- 1Department of Pediatrics, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Department of Pediatrics, Guangxi Clinical Research Center for Pediatric Diseases, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Objectives: To systematically analyze adverse events (AEs) in treatment of spinal muscular atrophy (SMA) with Nusinersen in children and adolescents.
Methods: The study is registered on PROSPERO (CRD42022345589). Databases were searched and literature relating to Nusinersen in the treatment of spinal muscular atrophy in children from the start of database establishment to December 1, 2022, was retrospectively analyzed. R.3.6.3 statistical software was used, and random effects meta-analysis was performed to calculate weighted mean prevalence and 95% confidence intervals (CI).
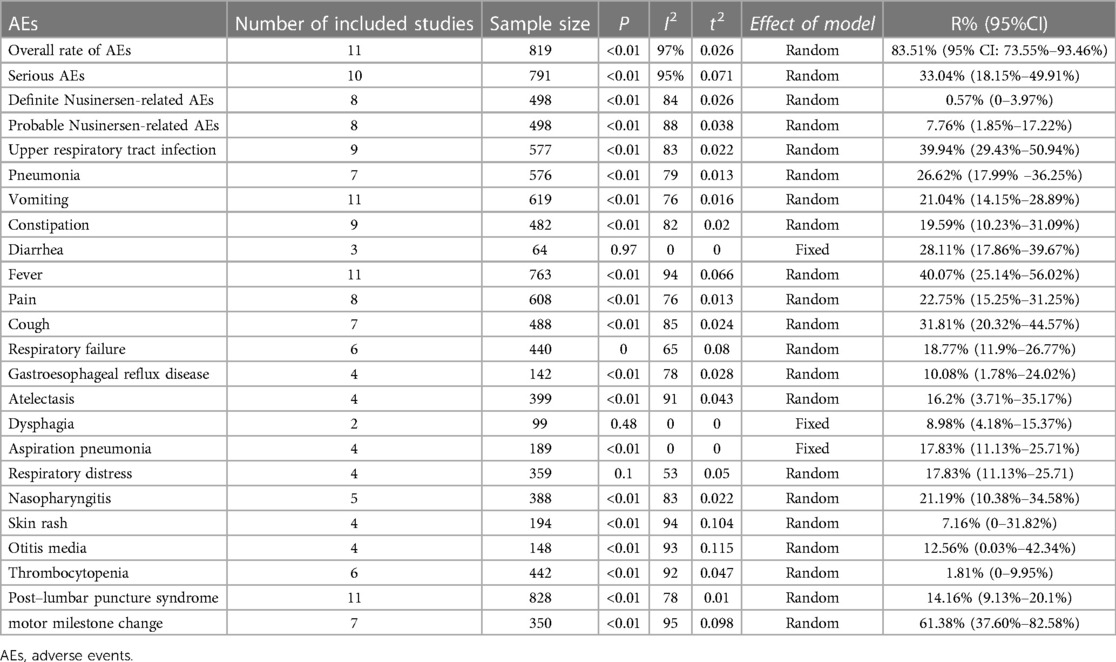
Results: In total, 15 eligible studies were included, with a total of 967 children. Rate of definite Nusinersen-related AEs was 0.57% (95% CI: 0%–3.97%), and probable Nusinersen-related AEs 7.76% (95% CI: 1.85%–17.22%). Overall rate of AEs was 83.51% (95% CI: 73.55%–93.46%), and serious AEs 33.04% (95% CI: 18.15%–49.91%). For main specific AEs, fever was most common, 40.07% (95% CI: 25.14%–56.02%), followed by upper respiratory tract infection 39.94% (95% CI: 29.43%–50.94%), and pneumonia 26.62% (95% CI: 17.99%–36.25%).
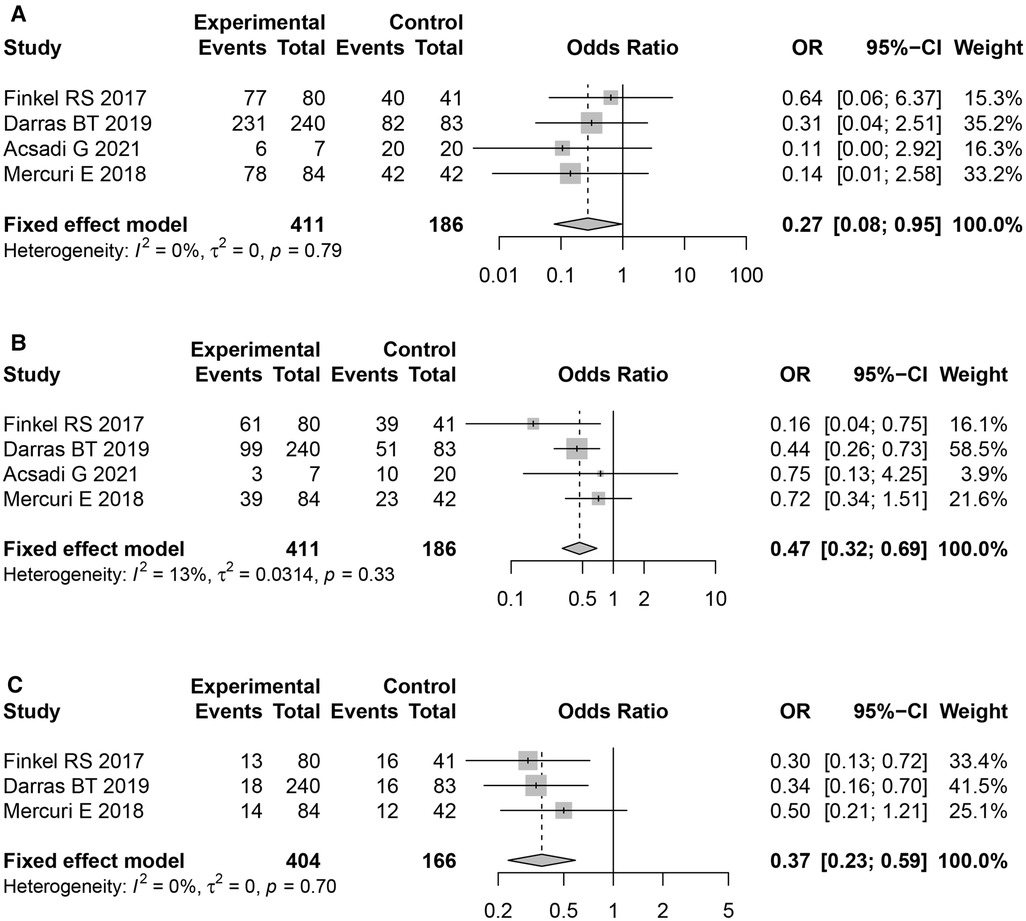
The difference in overall AE rates between the two groups (Nusinersen group and placebo group) was significant (OR = 0.27,95% CI: 0.08–0.95, P = 0.042). Moreover, incidence of serious adverse events, and fatal adverse events were both significantly lower than in the placebo group (OR = 0.47, 95%CI: 0.32–0.69, P < 0.01), and (OR = 0.37, 95%CI: 0.23–0.59, P < 0.01), respectively.
Conclusion: Nusinersen direct adverse events are rare, and it can effectively reduces common, serious, and fatal adverse events in children and adolescents with spinal muscular atrophy.
1. Introduction
Spinal muscular atrophy (SMA) is a progressive, autosomal recessive, motor neuron disease, which is caused by mutations on chromosome 5 of the SMN1 gene (1). The main clinical features of patients were progressive muscle weakness and muscle atrophy resulting from the degeneration of motor neurons in the anterior horn of spinal cord (2). The prevalence of SMA is 1–2 in 100,000 in developing countries (3). Furthermore, SMA is the most fatal genetic disease in children under 2 years of age; SMA affects the respiratory system, with respiratory failure being the most common cause of death (4). Nusinersen was the first approved disease-modifying treatment for SMA. It is an antisense oligonucleotide drug, which modifies pre-mRNA splicing to promote inclusion of SMN2 exon 7, which increases production of full-length SMN proteins (5). In clinical trials, Nusinersen was demonstrated to significantly improve both motor function and overall survival (6). As Nusinersen is unable to penetrate the blood-brain barrier, Nusinerson needs to be administered by repeat intrathecal injection at an injection dose of 12 mg (5 ml) each time. Typically, the first three doses are given 14 days apart, and a fourth dose given 30 days after the third dose. After this, maintenance therapy should be administered every 4 months, usually for more than 5 years (7). Platelet count, prothrombin time (PT), activated partial thromboplastin time (APTT) and urine protein should be measured before administration. Common adverse events of Nusinersen include respiratory infections and constipation, though some patients develop thrombocytopenia and coagulopathy, increasing their risk of nephrotoxicity (8). Nusinersen is proven to have significant clinical benefits for SMA in children, improving motor function.
Clinical use of a drug is associated with not only curative effect, but also the safety of the drug, which affects its clinical application. However, till date, there are no meta-analysis studies on the adverse events of nociception in children and adolescents. Therefore, this study used meta-analysis to systematically review existing published studies on adverse events during the treatment of SMA with Nusinersen in children and provide high-quality evidence for clinical practice.
2. Methods
The protocol of this systematic review and meta-analysis has been registered with the international prospective register of systematic reviews (PROSPERO) platform, following the PRISMA-P guidelines. The protocol is assigned the systematic review registration number, PROSPERO CRD42022345589, available from https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42022345589.
2.1. Search strategy and data extraction
PubMed, Web of Science, EMBASE, Medline, and Cochrane Library Databases were searched to collect literature relating to the use of Nusinersen for treatment of children with SMA. The search time limit was from the establishment of the database to December 1, 2022. At the same time as the online database and manual search were performed, the references of included literature were traced back. Both keywords and free text were used in the retrieval, and adjusted according to the different database characteristics. The search criteria for retrieval were as follows, consistently search for “Paediatrics” OR “Pediatrics” AND (Nusinersen) OR (SPINRAZA) AND (Spinal Muscular Atrophy) OR (SMA).
2.2. Literature screening and inclusion/exclusion criteria
In total, two researchers independently searched and screened the literature, collected data, and cross-checked. In the event of a dispute, a consensus was reached through discussion or negotiation with a further researcher. The inclusion criteria were defined firstly by study type, cohort study, case-control study, or case series analysis. Secondly, by research object, children who underwent Nusinersen treatment. Finally, by observation indicators, the number of samples and adverse events the study reported. Exclusion criteria included case reports, adult research, and either incomplete or missing data or an inability to obtain data from the study author.
2.3. Adverse event grade assessment
Adverse events were graded as mild or moderate (grades 1–2), and serious (grades 3–4) according to the Common Terminology Criteria for Adverse Events v5.0 (CTCAE v5.0) (U.S. Department of Health and Human Services; National Institutes of Health; National Cancer Institute, 2017) (9).
2.4. Literature quality evaluation
Quality assessment of the included studies was performed using different tools dependent on the study design. Randomized controlled trials were assessed following Cochrane collaboration handbook version 5.1.0. Case series quality assessments were performed following the IHE (Institute of Health Economics) quality appraisal checklist (Institute of Health Economics (IHE) (10).
2.5. Statistical analysis
R 3.6.3 statistical software was used for meta-analysis. Continuous variables were described by weight mean difference (WMD) and the 95% confidence interval (CI). Prior to meta-analysis, a normality test was performed to determine both before the original and the converted rate. The effect rate sizes for each independent sample were pooled to calculate both the incidence and 95% CI. Heterogeneity between the included studies was assessed by Q-test and I2 statistics. Dichotomous variables were described by relative risk (RR) and its 95% CI. In cases where there was no statistical heterogeneity among the results (P > 0.1 and I2 < 50%), the fixed-effects model was used for meta-analysis. Conversely, in cases indicative of statistical heterogeneity, the random-effects model was used for Meta-analysis (α = 0.05). Egger's method was then used to statistically test the publication deviation.
3. Results
3.1. Literature search
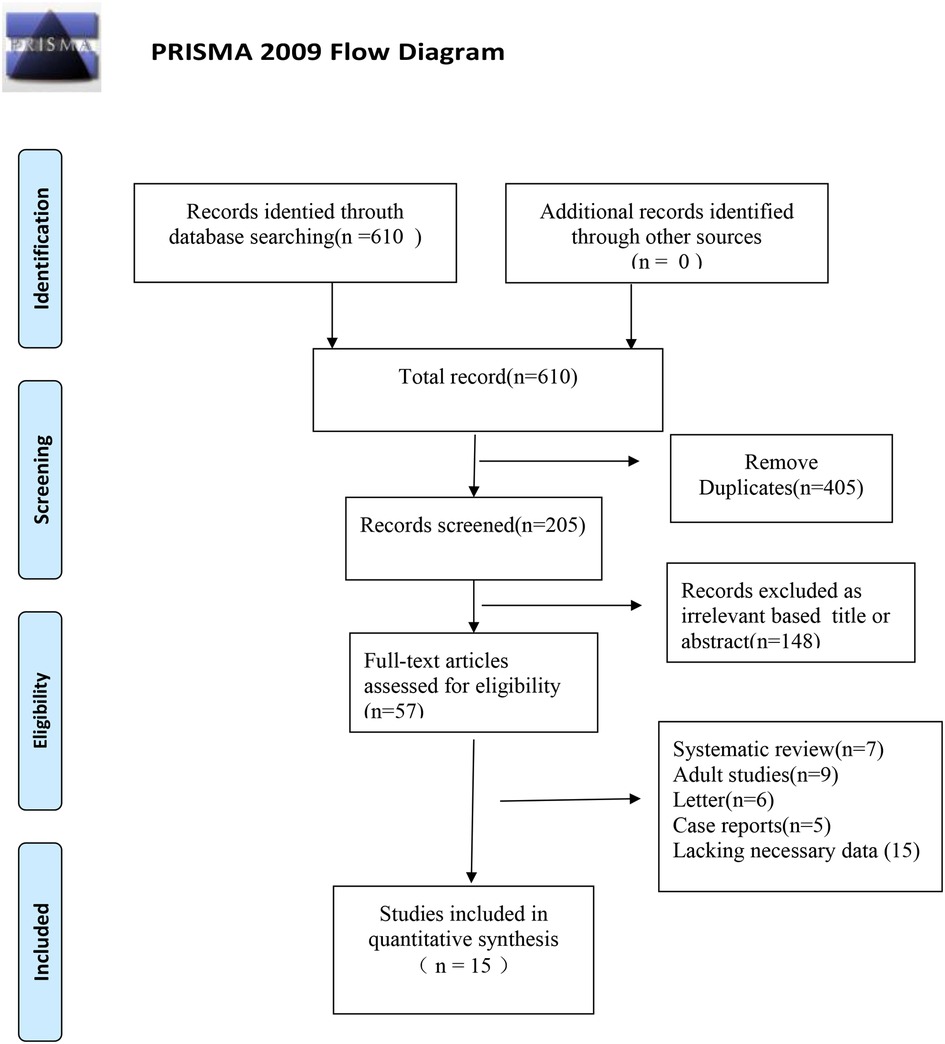
In total, 610 articles were retrieved from the literature databases. Endnote X9 was used to remove duplicate articles leaving 205 articles. After reading the titles and abstracts, 148 non-conforming articles were excluded, and 57 articles were included for double screening. After reading the full text, 15 articles (11–25) were included in the final review. The process and results of literature screening are shown in Figure 1.
3.2. Basic characteristics and quality evaluation of the study
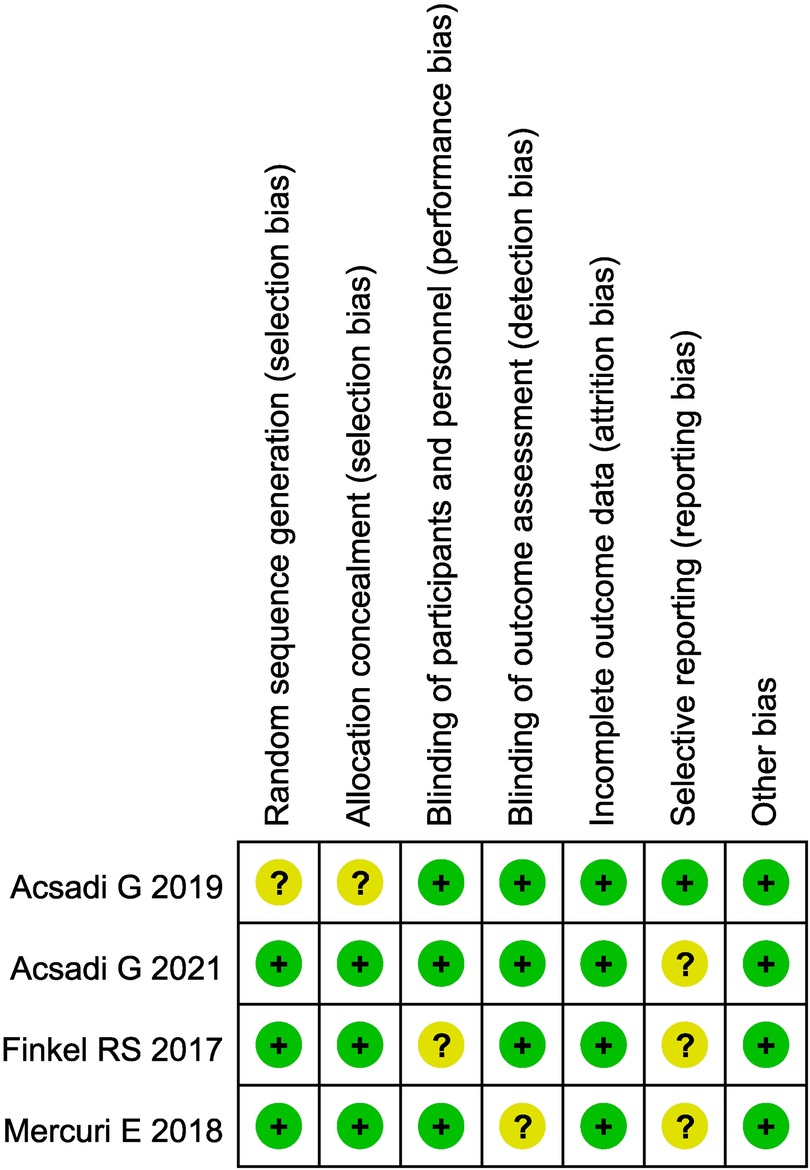
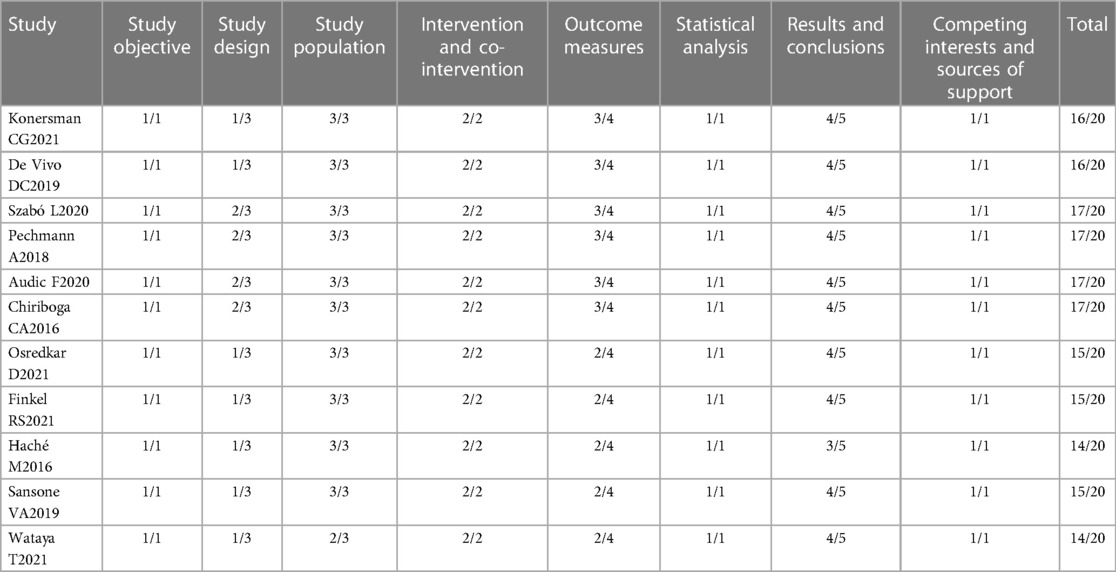
The basic characteristics and quality evaluation of the study included 15 articles in English, totaling 967 patients. There were 4 articles which were randomized controlled trials (RCT), the remaining 11 were case series. The basic characteristics of the included studies are shown in Table 1. Evaluation of the literature quality of the 4 RCTs is shown in Figure 2, and the 11 case series in Table 2.
3.3. Results of meta-analysis
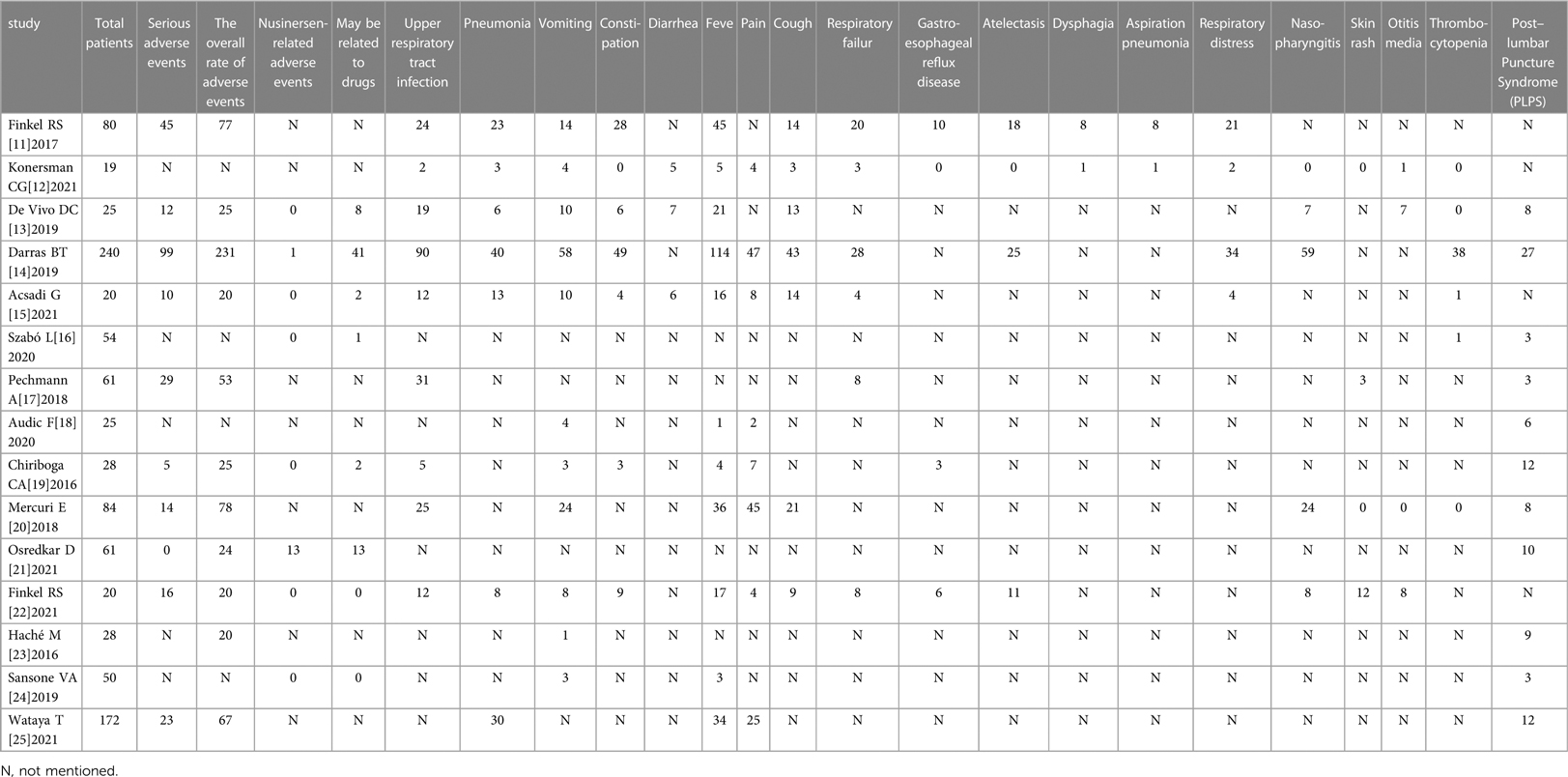
All the included literature studies were in English. Use of Nusinersen in the treatment of children with SMA had a motor milestone change of 61.38% (95%CI: 37.60%–82.58%). Most of the adverse events which occurred during Nusinersen treatment were related to the primary SMA disease and its related complications. The rate of definite Nusinersen-related adverse events was 0.57% (95% CI: 0%–3.97%), whilst the rate of probable Nusinersen-related adverse events was 7.76% (95% CI: 1.85%–17.22%). Moreover, the overall rate of adverse events was 83.51% (95% CI: 73.55%–93.46%), whilst the rate of serious adverse events was 33.04% (95% CI: 18.15%–49.91%). Among specific adverse events, fever was most common 40.07% (95% CI: 25.14%–56.02%), followed by upper respiratory tract infection 39.94% (95% CI: 29.43%–50.94%). Other specific adverse events included pneumonia 26.62% (95% CI: 17.99%–36.25%), pain 22.75% (95% CL: 15.25%–31.25%), respiratory failure 18.77% (95% CI: 11.9%–26.77%), respiratory distress 17.83% (95% CI: 11.13%–25.71%), aspiration pneumonia 17.83% (95% CI: 11.13%–25.71%), vomiting 21.4%, constipation 19.59%, diarrhea 28.11%, cough 31.81%, acid gastroesophageal reflux disease 10.08%, atelectasis 16.2%, dysphagia 8.98%, nasopharyngitis 21.19%, skin rash 7.16%, otitis media 12.56%, thrombocytopenia 1.81%, and post–lumbar puncture syndrome(PLPS) 14.16% (Table 3). Furthermore, a statistically significant difference in overall adverse event rates between the Nusinersen group and the placebo control group was observed [OR = 0.27,95% CI: 0.08–0.95, P = 0.042 (Figure 3A). The incidence of serious adverse events [OR = 0.47, 95%CI: 0.32–0.69, P < 0.01 (Figure 3B)], and fatal adverse events [OR = 0.47, 95%CI: 0.32–0.69, P < 0.01 (Figure 3B)], [OR = 0.37, 95%CI: 0.23–0.59, P < 0.01 (Figure 3C)] in the Nusinersen group were also significantly lower than in the placebo group.
3.4. Subgroup analysis
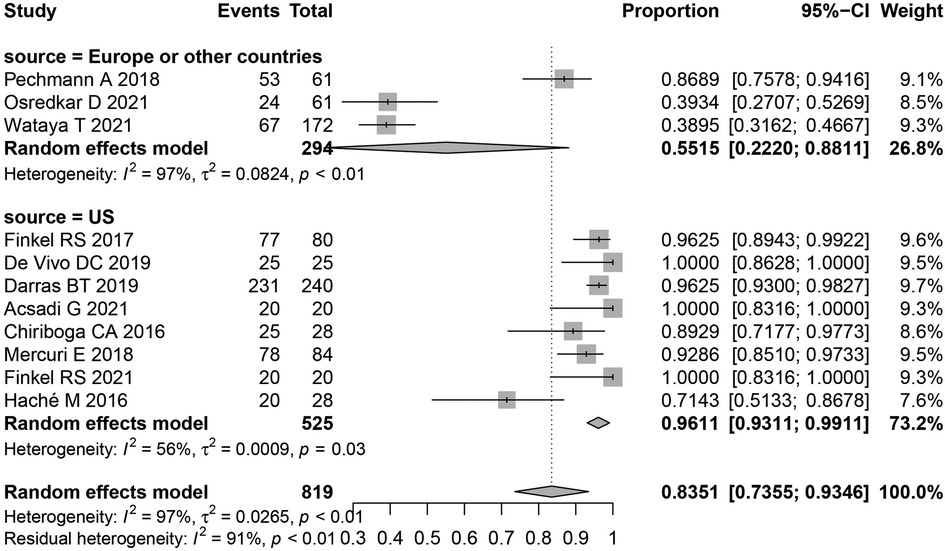
Most adverse events in this study were of high heterogeneity. Thus to determine the source of heterogeneity a subgroup analysis of overall adverse events was performed, subgroups included, United States, Europe, and other countries. The results following subgroup analysis were consistent with the overall results, indicating no significant differences between heterogeneity and overall heterogeneity in each subgroup. This suggests that the region studied was not the main source of heterogeneity (Figure 4).
4. Discussion
With the development and progression of SMA, muscle weakness can lead to abnormalities of the skeletal, respiratory, and digestive systems. Respiratory failure is the most common cause of death relating to SMA. For decades, treatment options for SMA have been limited to supportive physical, respiratory, and orthopedic corrective therapies, which aim to alleviate symptoms and improve residual function. Nusinersen was first approved by the U.S. Food and Drug Administration (FDA) in December 2016, as the first approved disease-modifying treatment for SMA, providing a new treatment hope (26). Nusinersen is an antisense oligonucleotide drug, which exerts its therapeutic effect through modification of pre-mRNA splicing to promote inclusion of SMN2 exon 7. This increases production of full-length SMN protein (27). Whilst Nusinersen has been shown to significantly improve motor function and overall survival, to date, it has not been curative, being effective only in relieving symptoms.
There have been no large-sample RCT studies of adverse events in pediatric patients with SMA treated with Nusinersen. In particular, long-term follow-up data of adverse events is lacking. In this meta-analysis study, the overall motor milestone change and adverse event rate in the treatment of SMA with Nusinersen were 61.38% and 83.51%, respectively. However, most adverse events were related to the primary SMA disease. In contrast, the rate of definite Nusinersen drug-related adverse events was 0.57% (95% CI: 0%–3.97%), and the rate of probable Nusinersen drug-related adverse events was 7.76% (95% CI: 1.85%–17.22%). The Darras BT (14) report showed that only 1 of the 240 patients with vomiting had a clear association with Nusinersen, whilst it was possible that Nusinersen caused headache (n = 9), fever (n = 8), back pain (n = 7), PLPS (n = 4), vomiting (n = 3) and tachycardia (n = 2). However, possibly, these effects could have been caused by the lumbar puncture procedure rather than Nusinersen. In a further study (14), 38 cases of thrombocytopenia were reported, though there were also thrombocytopenia cases in the SMA control group that did not receive Nusinersen. Therefore, it was not possible to assess whether thrombocytopenia was Nusinersen drug-induced or caused by SMA disease itself. However, Szab ól (16) reported 1 case of possible drug-induced thrombocytopenia. Moreover, Osredkar D (21) reported 13 cases of drug-related adverse reactions; however, all of them were mild adverse events, the majority of which occurred in the load stage of Nusinersen treatment.
Although most SMA patients treated with Nusinersen reported improvements in motor function, these improvements were not significant across all age groups. Moreover, whilst Nusinersin has received full contingent approval from the US FDA and the European Medicines Agency, Nusinersen is yet to be approved by other regulatory agencies in other countries, particularly in low and middle-income countries (28).
Nusinersen cannot cross the blood-brain barrier; therefore, Nusinersen is administered intrathecally. This presents challenges for pediatric patients with SMA, such as the need for repeated anesthesia/sedation. Especially in infants and young children, intrathecal administration increases anesthesia-related risks and the cost of surgical management. Moreover, intrathecal injection is associated with lower back pain, dizziness, and nausea. In this study, 14.16% of the PLPS were caused by the lumbar puncture procedure, rather than Nusinersen itself.
This review does have limitations. Firstly, there is a lack of long-term follow-up data of adverse events in children with SMA treated with Nusinersen, with the drug not having been officially approved for use in China until October 2019. Secondly, most of the included studies were case series with only small sample sizes. Different trial protocols may affect the true representation of the study population, thereby limiting the conclusions drawn in this review. Thirdly, there is large heterogeneity in the studies included in this meta-analysis, which may affect the accuracy of the results.
5. Conclusion
Currently, there is no cure for SMA; however, Nusinersen is the first choice and an effective and safe drug for SMA management, causing few directly drug-related adverse events. Moreover, Nusinersen can effectively reduce the incidence of general, serious, and fatal adverse events in SMA and its associated complications in children. This suggests that Nusinersen is worthy of continuation in SMA treatment, and further clinical investigation is warranted to maximize therapeutic benefits.
Author contribution
ZZ and and JW: reviewed the studies, extracted the data, and wrote the manuscript. ZZ and PZ: reviewed the draft, was involved in all stages of the review, and supervised the conduct of the study. HD: reviewed the studies and extracted the data. JW and ZZ: performed the statistical analysis of the studies and were involved in drafting the methods section of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge the authors of the included studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. (2015) 33(4):831–46. doi: 10.1016/j.ncl.2015.07.004
2. Singh RN, Singh NN. Mechanism of splicing regulation of spinal muscular atrophy genes. Adv Neurobiol. (2018) 20:31–61. doi: 10.1007/978-3-319-89689-2_2
3. Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. (2017) 12(1):124. doi: 10.1186/s13023-017-0671-8
4. Wijngaarde CA, Stam M, Otto LAM, van Eijk RPA, Cuppen I, Veldhoen ES, et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. (2020) 94(15):e1634–44. doi: 10.1212/WNL.0000000000009248
5. Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med. (2011) 3(72):72ra18. doi: 10.1126/scitranslmed.3001777
6. Chiriboga CA. Nusinersen for the treatment of spinal muscular atrophy. Expert Rev Neurother. (2017) 17(10):955–62. doi: 10.1080/14737175.2017.1364159
7. Hoy SM. Nusinersen: A review in 5q spinal muscular atrophy. CNS Drugs. (2021) 35(12):1317–28. doi: 10.1007/s40263-018-0545-1
8. Maggi L, Bello L, Bonanno S, Govoni A, Caponnetto C, Passamano L, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. (2020) 91(11):1166–74. doi: 10.1136/jnnp-2020-323822
9. CTCAE v5.0 incorporates certain elements of the MedDRA terminology. For further details on MedDRA refer to the MedDRA MSSO Web site, https://www.meddra.org/
10. Institute of Health Economics (IHE). Quality appraisal of case series studies checklist. Edmonton, AB: Institute of Health Economics (2014). Retrieved from http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about
11. Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. (2017) 377(18):1723–32. doi: 10.1056/NEJMoa1702752
12. Konersman CG, Ewing E, Yaszay B, Naheedy J, Murphy S, Skalsky A. Nusinersen treatment of older children and adults with spinal muscular atrophy. Neuromuscul Disord. (2021) 31(3):183–93. doi: 10.1016/j.nmd.2020.12.006
13. De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. (2019) 29(11):842–56. doi: 10.1016/j.nmd.2019.09.007
14. Darras BT, Farrar MA, Mercuri E, Finkel RS, Foster R, Hughes SG, et al. An integrated safety analysis of infants and children with symptomatic spinal muscular atrophy (SMA) treated with nusinersen in seven clinical trials. CNS Drugs. (2019) 33(9):919–32. doi: 10.1007/s40263-019-00656-w
15. Acsadi G, Crawford TO, Müller-Felber W, Shieh PB, Richardson R, Natarajan N, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: the EMBRACE study. Muscle Nerve. (2021) 63(5):668–77. doi: 10.1002/mus.27187
16. Szabó L, Gergely A, Jakus R, Fogarasi A, Grosz Z, Molnár MJ, et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: real world data from Hungarian patients. Eur J Paediatr Neurol. (2020) 27:37–42. doi: 10.1016/j.ejpn.2020.05.002
17. Pechmann A, Langer T, Schorling D, Stein S, Vogt S, Schara U, et al. Evaluation of children with SMA type 1 under treatment with nusinersen within the expanded access program in Germany. J Neuromuscul Dis. (2018) 5(2):135–43. doi: 10.3233/JND-180315
18. Audic F, de la Banda MGG, Bernoux D, Ramirez-Garcia P, Durigneux J, Barnerias C, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. (2020) 15(1):148. doi: 10.1186/s13023-020-01414-8
19. Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, et al. Results from a phase 1 study of nusinersen [ISIS-SMN(Rx)] in children with spinal muscular atrophy. Neurology. (2016) 86(10):890–7. doi: 10.1212/WNL.0000000000002445
20. Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. (2018) 378(7):625–35. doi: 10.1056/NEJMoa1710504
21. Osredkar D, Jílková M, Butenko T, Loboda T, Golli T, Fuchsová P, et al. Children and young adults with spinal muscular atrophy treated with nusinersen. Eur J Paediatr Neurol. (2021) 30:1–8. doi: 10.1016/j.ejpn.2020.11.004
22. Hoy SM. Nusinersen: first global approval. Drugs. (2017) 77(4):473–79. doi: 10.1007/s40265-017-0711-7
23. Haché M, Swoboda KJ, Sethna N, Farrow-Gillespie A, Khandji A, Xia S, et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J Child Neurol. (2016) 31(7):899–906. doi: 10.1177/0883073815627882
24. Sansone VA, Albamonte E, Salmin F, Casiraghi J, Pirola A, Bettinelli M, et al. Intrathecal nusinersen treatment for SMA in a dedicated neuromuscular clinic: an example of multidisciplinary and integrated care. Neurol Sci. (2019) 40(2):327–32. doi: 10.1007/s10072-018-3622-9
25. Wataya T, Takasaki S, Hoshino M, Makioka H, Nakamura G, Matsuda N. Real-world safety of nusinersen in Japan: results from an interim analysis of a post-marketing surveillance and safety database. Int J Neurosci. (2021):1–13. doi: 10.1080/00207454.2021.1995382
26. Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc Health. (2021) 5(7):491–500. doi: 10.1016/S2352-4642(21)00100-0
27. Messina S, Sframeli M. New treatments in spinal muscular atrophy: positive results and new challenges. J Clin Med. (2020) 9(7). doi: 10.3390/jcm9072222
Keywords: spinal muscular atrophy, nusinersen, SMA, meta, AES
Citation: Zhong Z-J, Zheng P-M, Dou H-H and Wang Ji-Gan (2023) Adverse events in the treatment of spinal muscular atrophy in children and adolescents with nusinersen: A systematic review and meta-analysis. Front. Pediatr. 11:1152318. doi: 10.3389/fped.2023.1152318
Received: 27 January 2023; Accepted: 7 April 2023;
Published: 25 April 2023.
Edited by:
Daria Diodato, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Marco Piastra, Catholic University of the Sacred Heart, ItalyVesna Brankovic, Child Neurology Association of Serbia, Serbia
© 2023 Zhong, Zheng, Dou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Gan Wang d2FuZ2ppZ2FuQDE2My5jb20=
†These authors have contributed equally to this work
 Zhi-Juan Zhong1,†
Zhi-Juan Zhong1,† Ji-Gan Wang
Ji-Gan Wang