
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 06 November 2023
Sec. Pediatric Cardiology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1154015
This article is part of the Research Topic Pediatric Exercise Medicine in Congenital and Acquired Heart Diseases View all 6 articles
 L. E. Scheffers1,2,3,4
L. E. Scheffers1,2,3,4 W. A. Helbing1
W. A. Helbing1 T. Pereira1
T. Pereira1 S. Walet5
S. Walet5 E. M. W. J. Utens6,7,8
E. M. W. J. Utens6,7,8 K. Dulfer9
K. Dulfer9 L. E. van den Berg10*
L. E. van den Berg10*  on behalf of the Rotterdam Exercise Team
on behalf of the Rotterdam Exercise Team
Introduction: Children and adolescents with a Fontan circulation are less physically active compared to healthy peers. In the current study, effects of a 12-week lifestyle intervention on fatigue, fears regarding exercise, caloric intake, rest energy expenditure (REE), and body composition were measured in children with a Fontan circulation.
Methods: This study was a semi-cross-over randomized controlled trial. The lifestyle intervention consisted of a 12-week high-weight resistance training (three supervised training sessions a week) supported by high-protein diet (>2 g/kg) and tailored recommended caloric intake. Fatigue (measured by the validated PedsQol Multidimensional Fatigue Scale), fears regarding exercise (measured on a fear thermometer), REE (measured using indirect calorimetry), caloric intake and body composition using air displacement plethysmography, and four-skinfold method were measured before and after the intervention and control period.
Results: Twenty-seven pediatric Fontan patients, median age 12.9 years (IQR: 10.5–16.2), of the included 28 patients successfully completed the program. Before training, both child- and parent-reported levels of fatigue were significantly worse on all domains (general, sleep/rest, and cognitive fatigue) compared to healthy peers. After training, parent-reported fatigue significantly improved on the general and cognitive fatigue domains [effect size +16 points (7–25), p < 0.001, and +10 points (2–17), p = 0.015, compared to the control period]. Before training, fear regarding exercise scored on the fear thermometer was low for both children and parents (median score 1 and 2, respectively, on a scale of 0–8). After training, child-reported fear decreased further compared to the control period [effect size −1.4 points (−2.3 to −0.6), p = 0.001]. At baseline, children had increased REE +12% compared to reference values, which did not change after exercise. Children ate an average of 637 calories below recommended intake based on REE, caloric deficit became smaller after the intervention, and protein intake increased compared to the control period [−388 calories (−674 to −102), p = 0.008, and +15 g (0.4–30), p = 0.044]. Body fat percentage did not change significantly.
Conclusion: A 12-week lifestyle intervention improved parent-reported fatigue symptoms in the children, further decreased child-reported fears, and increased caloric and protein intake.
Treatment of patients born with a single functional ventricle consists of a series of operations, leading to a Fontan circulation (1). Due to improvements in pre-, peri-, and post-operative care, this population is growing, and an increasing number of patients reach adulthood. In adulthood, increased risk of obesity and acquired cardiovascular disease in this population becomes more important (1–4). Regular physical activity is one of the most important contributors to prevent aforementioned morbidities, and also has numerous other health benefits, including higher energy levels and less fatigue (5). Several studies investigating physical activity levels in children with a Fontan circulation showed decreased physical activity levels and sports participation compared to healthy peers (6, 7). Multiple reasons for lower sports participation in children with congenital heart diseases (CHDs) have been identified, including fatigue, parental (and sport instructors) overprotection, fear regarding exercise, and (unnecessary) restricting physician recommendations (8–10). The study by Moola et al. found that fatigue, fear, and exclusion (not being able to perform on the same levels as healthy peers) further decreased the value children with a congenital heart disease ascribed to sport and physical activity (10). A systematic review published by our group showed that physical training in Fontan patients may lead to improvements in physical health and quality of life, with none of the included studies reporting negative outcomes related to the exercise programs (11). Interestingly, only one study investigated training effects on fears and none looked at exercise effects on fatigue, an important limiting factor in chronically ill children (12–14). We, therefore, investigated the effects of a lifestyle intervention on fatigue, fears regarding exercise, rest energy expenditure (REE), nutritional intake, and body composition in children with a Fontan circulation.
This was a semi-cross-over randomized controlled trial investigating effects of a 12-week lifestyle intervention on children with a Fontan circulation. This trial was conducted between December 2020 and July 2022 at the Department of Pediatric Cardiology, Erasmus MC Sophia Children’s Hospital, Netherlands. The study was performed in accordance with the Declaration of Helsinki. It was approved by the local Ethics Committee of Erasmus MC (NL.70912.078.19) and registered at the Dutch trial register (https://trialsearch.who.int/Trial2.aspx?TrialID=NL8181) as Trial NL8181. A detailed protocol of this study has been published previously (15).
Children with univentricular heart defects, who had a completed series of interventions resulting in the Fontan circulation, aged 6–18 years, were eligible for enrollment. Physical inability to perform a cardiopulmonary exercise test (CPET), participation in other exercise training programs, and contra-indications for exercise (a ventricular obstruction of >60 mmHg and arrhythmias) were exclusion criteria. All patients were recruited at the outpatient clinic of the Erasmus MC Sophia Children's Hospital. Signed informed consent was obtained from all participants and/or parents before enrollment.
Figure 1 shows the study design, visits, and measurements. Randomization was performed in blocks of four or six using Castor (Clinical electronic Data Capture) (16). All children were randomized in group A (start exercise) or group B (start control period, duration 6 weeks). Group A started the intervention immediately after the first assessment, and group B started after a period of 6 weeks during which they received “care as usual” (thus this 6 weeks period of group B serves as the control period for all patients with a 2:1 ratio). Each study visit consisted of two assessment days with at least 3 days and maximally 7 days in between during which patients continued normal daily life. The tailored lifestyle intervention was designed as previously described in our trial design paper (15). The tailored individual physical training program lasted 12 weeks and consisted of three supervised training sessions per week (supervision performed by a trained physiotherapist close to participant's home) lasting 45 min each. The standardized training program started with 10 min of walking on a treadmill with maximal incline, rowing, or cross trainer exercise, where after children performed progressive overload resistance training focused on the leg muscles. Each exercise was performed in three sets of six to eight repetitions. Exercises included squats, (single) standing/seated calf raises, hip trusts, step-ups, leg press, leg abduction, and leg adduction. The full training program can be found out in other study (17). In order to support muscle growth, patients received a high-protein diet of 2 g/kg per day and a recommended caloric intake per day, total energy expenditure (TEE) (calculated using the Schofield formula and based on measured REE and corrected for weight) including a brochure regarding healthy diet in children (designed by the “Voedingscentrum,” the Dutch government–supported nutritional center) (18). LES visited all first training sessions and a training (either live or via video connection) of each patient every 2 weeks, to monitor uniform execution of the training program. During the intervention, LES telephoned patients weekly to monitor safety, side effects, and assure compliance for both the training and dietary advice.
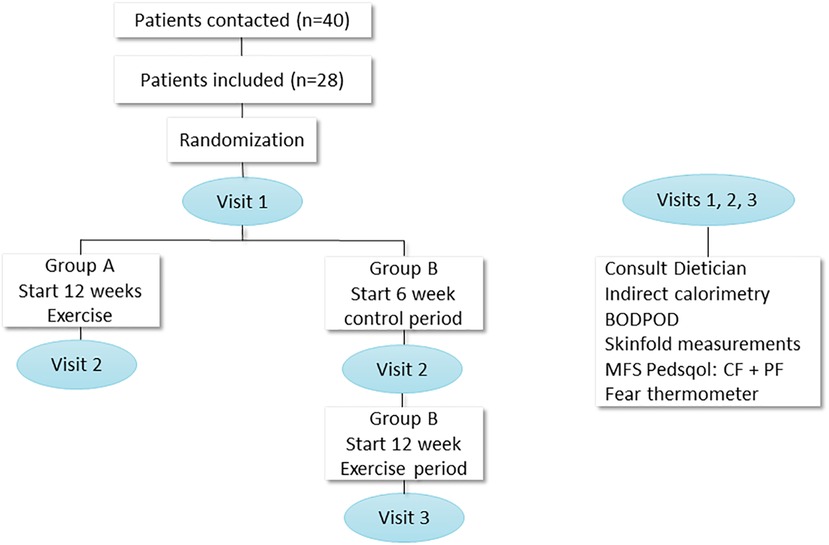
Figure 1. Study design with measurements. N, number; PedsQol, pediatric quality of life; MFS, multifatigue scale; CF, child form, PF, parent form; BODPOD, body composition measurement system.
The outcomes of the 12-week lifestyle intervention were fatigue, fear regarding exercise, and nutritional status (body composition, REE, and caloric intake).
The PedsQL Multidimensional Fatigue Scale (MFS) age-specific child form (CF) and parent form (PF), consisting of 18 items, divided into three domains, were used to assess fatigue in children/adolescents, with a higher score indicating less fatigue symptoms (19). Baseline outcomes were compared to previously published data in healthy Dutch children (n = 366) and their parents (n = 497) (20).
Children and parents had individual semi-structured interviews with a psychologist. They were asked to score fear regarding exercise on the fear anxiety thermometer, 0 (no fears at all) up to 8 (high anxiety) (Supplementary Data S1) (14).
At each visit, patients height and weight were measured, and body composition was assessed using a skinfold caliper (four skinfolds method) and air displacement plethysmography (ADP) on whole-body densitometry using the BOD POD (BOD POD body composition system, COSMED, Ltd, Concord, CA, USA). Body fat percentage (%BF) was calculated using the equations published by Brook, Drunin, Rahaman, and Wormsley et al. for the skinfold measurements, and the Lohman equation for the ADP (21–24). Measured body fat percentages using the skinfold method were compared with a large European reference group (25). As no large reference value studies using BOD POD currently exist in children, fat percentage measured by BOD POD were compared to body fat reference curves for children of McCarthy et al. (26). All patients filled in a detailed food diary for three consecutive days, which was checked by the dietician SW and used to calculate caloric intake and protein intake. Children underwent an indirect calorimetry (Quark-RMR, Cosmed, Italy) performed by the dietician. Measured REE was compared to predicted REE using the Schofield equation (27), whereas REE > 1.1 was defined is hypermetabolism and REE < 0.9 was defined as hypometabolism. TEE is calculated by multiplying measured REE with factors such as activity and growth (28).
Power calculation for this study was based on the primary study outcome of change in peak VO2 after the physical training program, and 28 patients with a Fontan circulation were included in this study (15). Data were collected in Castor (Clinical electronic Data Capture) (16), and all analyses were performed using IBM SPSS Statistics 25.0 (IBM Corp, Armonk, NY, USA). Patient characteristics were described using descriptive statistics. Baseline characteristics between groups were compared with the Mann–Whitney U and χ2 test for proportions. All data were analyzed as non-parametric due to the small sample size. Difference between baseline fatigue domains and healthy population were analyzed using the Wilcoxon one sample test. Differences over the exercise period and control period were analyzed using the Wilcoxon signed ranks test for proportions. A generalized equation approach model was used to compare change over the control to the exercise period [described as the effect size including 95% confidence interval (CI) and matching p-value] accounting for the correlation of the repeated measurements in the control/active group. The working correlation matrix was set as unstructured. The significance level was determined at p < 0.05.
In total, 40 patients were contacted to participate in the study (17). Reasons not to participate were too busy with school obligations (n = 6), parents were unable to bring the child to the hospital/physical therapist (n = 3), private circumstances (n = 1), already playing sports >3 times a week (n = 1), and one patient was scheduled for an operation. A total of 28 patients were included in the study, and 27 patients successfully completed the exercise intervention. The median age of the included patients was 12.9 (10.5–16.2) years, and 37% were female. Patient characteristics can be found in Table 1. Baseline echocardiograms showed a good systolic function in 15 and a moderate systolic function in the other patients. Atrioventricular valve insufficiency was mild in most patients (n = 13), moderate in four patients, and trivial or not present in the other patients. Three patients had mild aortic valve insufficiency, and other children did not have aortic valve insufficiency. Children showed no signs of arrhythmia at baseline.
Compliance was high, with a median training session attendance of 33 (32–34) out of 36 sessions. In total, 27 patients completed the exercise intervention. One patient dropped out after the first training session, as his parents were unable to bring him to the training sessions.
Before training, self- and parent-reported levels of fatigue in children/adolescents were significantly worse on all domains (general, sleep/rest, and cognitive fatigue) compared to healthy children (Table 2) (20). After training, parent-reported fatigue significantly improved on the general fatigue and cognitive fatigue domains compared to the control period [+16 points (7–25), p < 0.001, and +10 (2–17), p = 0.015]. Self-reported fatigue scores did not significantly improve after training.
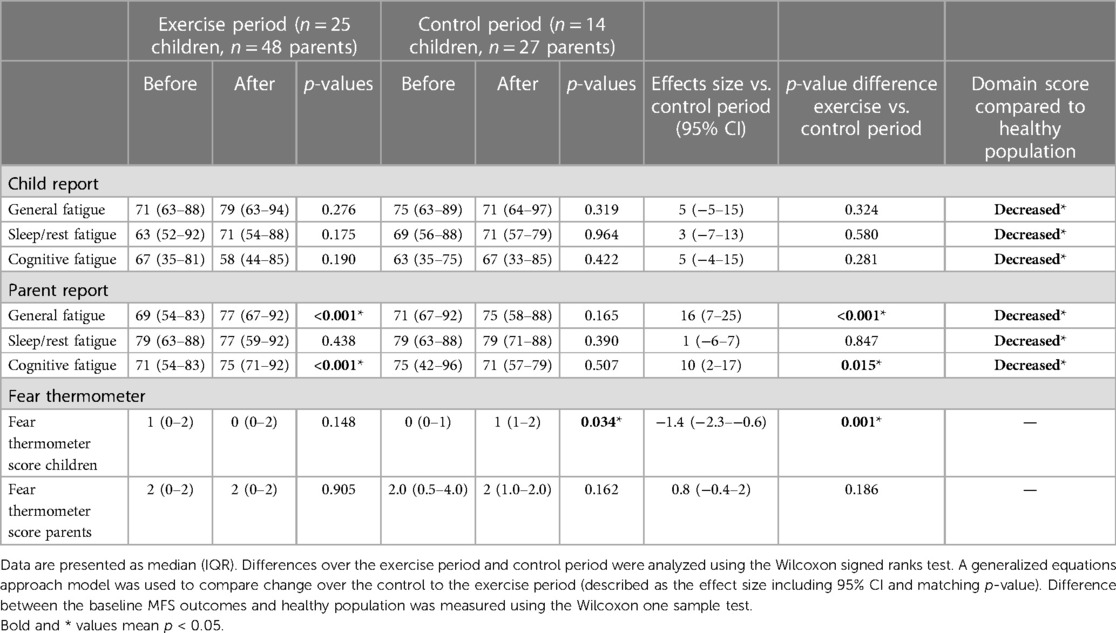
Table 2. Fatigue measured using the multidimensional fatigue scale and fear of exercise measured using the fear thermometer.
Before training, parents and children obtained low scores on the fear thermometer [1 (0–2) and 2 (0–2), respectively, on a scale from 0 to 8] (Table 2). After training, child-reported fear decreased significantly compared to the control period [effects size −1.4 points decrease (−2.3 to −0.6), p = 0.001].
Fontan patients showed normal growth compared to healthy children (Table 3). Body fat percentage did not change after the intervention (Table 3). Body fat percentage measured using the skinfold methods was lower compared to fat percentage measured using the BOD POD [9.1% (6.7–12.9) vs. 16.5% (13.3–22.5)]. At baseline, children had increased REE +12% compared to reference values, which did not change significantly after exercise. Based on measured REE, almost all children did consume too little calories, on average 637 below recommended TEE (=82% of recommended intake). After training, the caloric deficit became smaller and protein intake increased compared to the control period [−388 calories deficit (−674 to −102) = 89% of recommended intake, p = 0.008, and +15 g (0.4–30), p = 0.044, n = 19, as 8 children did not complete their food diary after training].
This study is the first to investigate the effects of a lifestyle intervention, in a—for the population of patients with a Fontan circulation—relatively large group of children/adolescents with a Fontan circulation on fatigue, fear of exercise, and nutritional status.
Although fatigue is widely investigated in children with various chronic diseases, remarkably few studies have assessed fatigue in children with CHD. We could only identify two previous studies, of which one is recently preprinted (version 1, research square): Vos et al. measured fatigue in 259 Dutch children with various CHD's using the same questionnaire as we did (29). In this study, 42% of the included single ventricle patients (n = 33) reported to experience fatigue (calculated by a deviation of at least 1 SD from healthy peers) (29). In the study by Bektas et al., 18% of children with a CHD (including seven Fontan patients) reported to have moderate to severe fatigue (30). In our study before training, both self and parent reports demonstrated higher levels of fatigue compared to healthy children on all domains (20). After training, two parent-reported domains improved significantly compared to the control period. The discrepancy between the parent and child reports might in part be explained by a lack of power in the child forms (parent reports n = 48, child reports n = 25). Interestingly, the preprinted study of de Vos et al. showed that only the number of previous surgeries and Watt/kg and VO2/kg measured during cardiopulmonary exercise testing significantly correlated with the level of fatigue in children with CHD (29). After our intervention, both Watt/kg and VO2/kg improved significantly, possibly contributing to the improved parent-reported fatigue.
Fear of exercise on the anxiety thermometer was already low for both children and parents before the intervention and decreased even more in children after the intervention compared to the control period. Children may have been nervous to start the exercise intervention or maybe became more aware of the impact of their disease on sports competencies by participating in the study. The only other study measuring the effects of training on fear regarding exercise in patients with a CHD also found a significant reduction in anxiety regarding exercise among adolescents (14).
Recently, evidence regarding unfavorable body composition (including high body fat percentages together with a decreased muscle mass) in both pediatric and adult Fontan patients is rising (31–34). Several studies found associations between unfavorable body composition in Fontan patients and adverse outcomes, including decreased exercise capacity and cardiac failure (35). In our study, children had a normal median body mass index (BMI) and body fat percentage measured by BOD POD indicating a lean to moderately lean posture compared to healthy peers (26, 33). Fat percentage measured by fourfold skinfold indicated a body fat percentage below the 50th percentile (25). After training, body fat percentage (and thus fat-free percentage) remained unchanged, which is in contrast to the large improvements we measured in strength of the children. Possibly assessment techniques for body composition were not sensitive enough to measure subtle changes in body composition (as might be expected during a period of only 12 weeks), and sufficient statistical power to measure these small differences is lacking. Also, other body composition measurement techniques such as dual-energy x-ray absorptiometry might be more sensitive; however, this technique is also more expensive and time consuming (36, 37). In addition, currently future studies could include nutritional biomarkers related to muscle mass and fat mass (such as adipocytokines, myokines) to measure exercise effects on nutritional status. Besides some small studies investigating REE in very young Fontan patients, aged 3.6 ± 2.6, immediately after Fontan surgery, no studies have reported upon REE in older Fontan patients before (38). This is remarkable, since it is well known that malnutrition (undernourishment) is a large problem in this population. Malnutrition may lead to longer hospital stays, higher mortality rates, and even worse neurodevelopmental and growth outcomes in the long term (39–42). A recent study by Sekhon et al. even showed that moderate to severe malnutrition is still present in 6.5%–12.9% of patients 10 years after the Fontan completion (43). Measured REE in children included in our study was increased compared to reference values (median 112% of predicted); simultaneously, children consumed a median of 649 calories (260–992) below their predicted TEE, increasing the caloric deficit further. After the intervention, median REE did not change compared to the control period. However, average caloric intake increased (thus caloric deficit decreased) by more than half compared to the control period and amount of protein (g) increased significantly, indicating that patients followed the dietary advice. We could not identify previous studies prescribing a (personalized) diet advice or even investigating the gap between intake and (recommended) TEE in Fontan children who passed the age of 6 years. Patients should be more closely monitored and more research is needed to determine the added value of proper nutritional management in this group, preventing large consequences of malnutrition on various health outcomes later in life.
Our study has several strengths and limitations. This study is the first to investigate the effects of a lifestyle intervention in a relatively large group of children with a Fontan circulation. Occasionally, investigated outcomes in Fontans were studied including fear regarding exercise, fatigue, and nutritional status. A weakness of our study is the small control group, of 14 patients. Also, researchers in the study could not be blinded during measurements due to the content of the study. Although we found a significant decrease in caloric deficit and increase in protein intake, indicating that patients followed the dietary advice, we cannot verify this with certainty. In addition, eight patients did not fill in the food diary properly (before or after) the intervention, leading to a smaller sample size. This is the first study performing the innovative and non-invasive whole-body densitometry using the BOD POD in Fontan patients to measure body composition. Although research investigating the BOD POD in adult patients claims high test–retest reliability, we occasionally saw large intra-patient differences in patients with mostly stable weight, BMI, and body fat percentage measured by the skinfold method (44, 45).
Overall the 12-week lifestyle intervention positively impacted participating children and their parents. Most children (and their parents) reported not to feel any hesitation to exercise (and let their child exercise) after the intervention. Parents reported their children to be more energetic, and also the children themselves stated that daily life activities including walking stairs and cycling to school took less effort. Besides positive effects on fear and fatigue, many parents reported the tailored diet advice to be very helpful, as most were unaware of the (unintended) caloric deficits of their children. Although measured body composition did not change significantly, children started to feel more muscular after the resistance training in comparison to before the intervention, which was consistent with our own clinical observations. When considering all results, we think pediatric cardiologists should actively encourage children with a Fontan circulation to participate in resistance training (or other fitting sports), and importantly more attention should be paid to proper nutritional management.
This study is the first to investigate the effects of a 12-week supervised lifestyle intervention in a relatively large group of children with a Fontan circulation on fatigue, fear, and nutritional status. The intervention improved parent-reported fatigue, decreased child-reported fear, and had positive impacts on caloric and protein intake.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by METC Erasmus MC Sophia's Children Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
LS, EU, WH, KD, LB, and the Rotterdam exercise team had the main role in the research protocol design. LS, LB, and SW performed all measurements. LS and TP did the statistical analyses. LS drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the “Stichting Vrienden van Sophia” (grant no. B19-01).
We would like to thank all patients for participating in our trial and all physiotherapists for training the patients; Merron Beyene, Emiel Samson, and Leah Dennebos for their contribution to the measurements and data management; Rizopoulos for his statistical advice; and K. F. M. Joosten for letting us use the BODPOD. The remaining members of the Rotterdam Exercise Team: J. C. Escher, M. W. Pijnenburg, A. T. van der Ploeg, J. Noske, A. van den Broek, and J. Olieman.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1154015/full#supplementary-material
1. van der Ven JPG, van den Bosch E, Bogers A, Helbing WA. State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Res. (2018) 7:F1000 Faculty Rev-935. doi: 10.12688/f1000research.13792.1
2. Plappert L, Edwards S, Senatore A, De Martini A. The epidemiology of persons living with Fontan in 2020 and projections for 2030: development of an epidemiology model providing multinational estimates. Adv Ther. (2022) 39(2):1004–15. doi: 10.1007/s12325-021-02002-3
3. Olsen M, Marino B, Kaltman J, Laursen H, Jakobsen L, Mahle W, et al. Myocardial infarction in adults with congenital heart disease. Am J Cardiol. (2017) 120(12):2272–7. doi: 10.1016/j.amjcard.2017.08.050
4. Pemberton VL, McCrindle BW, Barkin S, Daniels SR, Barlow SE, Binns HJ, et al. Report of the national heart, lung, and blood institute’s working group on obesity and other cardiovascular risk factors in congenital heart disease. Circulation. (2010) 121(9):1153–9. doi: 10.1161/CIRCULATIONAHA.109.921544
5. Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. (2019) 6:69. doi: 10.3389/fcvm.2019.00069
6. McCrindle BW, Williams RV, Mital S, Clark BJ. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. (2007) 92(6):509–14. doi: 10.1136/adc.2006.105239
7. Callegari A, Faeth K, Pfammatter C, Jung R, Berger F, Burkhardt B, et al. Physical activity in Fontan patients relates to quality of life and sleep quality. Front Cardiovasc Med. (2022) 9:915810. doi: 10.3389/fcvm.2022.915810
8. Longmuir PE, Corey M, McCrindle BW. Interactions with home and health environments discourage physical activity: reports from children with complex congenital heart disease and their parents. Int J Environ Res Public Health. (2021) 18(9). doi: 10.3390/ijerph18094903
9. van Deutekom AW, Lewandowski AJ. Physical activity modification in youth with congenital heart disease: a comprehensive narrative review. Pediatr Res. (2021) 89(7):1650–8. doi: 10.1038/s41390-020-01194-8
10. Moola F, Faulkner GE, Kirsh JA, Kilburn J. Physical activity and sport participation in youth with congenital heart disease: perceptions of children and parents. Adapt Phys Activ Q. (2008) 25(1):49–70. doi: 10.1123/apaq.25.1.49
11. Scheffers LE, Berg L, Ismailova G, Dulfer K, Takkenberg JJM, Helbing WA. Physical exercise training in patients with a Fontan circulation: a systematic review. Eur J Prev Cardiol. (2020) 28(11):1269–78. doi: 10.1177/2047487320942869
12. Nap-van der Vlist MM, Dalmeijer GW, Grootenhuis MA, van der Ent K, van den Heuvel-Eibrink MM, Swart JF, et al. Fatigue among children with a chronic disease: a cross-sectional study. BMJ Paediatr Open. (2021) 5(1):e000958.33665374
13. Dulfer K, Duppen N, Blom NA, van Dijk APJ, Helbing WA, Verhulst FC, et al. Effect of exercise training on sports enjoyment and leisure-time spending in adolescents with complex congenital heart disease: the moderating effect of health behavior and disease knowledge. Congenit Heart Dis. (2014) 9(5):415–23. doi: 10.1111/chd.12154
14. Dulfer K, Duppen N, Blom NA, Van Domburg RT, Helbing WA, Verhulst FC, et al. Effects of exercise training on behavioral and emotional problems in adolescents with tetralogy of Fallot or a Fontan circulation: a randomized controlled trial. Int J Cardiol. (2014) 172(3):e425–7. doi: 10.1016/j.ijcard.2013.12.244
15. Scheffers LE, Helbing WA, Utens E, Dieleman GC, Dulfer K, Noske J, et al. Study protocol of the exercise study: unraveling limitations for physical activity in children with chronic diseases in order to target them with tailored interventions-A randomized cross over trial. Front Pediatr. (2021) 9:791701. doi: 10.3389/fped.2021.791701
16. Castor Electronic Data Capture (2019). Available at: https://data.castoredc.com/. (Accessed January 1, 2022).
17. Utens EMWJ, Dulfer K, Hirsch A, Koopman LP, van den Berg LE. Rotterdam Exercise Team. Leg-focused high-weight resistance training improves ventricular stroke volume, exercise capacity and strength in young patients with a Fontan circulation. Eur J Prev Cardiol. (2023):zwad286. doi: 10.1093/eurjpc/zwad286
18. Stichting Voedingscentrum Nederland (2021). Available at: https://www.voedingscentrum.nl/nl.aspx (Accessed December 1, 2021).
19. Varni JW, Burwinkle TM, Szer IS. The PedsQL multidimensional fatigue scale in pediatric rheumatology: reliability and validity. J Rheumatol. (2004) 31(12):2494–500. PMID: 15570657.
20. Gordijn M, Cremers EM, Kaspers GJ, Gemke RJ. Fatigue in children: reliability and validity of the Dutch PedsQL™ multidimensional fatigue scale. Qual Life Res. (2011) 20(7):1103–8. doi: 10.1007/s11136-010-9836-9
21. Brook CG. Determination of body composition of children from skinfold measurements. Arch Dis Child. (1971) 46(246):182–4. doi: 10.1136/adc.46.246.182
22. Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. (1974) 32(1):77–97. doi: 10.1079/BJN19740060
23. Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. (1967) 21(3):681–9. doi: 10.1079/BJN19670070
24. Lohman TG, Going SB. Body composition assessment for development of an international growth standard for preadolescent and adolescent children. Food Nutr Bull. (2006) 27(4 Suppl Growth Standard):S314–25. doi: 10.1177/15648265060274S512
25. Nagy P, Kovacs E, Moreno LA, Veidebaum T, Tornaritis M, Kourides Y, et al. Percentile reference values for anthropometric body composition indices in European children from the IDEFICS study. Int J Obes. (2014) 38(2):S15–25. doi: 10.1038/ijo.2014.131
26. McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes. (2006) 30(4):598–602. doi: 10.1038/sj.ijo.0803232
27. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. (1985) 39(Suppl 1):5–41.4044297
28. van den Akker CHP. Eiwitten. In Joosten KFM, van Waardenburg D, Kneepkens CMF, editors. Werkboek voeding voor zieke kinderen (2edr ed.). VU University Press (2017). Available at: https://issuu.com/hinke5/docs/werkboek_voeding_def
29. de Vos DRH, Hoefnagels JW, Vlist MMNd, Breur JMPJ, Nijhof SL. Prevalence of fatigue in children with congenital heart disease and correlations with disease-specific factors, 21 November 2022, PREPRINT (Version 1). Researchsquare (2022).
30. Bektas İ, Kır M, Yıldız K, Genç Z, Bektas M, Ünal N. Symptom frequency in children with congenital heart disease and parental care burden in predicting the quality of life of parents in Turkey. J Pediatr Nurs. (2020) 53:e211–6. doi: 10.1016/j.pedn.2020.04.012
31. Cordina R, Meagher SO, Gould H, Rae C, Kemp G. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Comp Stud Heart. (2013) 99(20):1530–4. doi: 10.1136/heartjnl-2013-304249
32. Payne E, Garden F, d’Udekem Y, McCallum Z, Wightman H, Zannino D, et al. Body mass index trajectory and outcome post Fontan procedure. J Am Heart Assoc. (2022) 11(18):e025931. doi: 10.1161/JAHA.122.025931
33. Powell AW, Wittekind SG, Alsaied T, Lubert AM, Chin C, Veldtman GR, et al. Body composition and exercise performance in youth with a Fontan circulation: a bio-impedance based study. J Am Heart Assoc. (2020) 9(24):e018345. doi: 10.1161/JAHA.120.018345
34. Tran D, D’Ambrosio P, Verrall C, Attard C, Briody J, D’Souza M, et al. Body composition abnormalities in adults with a Fontan circulation. Heart Lung Circul. (2019) 28:S148–9. doi: 10.1016/j.hlc.2019.06.038
35. Cao JY, Tran D, Briody J, Attard C, Hassan EB, Simm P, et al. Impact of adiposity on clinical outcomes in people living with a Fontan circulation. Int J Cardiol. (2021) 329:82–8. doi: 10.1016/j.ijcard.2020.12.066
36. Branski LK, Norbury WB, Herndon DN, Chinkes DL, Cochran A, Suman O, et al. Measurement of body composition in burned children: is there a gold standard? JPEN J Parenter Enteral Nutr. (2010) 34(1):55–63. doi: 10.1177/0148607109336601
37. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. (2017) 104:101–5. doi: 10.1016/j.bone.2017.06.010
38. Mehta NM, Costello JM, Bechard LJ, Johnson VM, Zurakowski D, McGowan FX, et al. Resting energy expenditure after Fontan surgery in children with single-ventricle heart defects. J Parenter Enter Nutr. (2012) 36(6):685–92. doi: 10.1177/0148607112445581
39. Baldini L, Librandi K, D’Eusebio C, Lezo A. Nutritional management of patients with Fontan circulation: a potential for improved outcomes from birth to adulthood. Nutrients. (2022) 14(19). doi: 10.3390/nu14194055
40. Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, et al. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. (2013) 162(2):250–6.e2. doi: 10.1016/j.jpeds.2012.07.048
41. Anderson JB, Beekman RH 3rd, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. (2009) 138(2):397–404.e1. doi: 10.1016/j.jtcvs.2009.02.033
42. Ross FJ, Radman M, Jacobs ML, Sassano-Miguel C, Joffe DC, Hill KD, et al. Associations between anthropometric indices and outcomes of congenital heart operations in infants and young children: an analysis of data from the Society of Thoracic Surgeons database. Am Heart J. (2020) 224:85–97. doi: 10.1016/j.ahj.2020.03.012
43. Sekhon R, Foshaug RR, Kantor PF, Mansukhani G, Mackie AS, Hollander SA, et al. Incidence and impact of malnutrition in patients with Fontan physiology. JPEN J Parenter Enteral Nutr. (2023) 47(1):59–66. doi: 10.1002/jpen.2437
44. Tucker LA, Lecheminant JD, Bailey BW. Test-retest reliability of the bod pod: the effect of multiple assessments. Percept Mot Skills. (2014) 118(2):563–70. doi: 10.2466/03.PMS.118k15w5
Keywords: Fontan circulation, lifestyle intervention, fatigue, fears, body composition, rest energy expenditure
Citation: Scheffers LE, Helbing WA, Pereira T, Walet S, Utens EMWJ, Dulfer K and van den Berg LE (2023) A 12-week lifestyle intervention: effects on fatigue, fear, and nutritional status in children with a Fontan circulation. Front. Pediatr. 11:1154015. doi: 10.3389/fped.2023.1154015
Received: 30 January 2023; Accepted: 26 July 2023;
Published: 6 November 2023.
Edited by:
Patricia Longmuir, University of Ottawa, CanadaReviewed by:
Hedwig Hubertine Hövels-Gürich, University Hospital RWTH Aachen, Germany© 2023 Scheffers, Helbing, Pereira, Walet, Utens, Dulfer and van den Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: L. E. van den Berg bC5lLm0udmFuZGVuYmVyZ0BlcmFzbXVzbWMubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.