaving a total of 25 articles included in the analysis.
In Table 2, the studies' characteristics are presented. Nineteen articles described cross-sectional studies (76 percent) (15–17, 19–26, 28–33, 36, 40), 4 (16 percent) were case-control studies (34, 35, 37, 39), and 2 (8 percent) were cohort studies (27, 38). Fifteen studies (60 percent) had a prospective design (15–17, 20–28, 30, 31, 36, 40), 9 (36 percent) had a retrospective design (20, 33–35, 37–39), and one (4 percent) had both prospective and retrospective design (32). Thirty-two percent of the studies (n = 8) were performed in USA (21–23, 29, 34, 35, 37, 39), and the remaining studies were performed in China (n = 4) (20, 25, 36, 40), Israel (n = 3) (26, 28, 38), Netherlands (n = 3) (27, 30, 31), UK (n = 1) (15), France (n = 1) (24), Italy (n = 1) (16), Belgium(n = 1) (17), Germany (n = 1) (19), Spain (n = 1) (32), and Austria (n = 1) (33). From 25 studies included in the qualitative synthesis, 14 studies (56 percent) used two-dimensional (2D) ultrasound for prenatal examination of fetuses (15–17, 19–23, 26, 28, 31, 36, 39, 40), 4 (16 percent) used magnetic resonance imaging (MRI) (33–35, 38), 5 (20 percent) used three-dimensional (3D) ultrasound, 1 (4 percent) used both 2D ultrasound and MRI (37), 1 (4 percent) used both 2D and 3D ultrasound (32). The prenatal diagnosis of micrognathia can be performed as early as possible in the first trimester, while the second and third trimester of pregnancy were the main prenatal diagnosis period. The articles that were included in the qualitative synthesis describe 30 biometric parameters related to the mandible, including IFA, FNMA, maxilla-nasion-mandible angle (MNMA), mandibulomaxillary facia angle (MMFA), frontomaxillary/mandibulomaxillary facial angle (FMFA/MMFA), facial maxillary angle (FMA), mandibular protrusion, maxillary/mandibular protrusion, mandible length, anteroposterior diameter (APD), transverse diameter (TD), APD/TD, jaw index, mandible width (MDW), mandible/maxilla width (MDW/MXW), mandibular body length (MBL), chin index, mandibular corpus length, maxillary/mandibular corpus length, chin length, chin/philtrum length, mandibular vertebral length (MVL), lower-facial depth, mid-facial/lower-facial depth, mandibular curvature, maxillary/mandibular curvature, oropharyngeal space (OPS), fetal profile (FP) line, alveolar overjet (AO), mandibular gap.
This review provides a comprehensive overview of longitudinal changes of mandible-related parameters during fetal development and thereby provides references for prenatal diagnosis of fetal micrognathia. The 30 biometric parameters related to the mandible described in the articles in the qualitative synthesis can be divided into three categories, including angles on facial profile, direct measurements of mandibular biometry, and observational characteristics (Table 3).
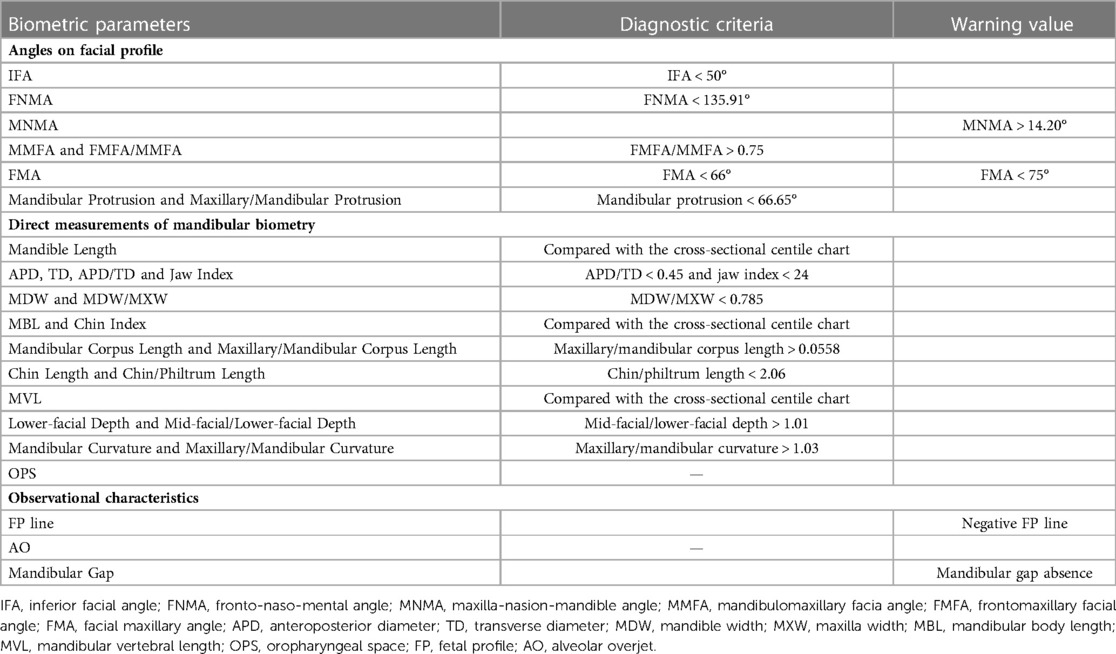
Table 3. The parameters to assess micrognathia using angles on facial profile, direct measurements of mandibular biometry and observational characteristics.
The IFA is measured as the angle between a line vertical to the forehead and a line joining the mentum and protrusive lip (Figure 2A). Of the 25 included studies, 10 measured the IFA of fetuses (19, 20, 24, 33–38, 40). Six of these studies performed the regression analysis between IFA and GA or between IFA and crown rump length (CRL). The study carried out by Li et al. showed a weak positive correlation between IFA and CRL from 11 to 13+6 weeks, and the median IFA was 66.5° with 25th and 75th percentiles of 62.5° and 70.4° (20). The study carried out by Lu et al. showed that the IFA increased slightly with gestation from 16 weeks till approximately 28–31 weeks, at 1° to 2° per week, and decreased minimally thereafter (36). Furthermore, in the study of Lu et al., the minimum value of IFA was not less than 60° (36). The other four studies with regression analysis showed that the IFA was independent of GA and CRL, and the IFA of normal fetuses ranged from 50.3° to 86.1°, suggesting that the normal IFA angle can be determined during pregnancy (24, 33, 38, 40). Overall, the IFA range always exceeded 50° throughout pregnancy. In Luedders et al.'s series of 54 prenatal ultrasound studies of fetuses with micrognathia, the average IFA of fetuses with micrognathia was 44.8° (range 27°-49°) (19). The case-control study carried out by Resnick et al. showed the IFA (<48°) is one of the three independent predictors of PRS (35). Therefore, we recommend that IFA < 50° should be used as the diagnostic criteria for micrognathia during prenatal examination.
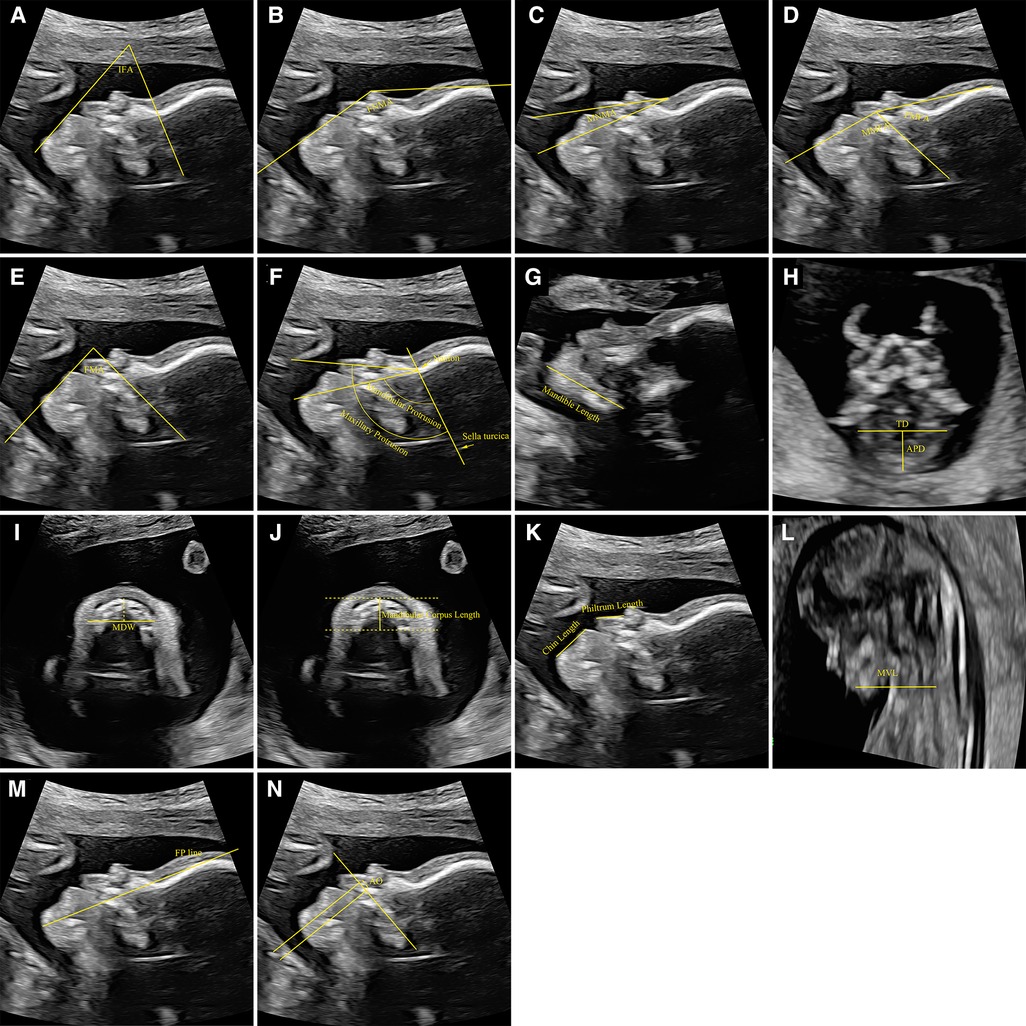
Figure 2. Measurement reference of biometric parameters related to the mandible. (A) The measurement of IFA, (B) the measurement of FNMA, (C) the measurement of MNMA, (D) the measurement of MMFA and FMFA, (E) the measurement of FMA, (F) the measurement of mandibular protrusion and maxillary protrusion, (G) the measurement of mandible length, (H) the measurement of APD and TD, (I) the measurement of MDW, (J) the measurement of mandibular corpus length, (K) the measurement of chin length and philtrum length, (L) the measurement of MVL, (M) the measurement of FP line, (N) the measurement of AO. IFA, inferior facial angle; FNMA, fronto-naso-mental angle; MNMA, maxilla-nasion-mandible angle; MMFA, mandibulomaxillary facia angle; FMFA, frontomaxillary facial angle; FMA, facial maxillary angle; APD, anteroposterior diameter; TD, transverse diameter; MDW, mandible width; MXW, maxilla width; MVL, mandibular vertebral length; FP, fetal profile; AO, alveolar overjet.
The FNMA is measured as the angle between a line from the most prominent bony part of the forehead to the nasal tip and a line from the most anterior point of the soft tissue of the mandible to the tip of the nose (Figure 2B). Of the 25 included studies, 5 measured the FNMA of fetuses (17, 19, 36, 39, 40). Three of these studies performed the regression analysis between FNMA and GA (or CRL). However, the regression relationships in the three studies are completely different, which may be related to the sample size and trimester difference included in the studies. Based on a small sample size of 81 non-randomly selected fetuses from 18 to 35 gestational weeks, Palit et al. proposed a single cut-off of 142° (5th percentile) across all gestation to define micrognathia (17), but 142° is just around the mean at 19 gestational weeks in the study of Lu et al. (36). The reliability of Palit et al. defined cut-off was also questioned by Luedders et al.'s study, in which the measurements of the FNMA in their cases of micrognathia (range 100°-134°) were far below 142°, and even many normal controls fell below 142° (19). In this review, the lower limit value of the normal FNMA is not less than 135.91°. Therefore, we recommend that FNMA < 135.91° should be used as the diagnostic criteria for micrognathia during prenatal examination.
The MNMA is the angle between the lines maxilla-nasion and mandible-nasion in the exact mid-sagittal plane for evaluation of anterior-posterior relationship of the upper and lower jaw. The nasion was defined as the most anterior point at the intersection of the frontal and nasal bones (when there was a little gap between the two of them, the anterior part of the frontal bone at that point was taken) (Figure 2C). Of the 25 included studies, 3 measured the MNMA of fetuses (30, 36, 40). All three studies showed that the MNMA was independent of GA and CRL. Jong-Pleij et al. and Lu et al. proposed that the MNMA can be used in middle and late pregnancy as a useful tool for assessing abnormal facial contours in the fetus, and their results were similar, (mean MNMA was 13.53° and 12.4°, respectively) (30, 36). However, the MNMA measured in the study of Ji et al. from 11 to 13 +6 weeks' gestation was far smaller, with mean only 9.55°±2.84° (5th Percentiles = 4.80°, 95th Percentiles = 14.20°) (40). Such huge gap presumably suggests that it is not easy to measure the MNMA accurately. Therefore, caution should be taken when using the MNMA to assess the micrognathia. Therefore, we recommend that the MNMA > 14.20° should be used as a warning value rather than a diagnostic criteria for micrognathia during prenatal examination.
The upper-anterior corner of the maxilla constitutes the common apex of the frontomaxillary facial angle (FMFA) and MMFA. The first line of the of the FMFA and MMFA coincides and is extended along the upper surface of the palate. The second line of the FMFA is drawn upwards from the anterior aspect of the maxilla at a point where the first line intercepts it. The second arm of the MMFA is drawn downwards and positioned so that the inner aspect of the line was flush with the upper anterior corner of the mandible (Figure 2D). The FMFA provides an objective way of evaluating the position of the maxilla with respect to the fetal forehead, while the MMFA provides an objective method for evaluating the location of the mandible with respect to the maxilla and could be used for early diagnosis of micrognathia. Of the 25 included studies, 2 measured the MMFA and calculated FMFA/MMFA (29, 40). Regression analysis of both studies showed that, in early pregnancy, the MMFA were negatively correlated with CRL while the FMFA/MMFA was independent of CRL. Borenstein et al. reported that the mean MMFA decreased with CRL from 114.5° at a CRL of 45 mm to 103.1° at a CRL of 84 mm (MMFA = 128.1-0.303CRL) with a FMFA/MMFA of 0.74 (29). Ji et al. reported that the mean MMFA ranged from 87° to 101.63° when the CRL was 45–84 mm (MMFA = 118.5–3.75CRL), and the FMFA/MMFA was fixed at 0.75 (40). In the two studies, the normal range of MMFA was wide while the FMFA/MMFA was relatively consistent. It is more reliable and convenient to use the FMFA/MMFA to identify the micrognathia. Therefore, we recommend that the FMFA/MMFA > 0.75 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The FMA is measured as the angle the line overlying the maxilla and a line across the upper lip and mentum tip (Figure 2E). Of the 25 included studies, 2 measured the FMA of fetuses (36, 39). Lu et al.'s study showed that FMA increased slightly with gestation from 16 weeks till approximately 28–31 weeks, at 1° to 2° per week, and decreased minimally thereafter, and the normal range of FMA was narrow with the lower limit value > 75° (36). Bruce et al.'s study showed that FMA was significantly smaller in the PRS group (63.2 ± 9.2°) compared to the control group (74.8 ± 6.1°), and 52% (n = 25) in the PRS group had an abnormally acute FMA (<66°) compared to 2% (n = 1) in the control group (39). Therefore, we recommend using FMA < 75° as the warning value and FMA < 66° as the diagnostic criteria for micrognathia during prenatal examination.
The degree of maxillary and mandibular protrusion was determined by the angle between the sella-nasion and nasion-anterior rims of the maxilla and mandible (Figure 2F). Only one study measured the mandibular protrusion (27). There is no correlation between the mandibular protrusion and GA, whereas the maxillary/mandibular protrusion was negatively correlated to GA. The mean mandibular protrusion was 67.25° (66.65° to 67.86°) (27). Therefore, we recommend that mandibular protrusion < 66.65° should be used as the diagnostic criteria for micrognathia during prenatal examination.
The mandible length was measured as the distance between the cartilaginous symphysis menti and the temporomandibular joint (Figure 2G). Of the 25 included studies, 3 measured the mandible length of fetuses (15, 20, 21). In these studies, the mandible length was plotted against GA and CRL as independent variables to build the growth charts, and was positively correlated with GA and CRL in all three trimesters. Considering that the mandible length changes greatly during pregnancy, it is necessary to compare the mandibular length measured during prenatal examination of the fetus with the cross-sectional centile chart for the mandible length if the mandibular length is used to screen micrognathia.
The TD of the mandible is measured as the distance from one external bone table to the other when the line between these just touched the anterior portion of the fetal hypopharynx. The APD is measured as the distance from the symphysis mentis to the middle of the TD (Figure 2H). The jaw index was then calculated as follows: APD/biparietal diameter × 100. Of the 25 included studies, 5 measured the APD (16, 20, 22, 33, 38) and 3 measured the TD of fetuses (16, 20, 22). All studies showed both APD and TD were positively correlated with GA. Zalel et al. reported that, in healthy fetuses at 11–31 weeks, there was a positive correlation between APD/TD and GA (28). However, the mean APD/ TD had a small range of 0.66 to 0.76. Li et al. reported that, in healthy fetuses at 11–13+6 weeks, the mean APD/TD was 0.56 with 5th and 95th percentiles of 0.45 and 0.75, while, the mean APD/TD in fetuses with micrognathia was <0.42 and significantly lower compared to healthy fetuses (20). The jaw index was calculated in 3 studies. In the study of Paladini et al., the mean jaw index was 32.2 with 5th and 95th percentiles of 24 and 41, and the index allowed a clear separation between micrognathic and normal fetuses (16). Only one fetus with micrognathia had a jaw index greater than 21, and only two fetuses without micrognathia had a jaw index less than 22 (16). The studies carried out by Nemec et al. and Kooiman et al. are consistent with Paladini et al. (33, 34). Considering that APD and TD changes greatly throughout pregnancy, we recommend that APD/TD < 0.45 and jaw index < 24 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The MDW and maxilla width (MXW) were measured on an axial plane caudal to the base of the cranium, at the level of the alveolus (dental arch). A line orthogonal to the sagittal axis was drawn 10 mm posteriorly to the anterior osteous border (approximately at the level of the canines). Measurements were obtained from one external bone table to the other (Figure 2I). The MDW/MXW was derived from these two measurements. Only one study measured the MDW and calculated the MDW/MXW (24). Both MDW and MXW were positively correlated with GA over the 18–28 gestational week period. The MDW/MXW was constant between 18 and 28 gestational weeks, and the mean value was 1.017 ± 0.116. In the study of Rotten et al., the mean ± 2 SD interval defined the normal population. Therefore, we recommend that MDW/MXW < 0.785 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The MBL is measured as the distance between the mandibular angles. The ratio of BPD vs. MBL was defined to be the chin index of the fetus. Only one study measured the MBL and calculated the chin index (25). The MBL was positively correlated with GA, whereas the chin index was negatively correlated to GA. In other words, the chin grows wider with the advancement of GA. Consequently, the subjective facial features change from a reverse triangular shape in early weeks, to an oval or square shape in later weeks. Considering that MBL and chin index changes greatly throughout pregnancy, it is necessary to compare the MBL and chin index measured during prenatal examination of the fetus with the cross-sectional centile chart for the MBL and chin index if the MBL and chin index are used to screen micrognathia during prenatal examination.
The mandibular corpus length was represented by the anterior-posterior border of the mandible, which was extended to the frontal rim of the mandibula-gonion (Figure 2J). Only one study measured the mandibular corpus length (27). The mandibular corpus length was positively correlated with GA, whereas the maxillary/mandibular corpus length was negatively correlated to GA. At 18–34 weeks of pregnancy, the mandibular corpus length changed greatly whereas the maxillary/mandibular corpus length had a small range of 0.0254 to 0.0558. Therefore, we recommend that the maxillary/mandibular corpus length > 0.0558 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The length of the philtrum was measured from the apex of the philtrum to the lower margin of the upper lip. The length of the chin was measured from the tip of the lower lip to the apex of the chin (Figure 2K). Of the 25 included studies, 2 measured the chin length and 1 calculated the chin/philtrum length (23, 26). Both the chin length and philtrum length were positively correlated with GA. Gull et al. reported the chin/philtrum length as a function of gestational age was relatively constant at 13–42 weeks of pregnancy, and was best described by the linear equation y = 0.0206x + 1.7931 with a range of 2.06–2.66. Therefore, we recommend that the chin/philtrum length < 2.06 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The MVL is measured as the distance between the mentum and the anterior vertebral line (Figure 2L). Only one study measured the MVL (38). The MVL was positively correlated with GA and had a wide range. It is necessary to compare the MVL measured during prenatal examination with the cross-sectional centile chart for the MVL if the MVL is used to screen micrognathia.
The mid-facial and lower facial depths were determined by the tragus-anterior rim of the maxilla and tragus-gnathion. Only one study measured the lower-facial depth (27).
Throughout pregnancy, the lower facial depths were positively correlated with GA and had a wide range, whereas the mid-facial/lower-facial depth was negatively correlated to GA and relatively constant with a range of 0.95–1.01. Therefore, we recommend that the mid-facial/lower-facial depth > 1.01 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The maxillary and mandibular curvature was represented by the curvature from the tragus-anterior rim of the maxilla, multiplied by two, and the curvature from the tragus-gnathion, multiplied by two. Only one study measured the mandibular curvature (27). Throughout pregnancy, the mandibular curvature was positively correlated with GA and changed greatly, whereas the maxillary/mandibular curvature was negatively correlated to GA and relatively constant with a range of 1.00–1.03. Therefore, we recommend that the maxillary/mandibular curvature > 1.03 should be used as the diagnostic criteria for micrognathia during prenatal examination.
The OPS was measured as the distance between the anterior and posterior walls of the oropharynx along a line connecting soft tissue overlying most concave point of mandibular symphysis mentis) and the tongue base located at the intersection of the base of tongue and epiglottis. In the study of Kooiman et al., the OPS was significantly smaller in the RPS group (4.3 ± 2.3 mm) than in the micrognathia group (5.8 ± 2.0 mm) and control group (7.1 ± 1.7 mm) (34). The OPS was also significantly smaller in the micrognathia group than in the control group. However, there is no cross-sectional study on the changes of OPS during pregnancy.
The FP line is a line that passes through the midpoint of the anterior border of the mandible and the nasion (Figure 2M). When the FP line passes lengthwise through the frontal bone, its position is defined as “zero”; when FP line passes the frontal bone anteriorly, its position is defined as negative; when the FP line passes the frontal bone posteriorly, its position is defined as positive. Of the 25 included studies, 3 measured the FP line (31, 36, 40). Jong-Pleij et al. reported that most seen was an FP line with position zero, and the positive FP line never occurred before 27 weeks’ gestation at 16–36 weeks of pregnancy (31). According to study carried out by Lu et al., the prevalence of the negative FP line decreased from 7.1% to 0% by 24 weeks and the positive type was observed from 22 weeks (36). The transition from the negative type to the positive type may result in a false positive result at early second trimester. For all that, the negative FP line should still be regarded as a warning value for micrognathia during prenatal examination.
To determine the AO, the maxillary plane was used as an easy to identify landmark. Two lines perpendicular to the maxilla were then created; the first aligned with the most anterior portion of the maxillary alveolar process (1), and the second aligned with the most anterior portion of the mandibular alveolar process (2). The distance between (1) and (2) was then measured as the alveolar overjet (AO) (Figure 2N). Only one study observed the AO (39). In the study of Bruce et al., the PRS group demonstrated significantly larger AO compared to the control group, 3.9 ± 1.4 mm and 2.1 ± 0.9 mm, respectively. However, there is no cross-sectional study on the AO during pregnancy.
The mandibular gap was measured from the midpoint of the echogenic edge of one mandibular bone to the other. Two study observed the mandibular gap (32, 40). In the study of Sepulveda et al., the mandibular gap was identified in all normal fetuses (32). In the study of Ji et al. 99.4% fetuses were observed the presence of the obvious mandibular gap, and 0.6% fetuses were observed the presence of the narrow mandibular gap (40). The absence of the mandibular gap should be regarded as a warning value for micrognathia during prenatal examination.
Most of the facial profile angles are independent of GA, or have a weak positive correlation with GA with a small changing amplitude throughout pregnancy. By comparing the value of these facial profile angles with corresponding diagnostic criterion or warning value in our review, they can be directly used as a reference for the diagnosis of micrognathia. In this review, a total of 5 facial profile angles can be directly used for the prenatal diagnosis of micrognathia, including IFA < 50°, FNMA < 135.91°, MNMA > 14.20°, FMA < 66°, Mandibular protrusion < 66.65°, while only one facial profile angle (MMFA) needs to be calculated as a ratio (FMFA/MMFA) to evaluate indirectly the mandibular abnormality, because the normal range of MMFA is wide and the FMFA/MMFA is relatively consistent. Direct measurements of mandibular biometry usually have a significant correlation with GA with a big changing amplitude throughout pregnancy. They are unavailable for the diagnosis of micrognathia unless compared with the cross-sectional centile chart, or calculated as a ratio such as APD/TD < 0.45, jaw index < 24, MDW/MXW < 0.785, maxillary/mandibular corpus length > 0.0558, chin/philtrum length < 2.06, mid-facial/lower-facial depth > 1.01, maxillary/mandibular curvature > 1.03. Observational characteristics can quickly indicate the presence of micrognathia, and are the most concise biometric parameters. There are two observational characteristics that can be used as warning value for micrognathia, such as the negative FP line and mandibular gap absence. Therefore, we recommend the observational characteristics and facial profile angles as the main diagnostic reference and direct measurements of mandibular biometry as the secondary diagnostic reference for the prenatal diagnosis of micrognathia.
In the included studies, only one evaluated the association between IFA measurements on ultrasound and MRI (37). There is a moderate positive correlation between IFA measurements on ultrasound and MRI. Nguyen et al. believed that a postnatal diagnosis of Robin sequence whose defining features is the micrognathia may be predictable by prenatal MRI (37). MR imaging has shown superiority over conventional sonographic methods in assessing fetal facial structures, as it is of high diagnostic value in cases of limited acoustic window (e.g., maternal obesity, oligohydramnios, and anterior spine position) (41). MRI, however, is a limited resource and requires recognition of risk factors to indicate referral. Ultrasound is a simple measurement equipment that indicates a high risk of micrognathia and prompts a confirmatory MRI study which may improve the availability and reliability of prenatal diagnosis of micrognathia.
In this review, four studies are from China, and the rest are from countries with Caucasian ethnic distribution. However, the limited sample size cannot be used to analyze the impact of ethnic groups on biometric parameters related to the mandible. Further research may be required to clarify the impact of ethnic groups on biometric parameters related to the mandible.
In conclusion, 30 biometric parameters related to the mandible measured from ultrasound or MRI images have been proposed for screening micrognathia. Among these parameters, 15 can obtain the simple and convenient diagnostic criteria or warning value for micrognathia, including the IFA < 50°, FNMA < 135.91°, MNMA > 14.20°, FMFA/MMFA > 0.75, FMA < 66°, mandibular protrusion < 66.65°, APD/TD < 0.45, jaw index < 24, MDW/MXW < 0.785, maxillary/mandibular corpus length > 0.0558, chin/philtrum length < 2.06, mid-facial/lower-facial depth > 1.01, maxillary/mandibular curvature > 1.03, negative FP line, mandibular gap absence. Based on these diagnostic criteria or warning value, clinicians can quickly make a preliminary judgment on facial deformities, to carry out cytologic examination to further clarify the diagnosis of micrognathia.
YL, XZ and XF conceived and designed the study. ZC, JC and JP conducted the literature search. ZC, ZW, YD, SM, and WD performed data extraction. ZC, YL and XZ conducted the literature quality assessment. ZC, JC and JP drafted the manuscript with the help of all the other authors. YL and XF reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Natural Science Foundation of Shaanxi Province (2021JM-239).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fped.2023.1161421/full#supplementary-material.
1. Vettraino IM, Lee W, Bronsteen RA, Harper CE, Aughton D, Comstock CH. Clinical outcome of fetuses with sonographic diagnosis of isolated micrognathia. Obstet Gynecol. (2003) 102(4):801–5. doi: 10.1016/s0029-7844(03)00672-0
2. Chen Q, Zhao Y, Qian Y, Lu C, Shen G, Dai J. A genetic-phenotypic classification for syndromic micrognathia. J Hum Genet. (2019) 64(9):875–83. doi: 10.1038/s10038-019-0630-4
3. Zhen L, Yang YD, Xu LL, Cao Q, Li DZ. Fetal micrognathia in the first trimester: an ominous finding even after a normal array. Eur J Obstet Gynecol Reprod Biol. (2021) 263:176–80. doi: 10.1016/j.ejogrb.2021.06.031
4. Hsieh ST, Woo AS. Pierre robin sequence. Clin Plast Surg. (2019) 46(2):249–59. doi: 10.1016/j.cps.2018.11.010
5. Logjes RJH, Haasnoot M, Lemmers PMA, Nicolaije MFA, van den Boogaard MH, Mink van der Molen AB, et al. Mortality in robin sequence: identification of risk factors. Eur J Pediatr. (2018) 177(5):781–9. doi: 10.1007/s00431-018-3111-4
6. Breik O, Umapathysivam K, Tivey D, Anderson P. Feeding and reflux in children after mandibular distraction osteogenesis for micrognathia: a systematic review. Int J Pediatr Otorhinolaryngol. (2016) 85:128–35. doi: 10.1016/j.ijporl.2016.03.033
7. Sadakah AA, Elshall MA, Farhat AA. Bilateral intra-oral distraction osteogenesis for the management of severe congenital mandibular hypoplasia in early childhood. J Craniomaxillofac Surg. (2009) 37(4):216–24. doi: 10.1016/j.jcms.2008.10.014
8. Hong P, Bezuhly M. Mandibular distraction osteogenesis in the micrognathic neonate: a review for neonatologists and pediatricians. Pediatr Neonatol. (2013) 54(3):153–60. doi: 10.1016/j.pedneo.2012.11.018
9. Mathew OP, Clark ML, Pronske ML, Luna-Solarzano HG, Peterson MD. Breathing pattern and ventilation during oral feeding in term newborn infants. J Pediatr. (1985) 106(5):810–3. doi: 10.1016/s0022-3476(85)80363-2
10. Dinwiddie R. Congenital upper airway obstruction. Paediatr Respir Rev. (2004) 5(1):17–24. doi: 10.1016/j.prrv.2003.10.001
11. Hong P, Bezuhly M, Mark Taylor S, Hart RD, Kearns DB, Corsten G. Tracheostomy versus mandibular distraction osteogenesis in Canadian children with pierre robin sequence: a comparative cost analysis. J Otolaryngol Head Neck Surg. (2012) 41(3):207–14. doi: 10.2310/7070.2012.00016
12. Hong P, Brake MK, Cavanagh JP, Bezuhly M, Magit AE. Feeding and mandibular distraction osteogenesis in children with pierre robin sequence: a case series of functional outcomes. Int J Pediatr Otorhinolaryngol. (2012) 76(3):414–8. doi: 10.1016/j.ijporl.2011.12.023
13. Hsieh YY, Chang CC, Tsai HD, Yang TC, Lee CC, Tsai CH. The prenatal diagnosis of pierre-robin sequence. Prenat Diagn. (1999) 19(6):567–9. doi: 10.1002/(SICI)1097-0223(199906)19:6%3C567::AID-PD581%3E3.0.CO;2-P
14. Kruse T, Neuschulz J, Wilhelm L, Ritgen J, Braumann B. Prenatal diagnosis of robin sequence: sensitivity, specificity, and clinical relevance of an Index for micrognathia. Cleft Palate Craniofac J. (2021) 58(8):1012–9. doi: 10.1177/1055665620972297
15. Chitty LS, Campbell S, Altman DG. Measurement of the fetal mandible–feasibility and construction of a centile chart. Prenat Diagn. (1993) 13(8):749–56. doi: 10.1002/pd.1970130809
16. Paladini D, Morra T, Teodoro A, Lamberti A, Tremolaterra F, Martinelli P. Objective diagnosis of micrognathia in the fetus: the jaw index. Obstet Gynecol. (1999) 93(3):382–6. doi: 10.1016/s0029-7844(98)00414-1
17. Zalel Y, Shapiro I, Weissmann-Brenner A, Berkenstadt M, Leibovitz Z, Bronshtein M. Prenatal sonographic features of triploidy at 12-16 weeks. Prenat Diagn. (2016) 36(7):650–5. doi: 10.1002/pd.4834
18. Kaufman MG, Cassady CI, Hyman CH, Lee W, Watcha MF, Hippard HK, et al. Prenatal identification of pierre robin sequence: a review of the literature and Look towards the future. Fetal Diagn Ther. (2016) 39(2):81–9. doi: 10.1159/000380948
19. Luedders DW, Bohlmann MK, Germer U, Axt-Fliedner R, Gembruch U, Weichert J. Fetal micrognathia: objective assessment and associated anomalies on prenatal sonogram. Prenat Diagn. (2011) 31(2):146–51. doi: 10.1002/pd.2661
20. Li H, Zhu Z. Ultrasonographic study of fetal mandibular markers during the first trimester in a Chinese population. J Obstet Gynaecol Res. (2022) 48(2):333–9. doi: 10.1111/jog.15121
21. Otto C, Platt LD. The fetal mandible measurement: an objective determination of fetal jaw size. Ultrasound Obstet Gynecol. (1991) 1(1):12–7. doi: 10.1046/j.1469-0705.1991.01010012.x
22. Watson WJ, Katz VL. Sonographic measurement of the fetal mandible: standards for normal pregnancy. Am J Perinatol. (1993) 10(3):226–8. doi: 10.1055/s-2007-994725
23. Sivan E, Chan L, Mallozzi-Eberle A, Reece EA. Sonographic imaging of the fetal face and the establishment of normative dimensions for chin length and upper lip width. Am J Perinatol. (1997) 14(4):191–4. doi: 10.1055/s-2007-994125
24. Rotten D, Levaillant JM, Martinez H, Ducou le Pointe H, Vicaut E. The fetal mandible: a 2D and 3D sonographic approach to the diagnosis of retrognathia and micrognathia. Ultrasound Obstet Gynecol. (2002) 19(2):122–30. doi: 10.1046/j.0960-7692.2001.00622.x
25. Tsai MY, Lan KC, Ou CY, Chen JH, Chang SY, Hsu TY. Assessment of the facial features and chin development of fetuses with use of serial three-dimensional sonography and the mandibular size monogram in a Chinese population. Am J Obstet Gynecol. (2004) 190(2):541–6. doi: 10.1016/j.ajog.2003.07.031
26. Gull I, Wolman I, Merlob P, Jaffa AJ, Lessing JB, Yaron Y. Nomograms for the sonographic measurement of the fetal philtrum and chin. Fetal Diagn Ther. (2005) 20(2):127–31. doi: 10.1159/000082436
27. Roelfsema NM, Hop WC, Wladimiroff JW. Three-dimensional sonographic determination of normal fetal mandibular and maxillary size during the second half of pregnancy. Ultrasound Obstet Gynecol. (2006) 28(7):950–7. doi: 10.1002/uog.3866
28. Zalel Y, Gindes L, Achiron R. The fetal mandible: an in utero sonographic evaluation between 11 and 31 weeks’ gestation. Prenat Diagn. (2006) 26(2):163–7. doi: 10.1002/pd.1363
29. Borenstein M, Persico N, Strobl I, Sonek J, Nicolaides KH. Frontomaxillary and mandibulomaxillary facial angles at 11+0 to 13+6 weeks in fetuses with trisomy 18. Ultrasound Obstet Gynecol. (2007) 30(7):928–33. doi: 10.1002/uog.5188
30. de Jong-Pleij EA, Ribbert LS, Manten GT, Tromp E, Bilardo CM. Maxilla-nasion-mandible angle: a new method to assess profile anomalies in pregnancy. Ultrasound Obstet Gynecol. (2011) 37(5):562–9. doi: 10.1002/uog.7768
31. de Jong-Pleij EA, Ribbert LS, Pistorius LR, Tromp E, Bilardo CM. The fetal profile line: a proposal for a sonographic reference line to classify forehead and mandible anomalies in the second and third trimester. Prenat Diagn. (2012) 32(8):797–802. doi: 10.1002/pd.3904
32. Sepulveda W, Wong AE, Viñals F, Andreeva E, Adzehova N, Martinez-Ten P. Absent mandibular gap in the retronasal triangle view: a clue to the diagnosis of micrognathia in the first trimester. Ultrasound Obstet Gynecol. (2012) 39(2):152–6. doi: 10.1002/uog.10121
33. Nemec U, Nemec SF, Brugger PC, Weber M, Bartsch B, Bettelheim D, et al. Normal mandibular growth and diagnosis of micrognathia at prenatal MRI. Prenat Diagn. (2015) 35(2):108–16. doi: 10.1002/pd.4496
34. Kooiman TD, Calabrese CE, Didier R, Estroff JA, Padwa BL, Koudstaal MJ, et al. Micrognathia and oropharyngeal space in patients with robin sequence: prenatal MRI measurements. J Oral Maxillofac Surg. (2018) 76(2):408–15. doi: 10.1016/j.joms.2017.07.163
35. Resnick CM, Kooiman TD, Calabrese CE, Zurakowski D, Padwa BL, Koudstaal MJ, et al. An algorithm for predicting robin sequence from fetal MRI. Prenat Diagn. (2018) 38(5):357–64. doi: 10.1002/pd.5239
36. Lu J, Sahota DS, Poon LC, Ting YH, Cheng YKY, Wang Y, et al. Objective assessment of the fetal facial profile at second and third trimester of pregnancy. Prenat Diagn. (2019) 39(2):107–15. doi: 10.1002/pd.5371
37. Nguyen JQN, Calabrese CE, Heaphy KJ, Koudstaal MJ, Estroff JA, Resnick CM. Can robin sequence be predicted from prenatal ultrasonography? J Oral Maxillofac Surg. (2020) 78(4):612–8. doi: 10.1016/j.joms.2019.10.015
38. Toren A, Spevac S, Hoffman C, Katorza E. What does the normal fetal face look like? MR imaging of the developing mandible and nasal cavity. Eur J Radiol. (2020) 126:108937. doi: 10.1016/j.ejrad.2020.108937
39. Bruce MK, Islam R, Anstadt EE, Kenkre TS, Pfaff MJ, Canavan T, et al. Mandibular measurements at the 20-week anatomy ultrasound as a prenatal diagnostic predictor of pierre robin sequence. Cleft Palate Craniofac J. (2023) 60(3):352–8. doi: 10.1177/10556656211064771
40. Ji X, Han Z, Wu Y, Zhang S. Study on normal reference value of fetal facial profile markers during the first trimester of pregnancy. Transl Pediatr. (2022) 11(1):99–107. doi: 10.21037/tp-21-573
Keywords: micrognathia, fetus, mandible, biometric parameters, prenatal diagnosis
Citation: Cang Z, Cui J, Pei J, Wang Z, Du Y, Mu S, Dou W, Fan X, Zhang X and Li Y (2023) Prenatal diagnosis of micrognathia: a systematic review. Front. Pediatr. 11:1161421. doi: 10.3389/fped.2023.1161421
Received: 8 February 2023; Accepted: 28 March 2023;
Published: 12 April 2023.
Edited by:
Zhiyong Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Shanbaga Wurjihu, Chinese Academy of Medical Sciences and Peking Union Medical College, China© 2023 Cang, Cui, Pei, Wang, Du, Mu, Dou, Fan, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Fan ZmFueGluZy42MTJAMTYzLmNvbQ== Xi Zhang amVzc2lpQDE2My5jb20= Yang Li bGl5YW5nenhAZm1tdS5lZHUuY24=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Orthopedics, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.